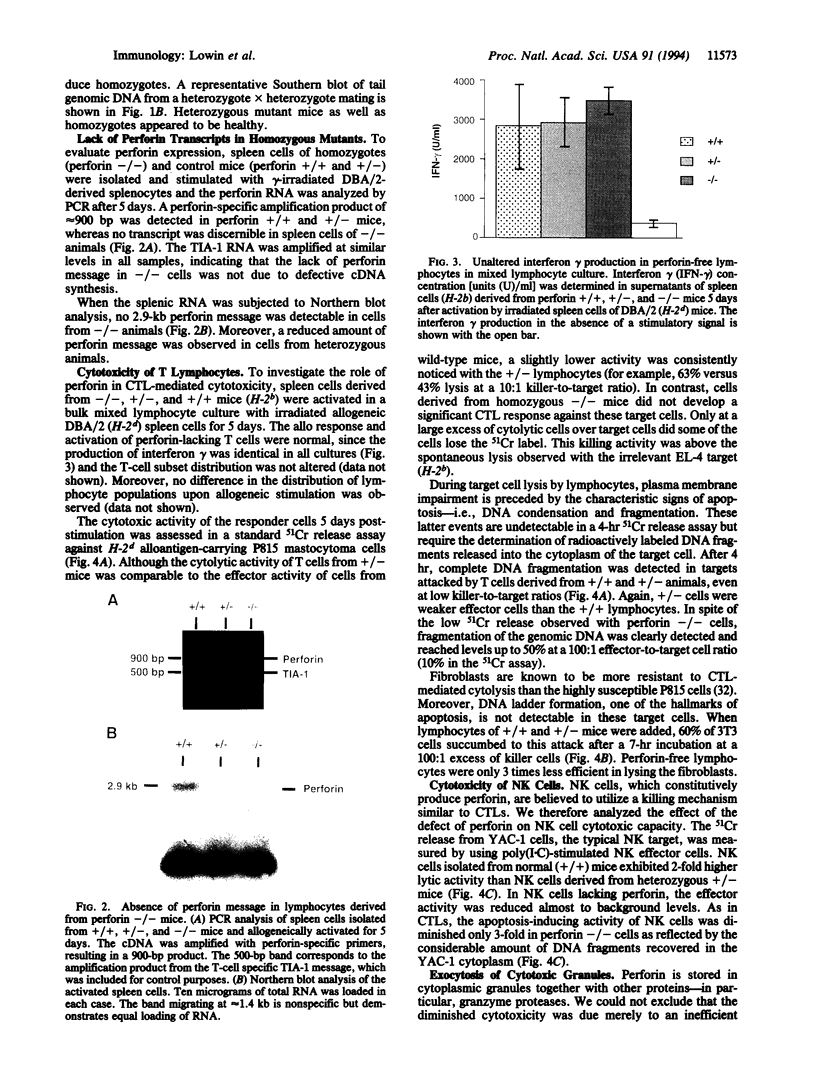
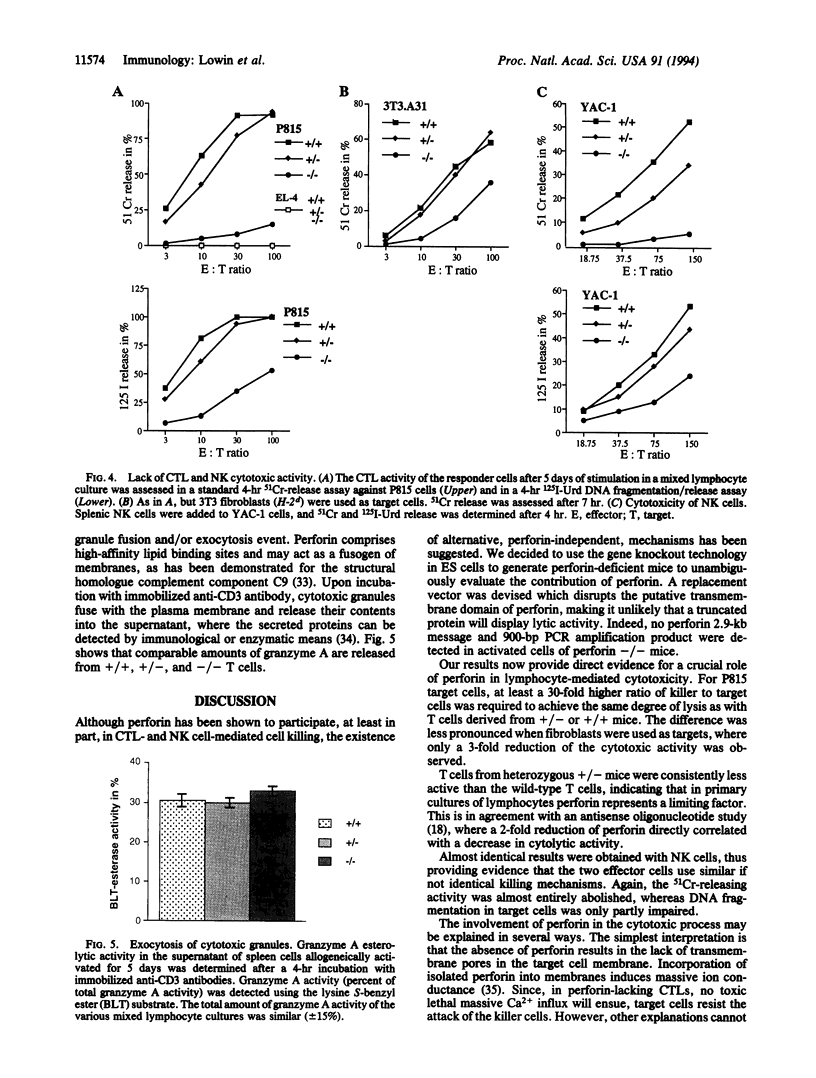
Abstract
Lymphocyte-mediated cytotoxicity has been proposed to consist of the polarized secretion of granule-stored perforin leading to target-cell lysis. Nevertheless, perforin-independent pathways were postulated to explain the cytolytic activity of apparently perforin-free lymphocytes and the DNA degradation found in dying target cells. To evaluate the role of perforin, we used gene targeting in embryonic stem cells to produce mice lacking perforin. Mice homozygous for the disrupted gene have no perforin mRNA. The mice are healthy. Activation and granzyme A secretion of perforin-free cytolytic T cells are unaltered. The killing activity of cytolytic T cells as well as natural killer (NK) cells, however, is impaired but not abolished. Approximately one-third of the killing activity remains when lysis of 3T3 fibroblast targets and the apoptotic cell death of YAC-1 NK targets are analyzed. We conclude that perforin is a crucial effector molecule in T cell- and NK cell-mediated cytolysis. However, alternative perforin-independent lytic mechanisms also exist.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Scarpellino L., Hertig S., Dupuis M., Tschopp J. Inhibition of lymphocyte mediated cytotoxicity by perforin antisense oligonucleotides. EMBO J. 1990 Dec;9(12):3815–3819. doi: 10.1002/j.1460-2075.1990.tb07599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Persechini P. M., Chang S., Liu C. C., Cohen J. J., Young J. D. Purified perforin induces target cell lysis but not DNA fragmentation. J Exp Med. 1989 Oct 1;170(4):1451–1456. doi: 10.1084/jem.170.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis M., Peitsch M. C., Hamann U., Stanley K. K., Tschopp J. Mutations in the putative lipid-interaction domain of complement C9 result in defective secretion of the functional protein. Mol Immunol. 1993 Jan;30(1):95–100. doi: 10.1016/0161-5890(93)90430-j. [DOI] [PubMed] [Google Scholar]
- Dupuis M., Schaerer E., Krause K. H., Tschopp J. The calcium-binding protein calreticulin is a major constituent of lytic granules in cytolytic T lymphocytes. J Exp Med. 1993 Jan 1;177(1):1–7. doi: 10.1084/jem.177.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromkowski S. H., Brown T. C., Masson D., Tschopp J. Lack of DNA degradation in target cells lysed by granules derived from cytolytic T lymphocytes. J Immunol. 1988 Aug 1;141(3):774–778. [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Granzymes, a family of serine proteases released from granules of cytolytic T lymphocytes upon T cell receptor stimulation. Immunol Rev. 1988 Mar;103:53–71. doi: 10.1111/j.1600-065x.1988.tb00749.x. [DOI] [PubMed] [Google Scholar]
- Krähenbühl O., Tschopp J. Debate: the mechanism of lymphocyte-mediated killing. Perforin-induced pore formation. Immunol Today. 1991 Nov;12(11):399–403. doi: 10.1016/0167-5699(91)90139-k. [DOI] [PubMed] [Google Scholar]
- Kägi D., Ledermann B., Bürki K., Seiler P., Odermatt B., Olsen K. J., Podack E. R., Zinkernagel R. M., Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994 May 5;369(6475):31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P., Schmidt R. E., Caulfield J. P., Hein A., Bartley G. T., Ritz J., Schlossman S. F., Austen K. F., Stevens R. L. Proteoglycans in cell-mediated cytotoxicity. Identification, localization, and exocytosis of a chondroitin sulfate proteoglycan from human cloned natural killer cells during target cell lysis. J Exp Med. 1985 Dec 1;162(6):1771–1787. doi: 10.1084/jem.162.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryanski J. L., MacDonald H. R., Cerottini J. C. Limiting dilution analysis of alloantigen-reactive T lymphocytes. IV. High frequency of cytolytic T lymphocyte precursor cells in MLC blasts separated by velocity sedimentation. J Immunol. 1980 Jan;124(1):42–47. [PubMed] [Google Scholar]
- Masson D., Tschopp J. Isolation of a lytic, pore-forming protein (perforin) from cytolytic T-lymphocytes. J Biol Chem. 1985 Aug 5;260(16):9069–9072. [PubMed] [Google Scholar]
- Miescher G. C., Schreyer M., MacDonald H. R. Production and characterization of a rat monoclonal antibody against the murine CD3 molecular complex. Immunol Lett. 1989 Dec;23(2):113–118. doi: 10.1016/0165-2478(89)90122-3. [DOI] [PubMed] [Google Scholar]
- Millard P. J., Henkart M. P., Reynolds C. W., Henkart P. A. Purification and properties of cytoplasmic granules from cytotoxic rat LGL tumors. J Immunol. 1984 Jun;132(6):3197–3204. [PubMed] [Google Scholar]
- Morelli L., Lusignan Y., Lemieux S. Heterogeneity of natural killer cell subsets in NK-1.1+ and NK-1.1- inbred mouse strains and their progeny. Cell Immunol. 1992 Apr 15;141(1):148–160. doi: 10.1016/0008-8749(92)90134-b. [DOI] [PubMed] [Google Scholar]
- Müller I., Kropf P., Etges R. J., Louis J. A. Gamma interferon response in secondary Leishmania major infection: role of CD8+ T cells. Infect Immun. 1993 Sep;61(9):3730–3738. doi: 10.1128/iai.61.9.3730-3738.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard H. L., Kane K. P., Mescher M. F., Clark W. R. Cytotoxic T lymphocyte mediated lysis without release of serine esterase. Nature. 1987 Nov 5;330(6143):71–72. doi: 10.1038/330071a0. [DOI] [PubMed] [Google Scholar]
- Peitsch M. C., Amiguet P., Guy R., Brunner J., Maizel J. V., Jr, Tschopp J. Localization and molecular modelling of the membrane-inserted domain of the ninth component of human complement and perforin. Mol Immunol. 1990 Jul;27(7):589–602. doi: 10.1016/0161-5890(90)90001-g. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Young J. D., Cohn Z. A. Isolation and biochemical and functional characterization of perforin 1 from cytolytic T-cell granules. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8629–8633. doi: 10.1073/pnas.82.24.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvier E., Luciani M. F., Golstein P. Fas involvement in Ca(2+)-independent T cell-mediated cytotoxicity. J Exp Med. 1993 Jan 1;177(1):195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellins K. S., Cohen J. J. Cytotoxic T lymphocytes induce different types of DNA damage in target cells of different origins. J Immunol. 1991 Aug 1;147(3):795–803. [PubMed] [Google Scholar]
- Shi L., Kam C. M., Powers J. C., Aebersold R., Greenberg A. H. Purification of three cytotoxic lymphocyte granule serine proteases that induce apoptosis through distinct substrate and target cell interactions. J Exp Med. 1992 Dec 1;176(6):1521–1529. doi: 10.1084/jem.176.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiver J. W., Henkart P. A. A noncytotoxic mast cell tumor line exhibits potent IgE-dependent cytotoxicity after transfection with the cytolysin/perforin gene. Cell. 1991 Mar 22;64(6):1175–1181. doi: 10.1016/0092-8674(91)90272-z. [DOI] [PubMed] [Google Scholar]
- Shiver J. W., Su L., Henkart P. A. Cytotoxicity with target DNA breakdown by rat basophilic leukemia cells expressing both cytolysin and granzyme A. Cell. 1992 Oct 16;71(2):315–322. doi: 10.1016/0092-8674(92)90359-k. [DOI] [PubMed] [Google Scholar]
- Takayama H., Trenn G., Humphrey W., Jr, Bluestone J. A., Henkart P. A., Sitkovsky M. V. Antigen receptor-triggered secretion of a trypsin-type esterase from cytotoxic T lymphocytes. J Immunol. 1987 Jan 15;138(2):566–569. [PubMed] [Google Scholar]
- Takayama H., Trenn G., Sitkovsky M. V. A novel cytotoxic T lymphocyte activation assay. Optimized conditions for antigen receptor triggered granule enzyme secretion. J Immunol Methods. 1987 Nov 23;104(1-2):183–190. doi: 10.1016/0022-1759(87)90502-3. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Tian Q., Streuli M., Saito H., Schlossman S. F., Anderson P. A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell. 1991 Nov 1;67(3):629–639. doi: 10.1016/0092-8674(91)90536-8. [DOI] [PubMed] [Google Scholar]
- Trapani J. A., Kwon B. S., Kozak C. A., Chintamaneni C., Young J. D., Dupont B. Genomic organization of the mouse pore-forming protein (perforin) gene and localization to chromosome 10. Similarities to and differences from C9. J Exp Med. 1990 Feb 1;171(2):545–557. doi: 10.1084/jem.171.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Trenn G., Takayama H., Sitkovsky M. V. Exocytosis of cytolytic granules may not be required for target cell lysis by cytotoxic T-lymphocytes. Nature. 1987 Nov 5;330(6143):72–74. doi: 10.1038/330072a0. [DOI] [PubMed] [Google Scholar]
- Tschopp J., Müller-Eberhard H. J., Podack E. R. Formation of transmembrane tubules by spontaneous polymerization of the hydrophilic complement protein C9. Nature. 1982 Aug 5;298(5874):534–538. doi: 10.1038/298534a0. [DOI] [PubMed] [Google Scholar]
- Tschopp J., Nabholz M. Perforin-mediated target cell lysis by cytolytic T lymphocytes. Annu Rev Immunol. 1990;8:279–302. doi: 10.1146/annurev.iy.08.040190.001431. [DOI] [PubMed] [Google Scholar]
- Ucker D. S., Obermiller P. S., Eckhart W., Apgar J. R., Berger N. A., Meyers J. Genome digestion is a dispensable consequence of physiological cell death mediated by cytotoxic T lymphocytes. Mol Cell Biol. 1992 Jul;12(7):3060–3069. doi: 10.1128/mcb.12.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Nathan C. F., Podack E. R., Palladino M. A., Cohn Z. A. Functional channel formation associated with cytotoxic T-cell granules. Proc Natl Acad Sci U S A. 1986 Jan;83(1):150–154. doi: 10.1073/pnas.83.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]