Abstract
Objective
Components of metabolic syndrome (MS) have been individually linked to colorectal cancer risk and prognosis; however, an understanding of the dominant mechanisms is lacking.
Materials and methods
Twenty-one patients (10 MS; 11 non-MS) with resectable colorectal cancer were prospectively enrolled. Patients were classified for MS by the World Health Organization criteria and tested for circulating vascular endothelial growth factor (VEGF), interleukin-6 (IL-6), insulin-like growth factor-1 (IGF-1), fasting insulin, and tumor expression of IGF-1 receptor (IGF-1R), insulin-receptor (IR) and receptor for advanced glycation end-products (RAGE). Circulating markers were re-tested 6 months after surgery.
Results
The MS group had significantly higher baseline and post-operative fasting insulin levels (p < 0.001 and 0.003). No differences were observed in circulating IL-6, VEGF, IGF-1 and free IGF-1. By immunohistochemistry (IHC), IGF-1R expression was significantly higher in tumor vs. normal tissues (p < 0.001) while IR expression showed no difference. Interestingly, 64% of tumors demonstrated high IR positivity in the vessels within or surrounding the tumor stroma, but not in the vessels away from the tumor. By reverse transcription polymerase chain reaction (RT-PCR), tumor IGF-1R over-expression (80%) was confirmed, but there was no difference between MS and non-MS patients. Tumor RAGE over-expression was found in 67% of patients and was equally distributed between the two groups.
Conclusions
Hyperinsulinemia was the only significant factor distinguishing patients with colorectal cancer who have MS. The preferential over-expression of IR in the peri-tumoral microvessels suggests that hyperinsulinemia might contribute to colorectal cancer growth by enhancing angiogenesis.
Keywords: Colorectal cancer, Metabolic syndrome, IGF-1, Insulin receptor, Hyperinsulinemia, Tumor vasculature, Elderly
1. Introduction
Older patients with cancer have on average 3 comorbidities.1–3 As an increasing amount of epidemiological evidence shows an impact of these comorbidities on cancer incidence and prognosis, it is essential for good quality care of the elderly to understand the mechanisms by which these diseases interact with cancer. One of the most prevalent comorbidities is metabolic syndrome. In the United States, this syndrome affects ~22% of the adult population, and its prevalence increases with age from 5% for subjects in their twenties to above 40% for people above the age of 60.4 The prevalence of obesity and MS is one of the great epidemics of the early 21st century. It has risen significantly over the last few decades and is expected to rise further.
The metabolic syndrome (MS), also called the insulin resistance syndrome, encompasses several metabolic and physiologic disturbances. In 1998, the World Health Organization (WHO) developed a definition of MS based on the individuals showing evidence of insulin resistance and at least 2 of 4 other factors, including hypertension, dyslipidemia, central obesity and microalbuminuria.5 In 2001, the National Cholesterol Education Program developed an alternative definition, which required 3 or more of the following 5 factors to be present: increased waist circumference, hypertriglyceridemia, low high-density lipoprotein (HDL) cholesterol, hypertension, and elevated fasting glucose.4 The MS and diabetes are known risk factors for colon cancer and these patients have a higher rate of relapse of their cancer as well.6–8 Several potential mechanisms have been proposed. However, their relative contribution to outcome in humans has not been assessed. It is important to identify the dominant mechanisms involved so that targeted therapeutic strategies can be designed. In the present study, we compared the circulating level or tissue expression of cancer-associated factors between older colorectal cancer (CRC) patients with and without MS. These included components of biologic pathways related to obesity,9 hyperlipidemia,10 insulin signaling,11 insulin-growth factor-1 (IGF-1) signaling,12 vascular endothelial growth factor (VEGF),13 inflammation,14,15 intratumoral immunity,16 and advanced glycation end products.17 We assessed the host parameters before surgery and 6 months after surgery in order to help differentiate those triggered when the tumor was present versus those present chronically.
2. Methods
2.1. Patients and Assessment
From March 2006 to July 2009, 21 patients who were 60 years or older with biopsy proven resectable CRC and planned for curative surgery at Moffitt Cancer Center were enrolled. The WHO criteria were adapted for classification of patients into MS vs. non-MS groups. Insulin resistance was defined as a homeostatic model assessment (HOMA) score18 greater than 1. The calculation was done by the downloadable calculator at http://www.dtu.ox.ac.uk/homacalculator/index.php. Receiving drugs for diabetes, hyperlipidemia, or hypertension were taken as evidence of the disease, if the corresponding laboratory values were not present at the time of study entry.
At baseline, history and physical (H&P), MS-related parameters, ECOG (Eastern Cooperative Oncology Group) performance status, Activities of Daily Living (ADL), instrumental ADL, comorbidity, medications and Cumulative Illness Rating Scale-Geriatric (CIRS-G) were recorded. Blood samples were drawn to assess metabolic parameters, and for the cytokine and circulating tissue marker assays. A second H&P, MS-related parameters, and blood sample collection were obtained 6 months after surgery, or 1 month after the last dose of chemotherapy if the patient was receiving adjuvant chemotherapy, whichever came last. Surgical tissue was collected at the time of surgery, tissue not used for clinical purposes was macrodissected, split into paraffin-embedded blocks and a flash frozen sample was saved for molecular analysis. Cases with <1.5 mm2 of tumor available for this study were not included. The same procedure was used for a sample of adjacent normal tissue.
2.2. Circulating Cytokine Level and Tissue Markers
Circulating levels of free IGF-1, total IGF-1, interleukin-6 (IL-6), and VEGF were determined by chemiluminescent or horseradish peroxidase based ELISA (enzyme-linked immunosorbent assay) kits. The free and total IGF-1 ELISA kits were purchased from Beckman Coulter (Brea, CA) and Alpco Diagnostics (Salem, NH), respectively. The IL-6 and VEGF chemiluminescent ELISAs were purchased from R&D Systems (Minneapolis, MN). The ELISAs were performed according to the manufacturer’s instructions. The IGF-1, free IGF-1, VEGF-1 and IL-6 ELISA immunoassays have been calibrated by the manufacturers to National Institute for Biological Standards and Control (NIBSC)/WHO international standards 02/254, 87/518, 02/286 and 89/548, respectively. Each manufacturer has checked specificity for each ELISA by spiking the assay with similar cytokines at a concentration of 200 ng/ml. The only reported cross-reactivity was a 5% value when VEGF165/PlGF was added to the VEGF-1 ELISA.
2.3. Immunohistochemistry (IHC)
Formalin-fixed, paraffin-embedded tissue was cut in 4-μm sections. IHC was done using a Ventana Discovery XT automated system (Ventana Medical Systems, Tucson, AZ) per manufacturer’s protocol. For IGF-1R staining, the mouse monoclonal antibody that reacts to IGF1-R alpha subunit (#AHR0321, Invitrogen, Carlsbad, CA) was used at a 1:25 concentration in Dako antibody diluent (Carpinteria, CA). For insulin-R staining, the anti-insulin-R mouse monoclonal antibody (#ab54268, Abcam, Cambridge, MA), was used at a prediluted concentration. The other 2 primary antibodies used were anti-CD68 mouse monoclonal antibody (#760-2931, Ventana, Tucson, AZ, at prediluted concentration) and anti-S100 rabbit monoclonal antibody (#760-2523, Ventana, Tucson, AZ, at prediluted concentration). The Ventana anti-mouse or anti-rabbit secondary antibody was then introduced. The detection system used was the Ventana OmniMap kit and slides were counter-stained with Hematoxylin. The positive control for insulin receptor and IGF-1 receptor was placental tissue; for CD-68, lymph node tissue, and for S-100, melanoma. The negative controls were the same tissues, with the primary antibody omitted during the incubation. The IGF-1R and IR stains were scored according to the Allred 8-unit system using the combination of a proportion score from 0 to 5 and an intensity score on a scale of 0 to 3.19 For the assessment of macrophages and Langerhans cell infiltration, microscopic evaluation of CD68 and S100 stained tumor sections was done at ×2000 magnification (10 × 100 ocular grid, observed area 0.25 mm2). Stained cells were counted in 5 fields of tumor area, containing the highest number of macrophages, selected from each slide. The sum of the total number of staining positive tumor cells was used for statistical analysis. All the slides were read by an experienced pathologist (DC) blinded to the metabolic syndrome status of the patients.
2.4. RNA Extraction and Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analysis
Total RNA was extracted utilizing RNeasy Kit (Qiagen, Hilden, Germany) as described in the manufacturer’s protocol. RNA absorbance was measured by NanoDrop. Complementary DNA (cDNA) synthesis was conducted utilizing total RNA extracted from both normal and tumor tissues at 20 μl in volume using a cDNA synthesis kit (I-script cDNA Synthesis kit, Bio-Rad, Hercules, CA). Primers for the RT-PCR assay were designed from the GenBank mRNA sequence (Table 1). 2 μl of cDNA per sample was used in each 25 μl PCR reaction. Housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control and added to the PCR reaction after 10th PCR cycles. 5 μl of PCR product was run in an 8% polyacrylamide gel to confirm the gene expression and the product size.
Table 1.
Primers and probes for RT-PCR.
| Gene | Product size | Primer/probe | Sequence |
|---|---|---|---|
| RAGE | 133 bp | Forward | 5′ AAAGCTTTCGGGAGTGGTCAGACT 3′ |
| Reverse | 5′ ATCTGTCTTCTTGCTC GCAGGGAT 3′ | ||
| IR | 182 bp | Forward | 5′ TTTGGGAAATCACCAGCTTGGCAG 3′ |
| Reverse | 5′ AATCTCCAGG AAGGTTGGCCTCAT 3′ | ||
| IGF-1 | 423 bp | Forward | 5′TGAAGATGCACA CCATGTCCTCCT3′ |
| Reverse | 5′TGCACTC CCTCTA CTTGCG TTCTT 3′ | ||
| IGF-1R | 267 bp | Forward | 5′ GCGGCTGGAAACTCTTCTACAACT 3′ |
| Reverse | 5′ TGGTCTT CTCACACATCGGCTTCT 3′ |
Abbreviations: RT-PCR: reverse transcription polymerase chain reaction; RAGE: receptor for advanced glycation end-products; IR: insulin-receptor; IGF-1: insulin-like growth factor-1; IGF-1R: insulin-like growth factor-1 receptor.
2.5. Statistical Analysis
MS and non-MS groups were compared with respect to their MS-related parameters, circulating cytokines, and tissue markers at 2 time points, i.e. before surgery and after surgery. Comparisons were made using values at each time point and difference scores between time points to assess whether scores changed differentially between groups. The expression levels of IGF-1R and IR in tumors were compared to those in normal tissues. Tumor infiltration by histiocytes and macrophages was compared between MS and non-MS groups. Between group comparisons were made using the Wilcoxon signed-ranks test. All the tests were 2-sided with a threshold of 0.05 for significance.
The protocol was approved by the Institutional Review Board of the University of South Florida.
3. Results
3.1. Clinicopathological Features
Patient clinicopathological characteristics are detailed in Table 2. Ten patients with MS and 11 patients without MS were enrolled. All the patients had baseline measurements including clinical parameters and lab work on the day of pre-operative evaluation. Three patients did not have post-op blood sample collection. Two of them were from MS group and 1 from the non-MS group. Besides the components of MS, our patients had a low level of comorbidity, with a mean of 0.8 for grade 3 comorbidities, and no grade 4 comorbidities.
Table 2.
Patient clinicopathological characteristics.
| Factor | No. of patients | % | Average scores |
|---|---|---|---|
| Median age, years | 73.8 (range 63–93) | ||
| Sex | |||
| Male | 12 | 57.1 | |
| Female | 9 | 42.9 | |
| ECOG PS (0/1) | |||
| Before Sx | 21/0 | 100/0 | |
| After Sx | 18/2 | 90/10 | |
| Differentiation | |||
| Well | 4 | 19.0 | |
| Moderate | 13 | 61.9 | |
| Poor | 4 | 19.0 | |
| Chemotherapy | |||
| Yes | 3 | 14.3 | |
| No | 18 | 85.7 | |
| Stage I/II/III | 6/11/4 | ||
| IADL | |||
| Before Sx | 27.9 | ||
| After Sx | 27.8 | ||
| ADL | |||
| Before Sx | 0 | ||
| After Sx | 0 | ||
| CIRS-G categories | 5 | ||
| CIRS-G score | 8.4 | ||
| CIRS-G severity | 1.6 | ||
| CIRS-G level 3 | 0.8 | ||
| CIRS-G level 4 | 0 | ||
ECOG PS: Eastern Cooperative Oncology Group performance status; ADL: activities of daily living (0 = independent); IADL: instrumental activities of daily living (max. score = 29); CIRS-G: cumulative illness rating scale for geriatrics; Sx, surgery.
3.2. Metabolic Syndrome Parameters
At baseline and/or post-op reassessment, multiple MS parameters including weight, body mass index, waist circumference, hip circumference, systolic and diastolic blood pressure, fasting insulin, and HDL were noted to be significantly different between the 2 groups (Table 3). The diastolic blood pressure was elevated in the MS group compared to the non-MS group only at baseline, but not at the 6-month follow-up. When the variation from pre-op to post-op (difference scores) was evaluated, the changes in fasting insulin level and systolic blood pressure (SBP) were significantly different between the 2 groups. The median decrease of fasting insulin was 1 μIU/ml in the MS group (p < 0.001) and the median increase of fasting insulin was 1.5 μIU/ml in the non-MS group (p = 0.003). The median reduction of SBP after surgery in MS group was 18 mm Hg and the median increase of SBP in non-MS group was 1 mm Hg (p = 0.03).
Table 3.
Summary of metabolic syndrome-related parameters.
| Median | MS (10) | Non-MS (11) | p Value | |
|---|---|---|---|---|
| Weight (kg) | Pre-Sx | 85 | 65.1 | 0.003 |
| Post-Sx | 85.2 | 65.3 | 0.002 | |
| BMI (kg/m2) | Pre-Sx | 29.2 | 22.7 | <0.001 |
| Post-Sx | 29.2 | 24.2 | 0.001 | |
| SBP (mm Hg) | Pre-Sx | 150.5 | 148 | NS |
| Post-Sx | 133 | 136 | NS | |
| DBP (mm Hg) | Pre-Sx | 80 | 73 | 0.04 |
| Post-Sx | 75 | 75 | NS | |
| Glucose (mg/dl) | Pre-Sx | 105 | 95 | NS |
| Post-Sx | 91.5 | 89 | NS | |
| Insulin (μIU/ml) | Pre-Sx | 11.5 | 4 | <0.001 |
| Post-Sx | 10.5 | 5.5 | 0.002 | |
| HbA1C (%) | Pre-Sx | 5.7 | 5.5 | NS |
| Post-Sx | 5.8 | 5.6 | NS | |
| Cholesterol (mg/dl) | Pre-Sx | 164.5 | 174 | NS |
| Post-Sx | 185 | 203 | NS | |
| TGG (mg/dl) | Pre-Sx | 140.5 | 75 | NS |
| Post-Sx | 133.5 | 111 | NS | |
| HDL (mg/dl) | Pre-Sx | 39 | 50 | 0.01 |
| Post-Sx | 41.5 | 54 | 0.036 | |
| LDL (mg/dl) | Pre-Sx | 108.9 | 99.6 | NS |
| Post-Sx | 100 | 115 | NS | |
| On HTN medication pre-Sx | 70% | 36.4% | NS | |
| Waist (cm) | 99 | 90 | 0.004 | |
| Hip (cm) | 110 | 102 | 0.004 | |
| Waist/hip ratio | 0.94 | 0.9 | NS | |
| HOMA | 1.5 | 0.7 | <0.001 | |
MS: metabolic syndrome; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; TGG: triglyceride; HDL: high density lipoprotein cholesterol; LDL: low density lipoprotein cholesterol; HTN: hypertension; HOMA: Homeostatic model assessment score; NS: not significant.
3.3. Circulating IL-6, VEGF-1 and IGF-1
There was no difference observed in terms of circulating IL-6, VEGF-1, total IGF-1 and free IGF-1 at baseline or after surgery between 2 groups (Table 4). As our pilot sample was small, we calculated whether a higher number of patients might detect a significant difference in cytokine levels. Based on the mean differences and standard deviations (effect sizes) observed between MS and non-MS patients, to observe statistically significant (p < 0.05) differences on the serum levels at 80% power, we would need for IGF-1: 292 patients; for free IGF-1: 468 patients; for IL-6: 124 patients; and for VEGF-1: 1396 patients. Since the goal of this project was to find dominant mechanisms able to explain a difference of about 20% in relapse at 5 years, we conclude that with the possible exception of IL-6, that mechanism is unlikely to be found in the serum cytokine levels.
Table 4.
Comparison of circulating cytokine and tissue markers between MS and non-MS groups.
| VEGF-1 (pg/ml)
|
IL-6 (pg/ml)
|
IGF-1 (ng/ml)
|
Free IGF-1 (pg/l)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Sx | Post-Sx | Pre-Sx | Post-Sx | Pre-Sx | Post-Sx | Pre-Sx | Post-Sx | ||
| MS | Median | 2.99 | 6.55 | 3.64 | 3.19 | 143.55 | 145.23 | 457.50 | 354.17 |
| Range | 57.30 | 32.14 | 278.32 | 8.91 | 264.15 | 111.00 | 982.23 | 678.00 | |
| non-MS | Median | 2.49 | 3.95 | 3.97 | 2.29 | 117.69 | 127.42 | 592.00 | 361.17 |
| Range | 38.25 | 39.53 | 3.12 | 7.86 | 206.42 | 154.35 | 431.00 | 1037.00 | |
MS: metabolic syndrome; VEGF: vascular endothelial growth factor; IGF-1: insulin-like growth factor-1; IGF-1R: IGF-1 receptor; Sx: surgery.
3.4. Immunohistochemistry
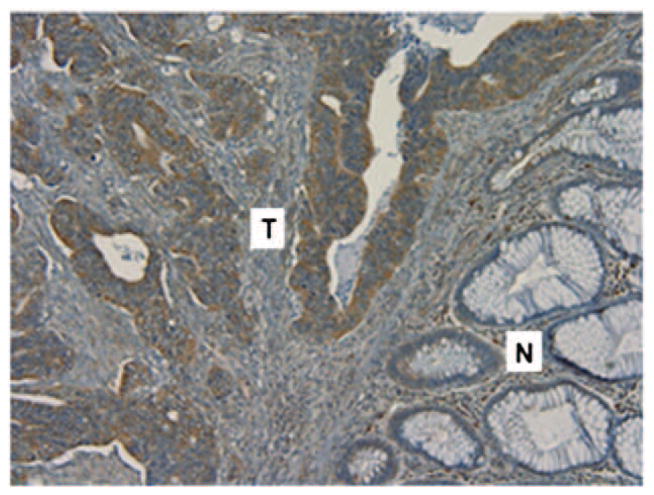
Thirty samples from surgical resection were subject to IHC analysis. Eleven tumor samples (1 adenoma, 1 high grade dysplasia, 1 nodal metastasis, and 8 primary invasive adenocarcinoma), including 7 MS patients and 4 non-MS patients, and 19 normal colon tissue, taken away from the tumor, from the resection specimens were stained. The results of the IHC studies are summarized in Table 5. IGF-1R expression measured by total Allred score was statistically significantly higher in tumor tissue (Fig. 1, p < 0.001). When separating MS and non-MS subgroups, the IGF-1R was over expressed in tumor samples vs. normal tissue in each group. This difference also was statistically significant (MS: p = 0.014; non-MS: p = < 0.001).
Table 5.
Summary of IHC staining results.
| Antibody | Median Allred score
|
||
|---|---|---|---|
| Tumor (11) | Normal (19) | p Value | |
| IGF-1R (all) | 7 | 4 | <0.001 |
| MSa | 7 | 4 | 0.014 |
| Non-MS | 6.5 | 4 | <0.001 |
| IR (all) | 2 | 5 | 0.05 |
| MSa | 3 | 4 | 0.54 |
| Non-MS | 0 | 5 | 0.02 |
| S100b | |||
| MS | 63.5 | 0.1 | |
| Non-MS | 34 | ||
| CD68c | |||
| MS | 198 | 0.52 | |
| Non-MS | 239 | ||
| % CD68d | |||
| MS | 71.4% | 0.27 | |
| Non-MS | 75% | ||
IHC: immunohistochemistry; HPF: high-power field (×100).
MS, N = 7 and non-MS, N = 4.
Tumor infiltration by histiocytes, sum of staining positive cells in 5 HPFs.
Tumor infiltration by macrophage, sum of staining positive cells in 5 HPFs.
% of cases with tumor cell staining positive for CD68.
Fig. 1.
IGF-1R expression in colon carcinoma. IGF-1R expression in colonic adenocarcinoma demonstrated by intense immunohistochemical in cell membranes of tumor cells, in contrast to the absence of staining in adjacent normal colonic mucosa. Original magnification × 10. N: normal mucosa; T: Adenocarcinoma.
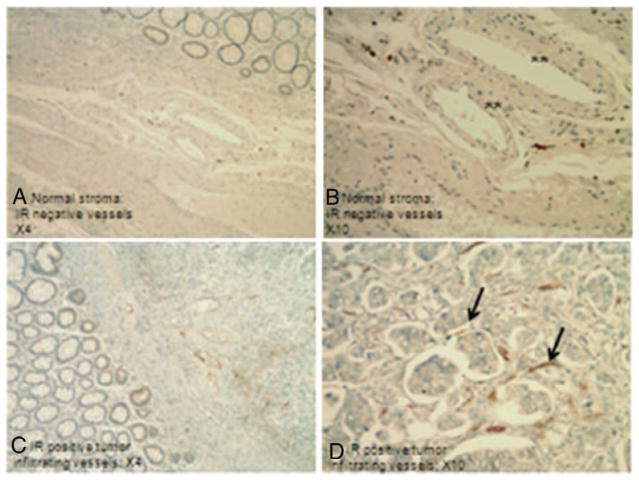
The insulin receptor was usually weak and focal or negative in tumors, but sometimes positive in normal tissue. By measurement of the total Allred score, IR was slightly over-expressed in normal tissue compared to tumor tissue (p = 0.05). There was a statistically significant IR over-expression in normal tissue in the non-MS group (p = 0.02), but not in the MS group (p = 0.54). There was strong and diffuse insulin receptor positivity in the vessels at the edge and within the tumors, but not in the vessels in the normal tissue (Fig. 2). This phenomenon was observed in 7 out of 11 tumors (3 from non-MS and 4 from MS patients). In all of these 7 samples, the peri-tumoral vessel IR IHC Allred scores were higher than the IHC Allred score of the vessels in the nondesmoplastic stroma. One sample of tumor metastasis to a lymph node had abundant intratumoral IR positive vessels. All the 30 samples were subsequently subject to insulin staining and they were negative (data not shown).
Fig. 2.
Immunohistochemical staining for the insulin receptor in normal stroma (A, B) and tumor infiltrating stroma (C, D). Neovessels in the tumor stroma are strongly positive (arrows). Endothelial cells in the non-tumor stroma show no or little IR staining (**).
There was no statistically significant difference between MS vs. non-MS groups in terms of macrophage or Langerhans cell infiltration. Tumor cells from all the samples except the adenoma also stained positive for CD68, with a mean Allred score of 5.
3.5. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Twenty-nine samples, including 16 tumor samples and 13 normal tissues, from 15 patients were subject to RT-PCR analysis (Table 6). The gene expression level in the tumor samples was assessed by qualitative comparison to the matching normal sample, if available, or randomly picked normal sample when matching normal was not available. Tumor IGF-1R over-expression (80%, 12/15) was confirmed, while IGF-1 was mostly (11/15, 73.3%) under-expressed in tumor tissues. IR also tended to be under-expressed in tumor tissues (14/15, 93.3%). Tumor RAGE over-expression was found in 67% (10/15) of patients and was evenly distributed between the two groups.
Table 6.
Summary of RT-PCR qualitative analysis of gene expression.
| MS | Non-MS | Overall | % | |
|---|---|---|---|---|
| RAGE | ||||
| Over-expression | 4 | 6 | 10 | 66.7 |
| Equivocal | 3 | 1 | 4 | 26.7 |
| Homologous deletion | 0 | 1 | 1 | 6.7 |
| IR | ||||
| Over-expression | 0 | 1 | 1 | 6.7 |
| Under-expression | 7 | 7 | 14 | 93.3 |
| IGF-R | ||||
| Over-expression | 7 | 5 | 12 | 80.0 |
| Equivocal | 0 | 3 | 3 | 20.0 |
| IGF-1 | ||||
| Under-expression | 6 | 5 | 11 | 73.3 |
| Equivocal | 1 | 3 | 4 | 26.7 |
RT-PCR: reverse transcription polymerase chain reaction; IR: insulin-receptor; RAGE: advanced glycation end-products; IGF-1: insulin-like growth factor-1; IGF-1R: insulin-like growth factor-1 receptor.
4. Discussion
A remarkable body of epidemiologic data collected over the past 2 decades indicates that the risk of colon cancer is elevated and the prognosis of colon cancer is worse in subjects with MS.6–8 The mechanism underlying these associations is unknown, but experimental and clinical investigations have indicated the possible involvement of various biological processes such as IGF-1R pathway,20,21 VEGF pathway,12 inflammation,22,23 intratumoral immune modulation16 and advanced glycation end-products and its receptors.17 The novel approach of our study was to observe these potential mechanisms of action in an integrated manner to determine the most relevant targets that may be subsequently studied for therapeutic intervention. Hyperinsulinemia stood out as the only significant factor distinguishing patients with MS from patients without MS (Fig. 3). This finding is consistent with the increasing body of literature suggesting that hyperinsulinemia seems to be the critical factor in the association of MS and colon cancer. Increased risk of colon cancer or excess of colon cancer deaths were found in patients with recently diagnosed diabetes or impaired glucose tolerance.24 C-peptide concentrations, which are a measure of insulin secretion, were found to be a stronger predictor of CRC risk than was the MS.20 Postprandial insulin25 and non-fasting C-peptide,20,26 a measure of hyperinsulinemia rather insulin-resistance, are stronger predictors of colon cancer risk than is the fasting insulin concentration.25,27 Finally, in one study, chronic insulin therapy was associated with a significantly increased risk of CRC among patients with type 2 diabetes.28 In addition to the epidemiologic evidence, mechanistic studies have also suggested direct mitogenic and proliferative effects of insulin on tumors.29 As mentioned in our results, although our numbers are small, there is little indication that the other cytokines tested: IL-6, IGF-1 and its free fraction, or VEGF-1 may play a dominant role in increasing the rate of relapse in patients with colorectal cancer. IL-6 values increase with age, but one can compare our study population of patients 60 years and older with the data from the InChianti study, which assessed Tuscan community-dwelling elderly age 65 and older. Our median values fall within the range of <4.18 pg/ml considered normal by the InChianti investigators.30 Although we cannot rule out a possible role of IL-6 based on our study, large cohorts provide unclear results as to the association of IL-6 levels with the metabolic syndrome. In the InChianti study, the median levels of IL-6 were 0.3 pg/ml higher in MS patients (p = 0.001).31 On the other hand, a Korean study did not show differences among elderly women with or without glucose intolerance and MS.32 Likewise, our median IGF-1 and VEGF values are not elevated compared to studies in non-cancer patients.20,33
Fig. 3.
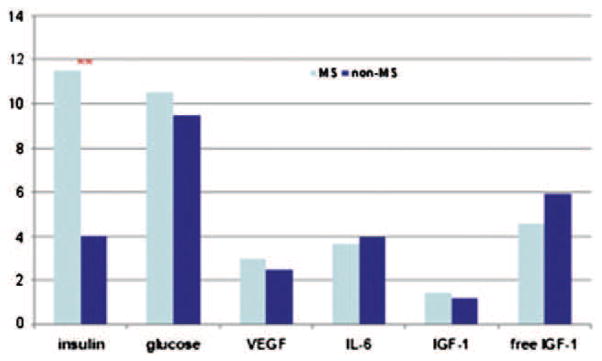
Comparison of median values of studied parameters between MS and non-MS groups. Pre-surgery measurements were used and the actual values were scaled into ×101. **: statistically significantly different by the Wilcoxon signed-ranks test (p < 0.001).
The other key finding in our study relates to the demonstration of IR over-expression in the peri-tumoral vessels. This phenomenon was previously reported by Rensing et al. in various tumor tissues including colon cancer.34 The same study and another study by Liu et al.35 also demonstrated through in vitro and in vivo experiments that insulin stimulates angiogenesis. These effects occur independently of VEGF/VEGFR signaling, but are dependent upon the insulin receptor itself. Downstream signaling pathways involve PI3K, AKT, sterol regulatory element-binding protein 1 (SREBP-1) and Rac1.35 Zhang et al. showed that IR down-regulated cancer cell induced xenograft tumors in mice had reduced growth, angiogenesis, lymphangiogenesis and metastasis compared with wild type cell xenografts.36 The hypothesis can be generated from the current evidence that peri-tumoral IR over-expression promotes tumor angiogenesis and leads to tumor growth and metastasis. Our finding of peri-tumoral vessels over-expressing IR in one metastatic lymph node supports the concept that IR may be involved in the process of metastasis. The persistent hyperinsulinemia found in MS patients may potentially represent an important driver of colorectal carcinogenesis in this group of patients. It is also notable that our finding of IGF1-R over-expression in CRCs is consistent with previous studies.12 All of these changes point to alterations of the insulin/IGF-1 pathways as major contributors to CRC carcinogenesis in MS patients.
Our study is limited by its small sample size, but despite the limited power of the study, hyperinsulinemia and IR expression alterations stood out and provided a potentially new molecular mechanism for CRC arising in patients with MS. Additional studies are in progress to further explore these processes. Confirmatory results would be of great interest as IGF-1 pathway inhibitors are now undergoing active development with figitumumab being one of those that are in advanced stage of development. In a recent phase III trial of this agent; however, a major side effect of IGF-1 pathway inhibition was the feedback hyperglycemia. This might potentially cause hyperinsulinemia and dampen the anti-tumor effect of the primary therapy.37 Our study calls more attention to this aspect of IGF-1 inhibition. Serum insulin levels may be considered as a biomarker of outcome in IGF-1 inhibitor trials and insulin-sensitizing agents could be considered as an adjunct therapy added to IGF-1 inhibition. A small molecule dual inhibitor of IR and IGF-1R that is under development can potentially address this issue as well.38,39 On the other hand, inhibition of the PI3k–AKT–mTOR pathway in the tumoral microvasculature might also be one possible explanation for the antitumoral effects of metformin, and metformin has the advantage of decreasing plasma insulin levels by decreasing hepatic glucose output and increasing insulin consumption by peripheral tissues, notably muscles.40
In conclusion, although limited by small sample size, our study supports a direct role of hyperinsulinemia more strongly than other aspects of the MS for its association with CRC. The preferential over-expression of IR in the peri-tumoral microvessels suggests that hyperinsulinemia might contribute to colon cancer growth by enhancing angiogenesis, in addition to its known proliferative effects. Given the growing prevalence of metabolic syndrome in the elderly (40% of people above age 60 in the US) and its impact on the risk of recurrence of colon cancer, which is diagnosed in patients with a median age of 71 years, it is necessary to explore further this study’s findings in larger cohorts. The understanding gained might be key to developing interventions to improve the prognosis of a significant number of older cancer patients.
Acknowledgments
We thank the Moffitt Cancer Center’s Pathology, Tissue Core and Molecular Biology Core facilities.
Footnotes
Funding: None.
Author Contributions
Concept and design: M. Extermann, D. Shibata, E. Siegel, D. Coppola.
Data collection: J. Liu, M. Druta, D. Shibata, I. Boler, D. Coppola, M. Extermann, Analysis and interpretation of the data: All authors.
Writing and approval: All authors.
Disclosures and conflict of interest statements
Financial disclosures: Martine Extermann: Study funding, Consultant: GTX. All other authors declare no conflict of interest.
References
- 1.Yancik R, Havlik RJ, Wesley MN, Ries L, Long S, Rossi WK, et al. Cancer and comorbidity in older patients: a descriptive profile. Ann Epidemiol. 1996;6(5):399–412. doi: 10.1016/s1047-2797(96)00063-4. Epub 1996/09/01. [DOI] [PubMed] [Google Scholar]
- 2.Yancik R, Wesley MN, Ries LA, Havlik RJ, Long S, Edwards BK, et al. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: a population-based study. Cancer. 1998;82(11):2123–2134. Epub 1998/06/04. [PubMed] [Google Scholar]
- 3.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16(4):1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. Epub 2002/01/16. [DOI] [PubMed] [Google Scholar]
- 5.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 6.Kabat GC, Kim MY, Peters U, Stefanick M, Hou L, Wactawski-Wende J, et al. A longitudinal study of the metabolic syndrome and risk of colorectal cancer in postmenopausal women. Eur J Cancer Prev. 2012;21(4):326–332. doi: 10.1097/CEJ.0b013e32834dbc81. Epub 2011/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen Z, Ye Y, Bin L, Yin M, Yang X, Jiang K, et al. Metabolic syndrome is an important factor for the evolution of prognosis of colorectal cancer: survival, recurrence, and liver metastasis. Am J Surg. 2010;200(1):59–63. doi: 10.1016/j.amjsurg.2009.05.005. Epub 2010/01/16. [DOI] [PubMed] [Google Scholar]
- 8.Stocks T, Lukanova A, Bjorge T, Ulmer H, Manjer J, Almquist M, et al. Metabolic factors and the risk of colorectal cancer in 580,000 men and women in the metabolic syndrome and cancer project (Me-Can) Cancer. 2011 Jun 1;117(11):2398–2407. doi: 10.1002/cncr.25772. Epub 2010/12/21. [DOI] [PubMed] [Google Scholar]
- 9.Meyerhardt JA, Tepper JE, Niedzwiecki D, Hollis DR, McCollum AD, Brady D, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J Clin Oncol. 2004;22(4):648–657. doi: 10.1200/JCO.2004.07.121. Epub 2004/02/18. [DOI] [PubMed] [Google Scholar]
- 10.Yamada K, Araki S, Tamura M, Sakai I, Takahashi Y, Kashihara H, et al. Relation of serum total cholesterol, serum triglycerides and fasting plasma glucose to colorectal carcinoma in situ. Int J Epidemiol. 1998;27(5):794–798. doi: 10.1093/ije/27.5.794. Epub 1998/12/05. [DOI] [PubMed] [Google Scholar]
- 11.Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2002;11(4):385–391. Epub 2002/04/03. [PubMed] [Google Scholar]
- 12.Hakam A, Yeatman TJ, Lu L, Mora L, Marcet G, Nicosia SV, et al. Expression of insulin-like growth factor-1 receptor in human colorectal cancer. Hum Pathol. 1999;30(10):1128–1133. doi: 10.1016/s0046-8177(99)90027-8. Epub 1999/10/26. [DOI] [PubMed] [Google Scholar]
- 13.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. Epub 2004/06/04. [DOI] [PubMed] [Google Scholar]
- 14.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292(12):1440–1446. doi: 10.1001/jama.292.12.1440. Epub 2004/09/24. [DOI] [PubMed] [Google Scholar]
- 15.Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diab Rep. 2005;5(1):70–75. doi: 10.1007/s11892-005-0071-7. Epub 2005/01/25. [DOI] [PubMed] [Google Scholar]
- 16.Kuniyasu H, Sasaki T, Sasahira T, Ohmori H, Takahashi T. Depletion of tumor-infiltrating macrophages is associated with amphoterin expression in colon cancer. Pathobiology. 2004;71(3):129–136. doi: 10.1159/000076467. Epub 2004/03/31. [DOI] [PubMed] [Google Scholar]
- 17.Kuniyasu H, Chihara Y, Takahashi T. Co-expression of receptor for advanced glycation end products and the ligand amphoterin associates closely with metastasis of colorectal cancer. Oncol Rep. 2003;10(2):445–448. Epub 2003/02/13. [PubMed] [Google Scholar]
- 18.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2192. doi: 10.2337/diacare.21.12.2191. Epub 1998/12/05. [DOI] [PubMed] [Google Scholar]
- 19.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11(2):155–168. Epub 1998/03/21. [PubMed] [Google Scholar]
- 20.Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91(7):620–625. doi: 10.1093/jnci/91.7.620. Epub 1999/04/15. [DOI] [PubMed] [Google Scholar]
- 21.Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, et al. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997;57(21):4667–4672. Epub 1997/11/14. [PubMed] [Google Scholar]
- 22.Makino T, Noguchi Y, Yoshikawa T, Doi C, Nomura K. Circulating interleukin 6 concentrations and insulin resistance in patients with cancer. Br J Surg. 1998;85(12):1658–1662. doi: 10.1046/j.1365-2168.1998.00938.x. Epub 1999/01/06. [DOI] [PubMed] [Google Scholar]
- 23.Belluco C, Nitti D, Frantz M, Toppan P, Basso D, Plebani M, et al. Interleukin-6 blood level is associated with circulating carcinoembryonic antigen and prognosis in patients with colorectal cancer. Ann Surg Oncol. 2000;7(2):133–138. doi: 10.1007/s10434-000-0133-7. Epub 2000/04/13. [DOI] [PubMed] [Google Scholar]
- 24.Hu FB, Manson JE, Liu S, Hunter D, Colditz GA, Michels KB, et al. Prospective study of adult onset diabetes mellitus (type 2) and risk of colorectal cancer in women. J Natl Cancer Inst. 1999;91(6):542–547. doi: 10.1093/jnci/91.6.542. Epub 1999/03/24. [DOI] [PubMed] [Google Scholar]
- 25.Rechler MM. Growth inhibition by insulin-like growth factor (IGF) binding protein-3—what’s IGF got to do with it? Endocrinology. 1997;138(7):2645–2647. doi: 10.1210/endo.138.7.5355. Epub 1997/07/01. [DOI] [PubMed] [Google Scholar]
- 26.Dy DY, Whitehead RH, Morris DL. SMS 201. 995 inhibits in vitro and in vivo growth of human colon cancer. Cancer Res. 1992;52(4):917–923. [PubMed] [Google Scholar]
- 27.Pollak MN, Polychronakos C, Guyda H. Somatostatin analogue SMS 201–995 reduces serum IGF-I levels in patients with neoplasms potentially dependent on IGF-I. Anticancer Res. 1989;9(4):889–891. Epub 1989/07/01. [PubMed] [Google Scholar]
- 28.Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127(4):1044–1050. doi: 10.1053/j.gastro.2004.07.011. Epub 2004/10/14. [DOI] [PubMed] [Google Scholar]
- 29.Lee WM, Lu S, Medline A, Archer MC. Susceptibility of lean and obese Zucker rats to tumorigenesis induced by N-methyl-N-nitrosourea. Cancer Lett. 2001;162(2):155–160. doi: 10.1016/s0304-3835(00)00635-2. Epub 2001/01/09. [DOI] [PubMed] [Google Scholar]
- 30.Zhu S, Patel KV, Bandinelli S, Ferrucci L, Guralnik JM. Predictors of interleukin-6 elevation in older adults. J Am Geriatr Soc. 2009;57(9):1672–1677. doi: 10.1111/j.1532-5415.2009.02426.x. Epub 2009/08/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuliani G, Galvani M, Maggio M, Volpato S, Bandinelli S, Corsi AM, et al. Plasma soluble gp130 levels are increased in older subjects with metabolic syndrome. The role of insulin resistance. Atherosclerosis. 2010;213(1):319–324. doi: 10.1016/j.atherosclerosis.2010.08.074. Epub 2010/09/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi KM, Lee J, Lee KW, Seo JA, Oh JH, Kim SG, et al. Comparison of serum concentrations of C-reactive protein, TNF-alpha, and interleukin 6 between elderly Korean women with normal and impaired glucose tolerance. Diabetes Res Clin Pract. 2004;64(2):99–106. doi: 10.1016/j.diabres.2003.10.007. Epub 2004/04/06. [DOI] [PubMed] [Google Scholar]
- 33.Voss MW, Erickson KI, Prakash RS, Chaddock L, Kim JS, Alves H, et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun. 2013;28:90–99. doi: 10.1016/j.bbi.2012.10.021. Epub 2012/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rensing KL, Houttuijn Bloemendaal FM, Weijers EM, Richel DJ, Buller HR, Koolwijk P, et al. Could recombinant insulin compounds contribute to adenocarcinoma progression by stimulating local angiogenesis? Diabetologia. 2010;53(5):966–970. doi: 10.1007/s00125-010-1687-y. Epub 2010/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Petreaca M, Martins-Green M. Cell and molecular mechanisms of insulin-induced angiogenesis. J Cell Mol Med. 2009;13(11–12):4492–4504. doi: 10.1111/j.1582-4934.2008.00555.x. Epub 2009/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Fagan DH, Zeng X, Freeman KT, Sachdev D, Yee D. Inhibition of cancer cell proliferation and metastasis by insulin receptor downregulation. Oncogene. 2010;29(17):2517–2527. doi: 10.1038/onc.2010.17. Epub 2010/02/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jassem J, Langer CJ, Karp DD, Mok T, Benner RJ, Green SJ, et al. Randomized, open label, phase III trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with non-small cell lung cancer (NSCLC) J Clin Oncol. 2010;28(15s Suppl):abstr 7500. doi: 10.1200/JCO.2013.54.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang F, Hurlburt W, Greer A, Reeves KA, Hillerman S, Chang H, et al. Differential mechanisms of acquired resistance to insulin-like growth factor-i receptor antibody therapy or to a small-molecule inhibitor, BMS-754807, in a human rhabdomyosarcoma model. Cancer Res. 2010;70(18):7221–7231. doi: 10.1158/0008-5472.CAN-10-0391. Epub 2010/09/03. [DOI] [PubMed] [Google Scholar]
- 39.Carboni JM, Wittman M, Yang Z, Lee F, Greer A, Hurlburt W, et al. BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol Cancer Ther. 2009;8(12):3341–3349. doi: 10.1158/1535-7163.MCT-09-0499. Epub 2009/12/10. [DOI] [PubMed] [Google Scholar]
- 40.United Kingdom Prospective Diabetes Study (UKPDS). . 13: Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ. 1995;310(6972):83–88. Epub 1995/01/14. [PMC free article] [PubMed] [Google Scholar]