Abstract
Purpose
To determine whether presenting sonographic features of invasive ductal carcinomas (IDC) are associated with patient age, tumor histologic grade, and hormonal receptor status.
Methods
Sonographic features of 101 consecutive cases of IDC seen at ultrasound were retrospectively assessed based on the BI-RADS criteria of posterior acoustic appearance, tumor margins, and echogenicity. Associations between sonographic features and tumor characteristics were statistically evaluated with attention to patient age.
Results
IDC with shadowing compared with unchanged posterior acoustic appearance were significantly more likely to be of low histologic grade (Odds Ratio [OR] = 5.00; p < 0.05) and estrogen receptor (ER) -positive (OR = 10.00; p < 0.05). Conversely, posterior enhancement was associated with ER-negative status (OR = 4.45; p < 0.01), particularly among patients younger than 60 years of age (OR = 5.36, p < 0.05). Circumscribed tumors were more often high grade, particularly among older women (p < 0.01), and hormone receptor--negative regardless of age group. Among older women, tumors with mixed echogenicity tended to be high grade and progesterone receptor--negative (p values < 0.05). Noncircumscribed borders were observed for all tumors with posterior shadowing, and 97% of such tumors were also ER positive.
Conclusions
Sonographic features were significantly associated with tumor grade and hormone receptor status, with some differences based on patient age. Specifically, the presence of posterior shadowing was associated with lower histologic grade and ER-positive status, especially in older patients. In contrast, we found that posterior acoustic enhancement was more commonly associated with ER-negative status, especially in younger patients.
Keywords: invasive ductal carcinoma, ultrasound, histologic grade, breast cancer, breast, cancer, hormonal receptors
Treatment and prognosis for breast cancer varies based on histologic grade and biological markers such as estrogen receptor (ER), progesterone receptor (PR), and human epithelial growth factor receptor 2 (HER-2).1,2 Accurate and early characterization of breast cancer, based on these prognostic indicators, is helpful in treatment planning. Ultrasound is a commonly used adjunct to mammography in the evaluation of clinically or mammographically suspicious breast lesions.3 Sonography provides information on acoustic properties useful in characterizing a breast mass, which assists in the diagnosis and management of breast lesions.4 Using acoustic properties to predict biologic features such as histologic grade and hormone receptor expression allows more confident early treatment and management. For example, lesions with characteristics suggestive of a high-grade tumor, which are more prone to have lymph node metastases,1 may influence the decision to perform fine-needle aspiration of lymph nodes with borderline appearance at the time of ultrasound-guided biopsy. Additionally, sophisticated tests such as receptor analyses are not readily available outside the United States, whereas ultrasound is a commonly used inexpensive modality. Being able to predict grade and receptor status may allow clinicians to be more selective and cost efficient in evaluating breast lesions.
Previous attempts to correlate ultrasound characteristics of invasive ductal carcinoma (IDC), the most common type of breast cancer, with prognostic indicators have been inconclusive or contradictory.5,6 This may be in part due to age-related differences in the ultrasound characteristics of breast tissue.7 To our knowledge, no study has addressed the impact of patient age on the relationships between ultrasound characteristics and breast tumor biologic features. Therefore, the purpose of this retrospective study was to investigate the associations between sonographic features and biologic features of invasive ductal carcinomas and whether they are impacted by patient age.
MATERIALS AND METHODS
Institutional Review Board approval was obtained from our institution.
Patient Selection
Female breast cancer patients diagnosed during 2008 and 2009 with IDC, not otherwise specified, were identified from our institution’s tumor registry. Clinical data, ultrasound images, and pathologic data were reviewed for the 101 patients who had ultrasound images available for study.
Ultrasound
Ultrasound scans were performed using either a General Electric LOGIQ 7 or a General Electric LOGIQ 9 unit (General Electric Healthcare, Milwaukee, WI) with a 10–14-MHz linear transducer. Real-time spatial compounding was not utilized to avoid suppression of potentially beneficial artifacts, such as acoustic enhancement. Ultrasound tumor images were reviewed for this analysis by two radiologists and characterized by consensus. The sonographic characteristics of posterior acoustic features, tumor margins, and echogenicity were assessed according to BI-RADS criteria.8 Posterior features were classified as shadowing if echogenicity posterior to the tumor was mostly darker than surrounding breast tissue at the same depth, enhancement if mostly brighter, and no change if not significantly different than surrounding breast tissue at the same depth. Margins were categorized as circumscribed if well-defined or sharp, with an abrupt transition between lesion and surrounding tissue. Tumor echogenicity was classified as hypoechoic if uniformly darker than surrounding breast tissue, hyperechoic if uniformly brighter, and mixed if the tumor contained both hypoechoic and hyperechoic components.
Histologic Analysis
The resected tumors were fixed, stained, and examined to determine histologic type based on World Health Organization criteria.9 Tumor grade was classified according to the Notting-ham’s grading system 1–3 for invasive cancers.10 For the purpose of this study, grades 1 and 2 were considered lower grade, whereas grade 3 was considered higher grade. E-cadherin stains were used to differentiate lobular from ductal cancers in cases of an equivocal histological appearance. Estrogen and progesterone receptor status were identified using immunohistochemistry stains. On pathology results, the ER or PR status was classified as positive if nuclear staining was present in >10% of nuclei, borderline if nuclear staining was between 1% and 10%, and negative if staining was seen in <1% of nuclei. For the purpose of this study, the few tumors with borderline hormone receptor expression status were considered negative due to their similar clinical behavior. HER-2 (proto oncogene Neu) receptor status was initially tested by immunohistochemical stains (Dako Hercep Test). Membrane staining seen in 0% to <10% of the invasive tumor cells was considered 0 (negative), partial membrane staining in >10% cells was considered 1+ (negative), complete membrane staining of >10% cells was considered as 2+ (equivocal), and strong membrane staining of >30% cells was considered 3+ (positive). Equivocal cases were further assessed by fluorescence in situ hybridization test (Abbott Laboratories, Abbot Park, IL), in which an HER-2 gene copy number per chromosome 17 centromere ratio of >2.2 was consistent with gene amplification and <1.8 was considered unamplified. A ratio between 1.8 and 2.2 was considered equivocal.
Statistical Methods
Descriptive statistics were generated for clinicopathologic and ultrasound characteristics. Associations between characteristics were examined using Fisher’s exact test and with regression modeling techniques (logistic, with and without interaction terms; polytomous/multinomial where outcome was multinomial; and exact logistic regression to handle zero cells). We also used recursive partitioning to explore interactions that might be predictive of clinicopathologic features for inclusion in multivariable regression models. Associations between age and ultrasound features were explored in regression modeling with age as a continuous variable and various age categories/quantiles, and using interaction terms. Tests of statistical significance were two-tailed, with alpha 0.05. Results presented are statistically significant unless otherwise stated and p values are provided in the text only if not also given in a table. We used Stata/IC statistical software version 10.0 (STATACorp LP, College Station, TX) for all reported analyses, plus the rpart11 package in R v.2.13.112 to explore classification trees.
RESULTS
Patient Population and Clinicopathologic Characteristics
Study population (n = 101), tumor characteristics, and ultrasound imaging features are presented in Table 1. Mean age at diagnosis was 60.1 ±10.3 years (range, 34–88; median, 60). A total of 60% were European American, 36% were African American, and 4% were Hispanic. Tumors diagnosed in African-American patients were significantly more likely to be of ER expression--negative status and higher histologic grade, typical of female invasive breast cancer cases in South Carolina13; however, imaging features did not vary significantly by race (data not shown). HER-2 status was not significantly associated with any sonographic feature and therefore is not detailed in this report.
TABLE 1.
Patient, Tumor, and Ultrasound Characteristics
| All Cases (N = 101) N (%) | Age <60 years (N = 48) N (%) | Age 60+ years (N = 53) N (%) | p Value* | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age (years) | ||||
| Mean ± SD | 60.1 ± 10.3 | 51.6 ± 6.3 | 67.7 ± 6.6 | — |
| Median; range | 60; 34–88 | 52; 34–59 | 66; 60–88 | |
| Race: White | 61 (60.4%) | 29 (60.4%) | 32 (60.4%) | NS |
| Tumor characteristics | ||||
| Histologic grade | ||||
| I | 19 (19.2%) | 8 (17.0%) | 11 (21.1%) | NS |
| II | 47 (47.5%) | 20 (42.6%) | 27 (51.9%) | |
| III | 33 (33.3%) | 19 (40.4%) | 14 (26.9%) | |
| Not available | 2 | 1 | 1 | |
| ER/PR expression status | ||||
| ER+/PR+ | 57 (58.8%) | 27 (57.4%) | 30 (60.0%) | NS |
| ER+/PR2 | 13 (13.4%) | 5 (10.6%) | 8 (16.0%) | |
| ER2/PR+ | 5 (5.2%) | 4 (8.5%) | 1 (2.0%) | |
| ER2/PR2 | 22 (22.7%) | 11 (23.4%) | 11 (22.0%) | |
| Not available | 4 | 1 | 3 | |
| HER2 amplification | ||||
| Positive (3+) | 8 (8.5%) | 6 (13.0%) | 2 (4.2%) | NS |
| Negative (0, 1+, 2+) | 86 (91.5%) | 40 (87.0%) | 46 (95.8%) | |
| Not available | 7 | 2 | 5 | |
| Sonographic characteristics | ||||
| Posterior acoustic features | ||||
| No change | 44 (43.6%) | 19 (36.6%) | 25 (47.2%) | <0.05 |
| Shadowing | 33 (32.7%) | 12 (25.0%) | 21 (39.6%) | |
| Enhancement | 24 (23.8%) | 17 (35.4%) | 7 (13.2%) | |
| Margins | ||||
| Noncircumscribed | 83 (82.2%) | 43 (89.6%) | 40 (75.5%) | NS |
| Circumscribed | 18 (17.8%) | 5 (10.4%) | 13 (24.5%) | |
| Echogenicity | ||||
| Hypoechoic | 91 (90.1%) | 44 (91.7%) | 47 (88.7%) | NS |
| Hyperechoic | 0 | 0 | 0 | |
| Mixed | 10 (9.9%) | 4 (8.3%) | 6 (11.3%) | |
Student’s t test, χ2 test, or Fisher exact test where any cell had five or fewer cases. A p value is not provided for age, as being inappropriate.
Abbreviation: NS, not significant.
Sonographic Characteristics
In addition to results with all cases combined, analyses are presented stratified by age (using median age of 60 years as cut-point) because of clinical relevance, even though the interactions with age were not statistically significant.
Posterior Acoustic Features
Among women of all ages combined, unchanged posterior signal (44 cases, 43.6%; Table 1) was more common than either shadowing (33 cases, 32.7%) or enhancement (24 cases, 23.8%). Tumors with shadowing or unchanged posterior acoustic properties were more often of lower histologic grade and positive ER status (Table 2). Enhancement was associated with negative ER status (Figure 1) but did not distinguish grade or PR status. With unchanged posterior signal as the referent for regression modeling, shadowing was significantly associated with positive ER status and enhancement with negative ER status. In age-stratified analysis, among women of age 60 years and older, where enhancement was uncommon, shadowing strongly predicted positive ER status and also lower grade.
TABLE 2.
Ultrasound Characteristics and Clinicopathologic Features
| Histologic Grade
|
ER Expression Status
|
PR Expression Status
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade I, II (N = 66) N (%)* |
Grade III (N = 33) N (%) |
p Value† | Odds Ratio G III vs G I, II (p Value) | Positive (N = 70) N (%)* |
Negative (N = 27) N (%) |
p Value | Odds Ratio Neg. vs Pos. (p Value) | Positive (N = 62) N (%)* |
Negative (N = 35) N (%) |
p Value | Odds Ratio Neg. vs Pos. (p Value) | |
| All cases combined | ||||||||||||
| Posterior acoustic properties | ||||||||||||
| No change | 27 (64.3%) | 15 (35.7%) | <0.05 | 1 | 32 (74.4%) | 11 (25.6%) | <0.001 | 1 | 29 (67.4%) | 14 (32.6%) | NS | 1 |
| Shadowing | 27 (81.8%) | 6 (18.2%) | 0.40 (NS) | 29 (96.7%) | 1 (3.3%) | 0.10 (<0.05) | 22 (73.3%) | 8 (26.7%) | 0.75 (NS) | |||
| Enhancement | 12 (50.0%) | 12 (50.0%) | 1.80 (NS) | 9 (37.5%) | 15 (62.5%) | 4.45 (<0.01) | 11 (45.8%) | 13 (54.2%) | 2.45 (NS) | |||
| Margins | ||||||||||||
| Noncircumscribed | 58 (71.6%) | 23 (28.4%) | <0.05 | 1 | 64 (81.0%) | 15 (19.0%) | <0.001 | 1 | 58 (73.4%) | 21 (27.6%) | <0.001 | 1 |
| Circumscribed | 8 (44.4%) | 10 (55.6%) | 3.15 (<0.05) | 6 (33.3%) | 12 (66.7%) | 8.53 (<0.001) | 4 (22.2%) | 14 (77.8%) | 9.67 (<0.001) | |||
| Echogenicity | ||||||||||||
| Hypoechoic | 61 (67.8%) | 29 (32.2%) | NS | 1 | 63 (72.4%) | 24 (27.6%) | NS | 1 | 58 (66.7%) | 29 (33.3%) | NS | 1 |
| Mixed | 5 (55.6%) | 4 (44.4%) | 1.68 (NS) | 7 (70.0%) | 3 (30.0%) | 1.12 (NS) | 4 (40.0%) | 6 (60.0%) | 3.00 (NS) | |||
| Age <60 years | ||||||||||||
| Posterior acoustic properties | ||||||||||||
| No change | 12 (66.7%) | 6 (33.3%) | NS | 1 | 15 (79.0%) | 4 (21.0%) | <0.05 | 1 | 14 (73.7%) | 5 (26.3%) | NS | 1 |
| Shadowing | 8 (66.7%) | 4 (33.3%) | 1.00 (NS) | 10 (90.9%) | 1 (9.1%) | 0.38 (NS) | 8 (72.7%) | 3 (27.3%) | 1.05 (NS) | |||
| Enhancement | 8 (47.1%) | 9 (52.9%) | 2.25 (NS) | 7 (41.2%) | 10 (58.8%) | 5.36 (<0.05) | 9 (52.9%) | 8 (47.1%) | 2.49 (NS) | |||
| Margins | ||||||||||||
| Noncircumscribed | 25 (59.5%) | 17 (40.5%) | NS | 1 | 31 (73.8%) | 11 (26.2%) | <0.05 | 1 | 30 (71.4%) | 12 (28.6%) | <0.05 | 1 |
| Circumscribed | 3 (60.0%) | 2 (40.0%) | 0.98 (NS) | 1 (20.0%) | 4 (80.0%) | 11.27 (<0.05) | 1 (20.0%) | 4 (80.0%) | 10.00 (<0.05) | |||
| Echogenicity | ||||||||||||
| Hypoechoic | 25 (56.8%) | 19 (43.2%) | NS | 1 | 28 (65.1%) | 15 (34.9%) | NS | 1 | 28 (65.1%) | 15 (34.9%) | NS | 1 |
| Mixed | 3 (100%) | 0 | 0.36 (NS)† | 4 (100%) | 0 | 0.37 (NS)† | 3 (75.0%) | 1 (25.0%) | 0.62 (NS) | |||
| Age 60+ years | ||||||||||||
| Posterior acoustic properties | ||||||||||||
| No change | 15 (62.5%) | 9 (37.5%) | <0.05 | 1 | 17 (70.8%) | 7 (29.2%) | <0.001 | 1 | 15 (62.5%) | 9 (37.5%) | NS | 1 |
| Shadowing | 19 (90.5%) | 2 (9.5%) | 0.18 (<0.05) | 19 (100%) | 0 | 0.10 (<0.05) | 14 (73.7%) | 5 (26.3%) | 0.60 (NS) | |||
| Enhancement | 4 (57.1%) | 3 (42.9%) | 1.25 (NS) | 2 (28.6%) | 5 (71.4%) | 5.68 (NS) | 2 (28.6%) | 5 (71.4%) | 4.17 (NS) | |||
| Margins | ||||||||||||
| Noncircumscribed | 33 (84.6%) | 6 (15.4%) | <0.01 | 1 | 33 (89.2%) | 4 (10.8%) | <0.01 | 1 | 28 (75.7%) | 9 (24.3%) | <0.01 | 1 |
| Circumscribed | 5 (38.5%) | 8 (61.5%) | 8.80 (<0.01) | 5 (38.5%) | 8 (61.5%) | 13.20 (<0.01) | 3 (23.1%) | 10 (76.9%) | 10.37 (<0.01) | |||
| Echogenicity | ||||||||||||
| Hypoechoic | 36 (78.3%) | 10 (21.7%) | <0.05 | 1 | 35 (79.6%) | 9 (20.4%) | NS | 1 | 30 (68.2%) | 14 (31.8%) | <0.05 | 1 |
| Mixed | 2 (33.3%) | 4 (66.7%) | 7.20 (<0.05) | 3 (50.0%) | 3 (50.0%) | 3.89 (NS) | 1 (16.7%) | 5 (83.3%) | 10.71 (<0.05) | |||
Row percentages total 100%.
Fisher exact test was used if any cells included five or fewer cases (yielding a more conservative p value). Otherwise, χ2 test was used. Exact logistic regression was used to accommodate a zero cell.
Abbreviation: NS, not significant.
FIGURE 1.
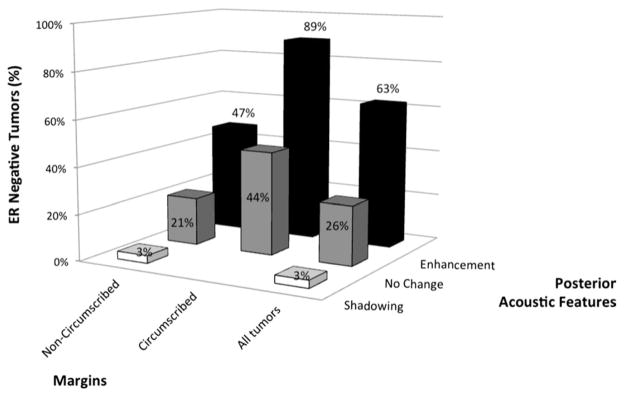
Estrogen receptor status, posterior acoustic features, and tumor margins.
Tumor Margins
Analysis of tumor margins revealed that most tumors (N = 83; 82%) were noncircumscribed. Circumscribed tumors were significantly more likely to be high grade (compared with lower grade) and to be ER-negative and/or PR-negative. When stratified by age, the significant association with grade was restricted to older women but was observed for ER and PR status regardless of age group.
Echogenicity
Most tumors (N = 91; 90%) were hypoechoic. No uniformly hyperechoic tumor was found in our case series. Tumor echogenicity characteristics were not associated with any particular clinicopathologic features when compared for all ages. However, when stratified by age, among older women, mixed echogenicity was significantly associated with high histologic grade and negative PR status.
Multivariable Prediction of Hormone Receptor Status and Grade Based on Sonographic Features
We found substantial differences in several odds ratios between univariable (Table 2) and multivariable models (Table 3), implying underlying correlations between sonographic features and tumor characteristics, although no statistically significant interactions were identified by regression modeling or suggested by recursive partitioning analysis.
TABLE 3.
Multivariable Regression Model of Associations Between Sonographic Characteristics, Histologic Grade, and Hormone Receptor Status
| Histologic Grade (N = 99) (Grade 3 vs Grades 1 and 2)
|
Estrogen Receptor Status (N = 97) (Negative vs Positive)
|
Progesterone Receptor Status (N = 97) (Negative vs Positive)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p Value | Odds Ratio | 95% CI | p Value | Odds Ratio | 95% CI | p Value | |
| Posterior features | |||||||||
| Shadowing | 0.60 | 0.19–1.92 | 0.387 | 0.16 | 0.02–1.41 | 0.099 | 1.35 | 0.43–4.22 | 0.606 |
| Enhancement | 1.24 | 0.41–3.74 | 0.701 | 4.00 | 1.26–12.78 | 0.019 | 2.20 | 0.64–7.51 | 0.209 |
| Circumscribed margins | 3.60 | 0.99–13.12 | 0.052 | 5.66 | 1.37–23.36 | 0.017 | 8.36 | 2.12–32.97 | 0.002 |
| Mixed echogenicity | 1.39 | 0.30–6.43 | 0.677 | 0.64 | 0.10–4.08 | 0.638 | 2.51 | 0.54–11.78 | 0.242 |
| Age | 0.94 | 0.89–0.99 | 0.017 | 0.98 | 0.93–1.04 | 0.586 | 0.96 | 0.31–2.67 | 0.934 |
Abbreviation: CI, confidence interval.
In multivariable regression models including all three sonographic features plus patient age, no sonographic feature independently and significantly predicted histologic grade, although we did observe an almost significant trend toward high grade with circumscribed margins (p = 0.052), consistent with univariable analyses. Negative PR status was predicted by circumscribed margins. However, ER status was significantly predicted by the independent characteristics of posterior features and tumor margins. This is illustrated in Figure 1, where most tumors with enhancement, and particularly with both enhancement and circumscribed margins, were ER negative (Spearman’s ρ correlation = 0.368; p < 0.001). By contrast, all tumors with posterior shadowing also had noncircumscribed borders and 97% of such tumors with known ER status were ER positive.
Although noncircumscribed borders and hypoechogenicity were more commonly associated with low-grade and hormone receptor-positive tumors, particularly among older women, these sonographic features were not statistically significantly correlated, perhaps in part due to small numbers.
DISCUSSION
A major role of breast ultrasound is to assist in the diagnosis and characterization of breast lesions, specifically differentiating between benign and malignant breast masses. Being able to predict the likelihood of histologic grade or hormone receptor status by imaging characteristics may also have implications for management.14 Results from previous studies attempting to correlate ultrasound characteristics and tumor grade have varied. Historically, the majority of malignant breast masses (70–80%) were felt to have posterior acoustic shadowing at ultrasound.15 However, it is now widely accepted that IDC may have a variable posterior acoustic appearance ranging from shadowing to unchanged to enhancement. The results of this study revealed associations with posterior acoustic properties and perhaps echogenicity were impacted by age. Specifically, the presence of posterior shadowing was associated with lower histologic grade and ER-positive status, especially in older patients. In contrast, we found that posterior acoustic enhancement was more commonly associated with negative ER status, particularly in younger patients.
Correlating posterior acoustic properties and histology is a complex multifactorial problem likely dependent on factors such as cellular structure, stromal reaction, and the way the tumor cells form the interfaces with the surrounding breast tissue.16–18 These factors may vary with histologic subtype, grade of tumor, and other factors such as patient age and type of the parenchymal breast tissue in which the tumor resides.
Traditionally, a malignant breast mass was expected to exhibit poorly defined or spiculated margins and the presence of this finding may be considered a poor prognostic indicator in prospectively differentiating benign from malignant masses. However, studies have since demonstrated well-defined margins are more likely to represent higher grade tumors,6 and posterior enhancement to represent high-grade and negative ER status.19 Our results are consistent with these findings, although in our patients it was noncircumscribed margins that predicted lower grade, the associations with circumscribed tumors being more equivocal. Stronger relationships were observed between circumscribed tumors and negative hormone receptor expression status, and with high grade in older patients. The combination of enhancement and circumscribed margins was highly predictive of ER-negative status. Conversely, posterior shadowing with noncircumscribed borders strongly predicted ER-positive status.
A hypoechoic or mixed echogenic appearance has been described with most malignant breast masses.20 Among our cases, mixed echogenicity was rare (10% of all cases). No uniformly hyperechoic tumors were present in our study, possibly related to the relatively small patient population given that this appearance has been reported in only a minority (4.1%) of tumors.7 A statistical association was found with tumor grade and with PR status, in contrast to reports by others,7 but this was only among older women and based on small numbers.
Our analyses do not indicate that combining all three sonographic features will greatly enhance predictive capability of tumor grade or ER status, because a majority of models identified only one sonographic feature and no statistically significant interaction between features was observed.
Our results were based on retrospective review and to avoid bias we included all consecutive patients with IDC undergoing sonographic evaluation. One disadvantage of ultrasound is its high intra- and interobserver variability.21 To minimize this variability, we had two radiologists interpret the sonographic findings by consensus. An additional limitation of this study is the relatively small sample size, specifically, the small number of younger patients (only 14 younger than 50 years of age) and of tumors with circumscribed borders or nonhypoechogenicity. Given the hormonal influence on the breast in terms of sonographic appearance and the average age at menopause in the US of 51.3 years,22 more patients in the younger age group would have been beneficial. Future work should focus on clinicopathologic predictors of sonographic characteristics specific to younger patients and include more variability in HER-2 status.
In conclusion, sonographic features were found to be significantly associated with tumor grade and hormone receptor status, with some differences based on patient age. Almost half of the tumors examined evidenced no change in posterior features, but among the remainder shadowing was associated with lower histologic grade and positive ER status; in contrast, enhancement was more commonly observed with negative ER status. Circumscribed tumors were significantly associated with high-grade and negative hormone receptor expression status, particularly in older patients. Enhancement and circumscribed margins combined were especially predictive of ER-negative status; conversely, posterior shadowing with noncircumscribed borders strongly predicted ER-positive status. This knowledge may allow clinicians to predict histologic grade and hormone receptor status more accurately based on sonographic properties, allowing earlier planning for future imaging,19 procedures, or surgery. Additionally, these findings may allow for more selective and cost-efficient evaluation of breast lesions in settings where receptor expression analysis may not be readily available.
References
- 1.Kurosumi M, Tabei T, Inoue K, et al. Prognostic significance of scoring system based on histological heterogeneity of invasive ductal carcinoma for node-negative breast cancer patients. Oncol Rep. 2003;10:833. [PubMed] [Google Scholar]
- 2.Stavros AT, Thickman D, Rapp CL, et al. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995;196:123. doi: 10.1148/radiology.196.1.7784555. [DOI] [PubMed] [Google Scholar]
- 3.Teh W, Wilson AR. The role of ultrasound in the breast cancer screening. A concensus statement by the European Group for Breast Cancer Screening. Eur J Cancer. 1998;34:449. doi: 10.1016/s0959-8049(97)10066-1. [DOI] [PubMed] [Google Scholar]
- 4.Fornage BD. Sonography of breast cancer. In: Winchester DJ, Winchester DP, Hudis CA, et al., editors. Breast Cancer. Ontario: BC Decker; 2006. p. 137. [Google Scholar]
- 5.Kijima Y, Yoshinaka H, Koriyama C, et al. Ultrasound examination is useful for prediction of histologic type in invasive ductal carcinoma of the breast. Ultrasound Med Biol. 2008;34:517. doi: 10.1016/j.ultrasmedbio.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Lamb PM, Perry NM, Vinnicombe SJ, et al. Correlation between ultrasound characterisitcs, mammographic findings and histological grade in patients with invasive ductal carcinoma of the breast. Clin Radiol. 2000;55:40. doi: 10.1053/crad.1999.0333. [DOI] [PubMed] [Google Scholar]
- 7.Watermann DO, Tempfer CB, Hefler LA, et al. Ultrasound criteria for ductal invasive breast cancer are modified by age, tumor size, and axillary lymph node status. Breast Cancer Res Treat. 2005;89:127. doi: 10.1007/s10549-004-1478-6. [DOI] [PubMed] [Google Scholar]
- 8.Costantini M, Belli P, Lombardi R, et al. Characterization of solid breast masses: use of the sonographic breast imaging reporting and data system lexicon. J Ultrasound Med. 2006;25:649. doi: 10.7863/jum.2006.25.5.649. [DOI] [PubMed] [Google Scholar]
- 9.The World Health Organization histological typing of breast tumors, 2nd ed. The World Health Organization. Am J Clin Pathol. 1982;78:806. doi: 10.1093/ajcp/78.6.806. [DOI] [PubMed] [Google Scholar]
- 10.Elston CW. Classification and grading of invasive breast carcinoma. Verh Dtsch Ges Pathol. 2005;89:35. [PubMed] [Google Scholar]
- 11.Therneau TM, Atkinson B. R port by Brian Ripley. Recursive Partitioning. [Accessed November 30, 2011];R package version 3.1–48. 2010 http://CRAN.R-project.org/package=rpart.
- 12.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2009. [Accessed December 3, 2011]. http://www.Rproject.org. [Google Scholar]
- 13.Cunningham JE, Montero AJ, Garrett-Mayer E, et al. Racial differences in the incidence of breast cancer subtypes defined by combined histologic grade and hormone receptor status. Cancer Causes Control. 2010;21:399. doi: 10.1007/s10552-009-9472-2. [DOI] [PubMed] [Google Scholar]
- 14.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein SP, Conant EF, Mies C, et al. Posterior acoustic shadowing in benign breast lesions: sonographic-pathologic corelation. J Ultrasound Med. 2004;23:73. doi: 10.7863/jum.2004.23.1.73. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi T. Diagnostic ultrasound in breast cancer: analysis of retrotumorous echo patterns correlated with sonic attenuation by cancerous connective tissue. J Clin Ultrasound. 1979;7:471. doi: 10.1002/jcu.1870070611. [DOI] [PubMed] [Google Scholar]
- 17.Kossoff G. Causes of shadowing in breast sonography. Ultrasound Med Biol. 1988;14:211. doi: 10.1016/0301-5629(88)90063-4. [DOI] [PubMed] [Google Scholar]
- 18.Gozzi G, Cressa C, Bazzocchi M, et al. Causes of attenuation of the sound waves in neoplasm of the breast. Histologic and echographic correlation study. Radiol Med. 1986;72:195. [In Italian] [PubMed] [Google Scholar]
- 19.Shin HJ, Kim HH, Huh MO, et al. Correlation between mammographic and sonographic findings and prognostic factors in patients with node-negative invasive breast cancer. Br J Radiol. 2011;84:19. doi: 10.1259/bjr/92960562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SH, Seo BK, Lee J, et al. Correlation of ultrasound findings with histology, tumor grade, and biological markers in breast cancer. Acta Oncol. 2008;47:1531. doi: 10.1080/02841860801971413. [DOI] [PubMed] [Google Scholar]
- 21.Baker JA, Kornguth PJ, Soo MS, et al. Sonography of solid breast lesions: observer variability of lesion description and assessment. AJR Am J Roentgenol. 1999;172:1621. doi: 10.2214/ajr.172.6.10350302. [DOI] [PubMed] [Google Scholar]
- 22.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14:103. doi: 10.1016/0378-5122(92)90003-m. [DOI] [PubMed] [Google Scholar]


