Abstract
Leukosialin (CD43) is a major glycoprotein of T lymphocytes whose extracellular domain of 224 amino acids contains on average one O-linked carbohydrate unit per three amino acids. This suggests an unfolded structure for the extracellular domain which has now been established to extend to a length of 45 nm by transmission electron microscopy following low angle rotary shadowing. The antigenicity of rat leukosialin has been studied using nine monoclonal antibodies (MAbs) whose binding is differentially affected by the cell type on which leukosialin is expressed and by the removal of sialic acid. From these observations it appears that the epitopes are affected by glycosylation, yet seven of the nine MAbs reacted clearly with the extracellular domain of leukosialian expressed in an unglycosylated form in Escherichia coli. The MAbs showing this positive reaction included three of the four antibodies whose epitopes were affected by neuraminidase treatment of leukosialin. It thus appears that linear protein epitopes are recognized and that some of these can be modified in the native structure by glycosylation. The positions of the antigenic determinants have been mapped by expressing fusion proteins of different lengths and the identity of one epitope was proven by the binding of two MAbs to an octapeptide expressed as a fusion protein. For three MAbs, the location of epitopes in the native protein was confirmed by electron microscopy of shadowed leukosialin--Fab complexes. Overall it is concluded that leukosialin is a major component at the periphery of the T lymphocyte and that despite its high level of glycosylation, protein determinants are exposed that could be ligands in cell interactions.
Full text
PDF
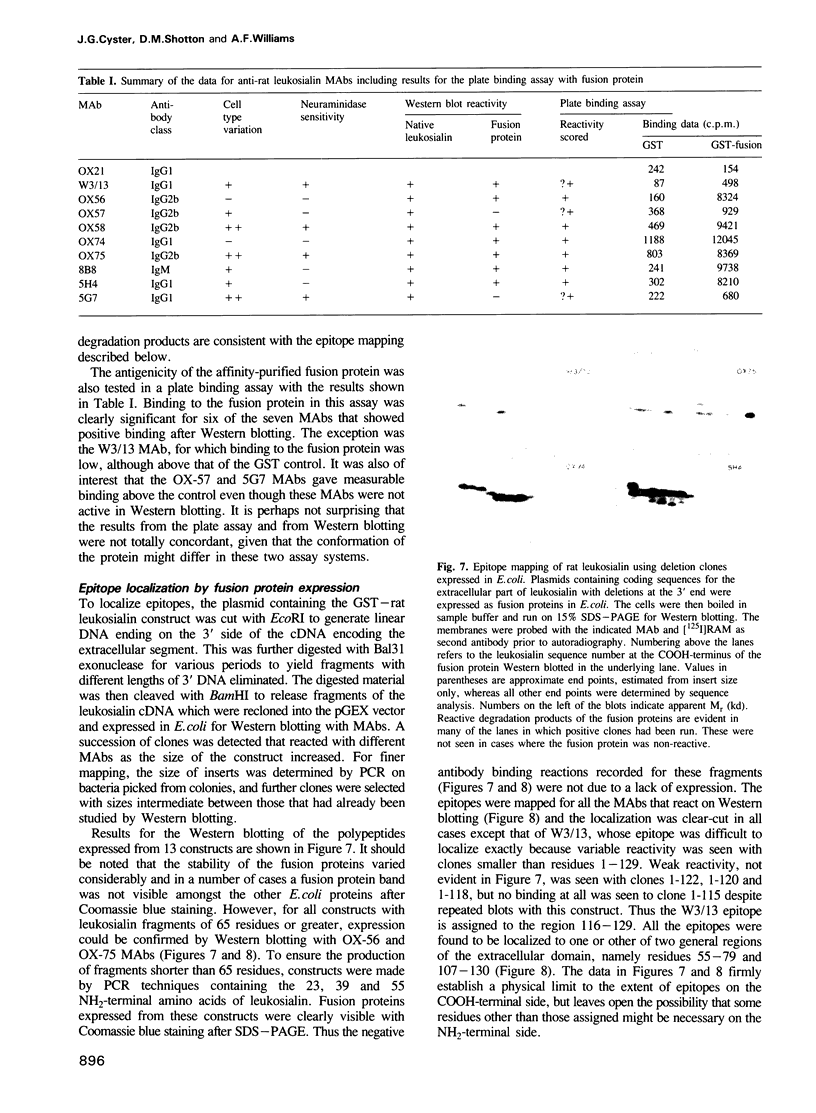
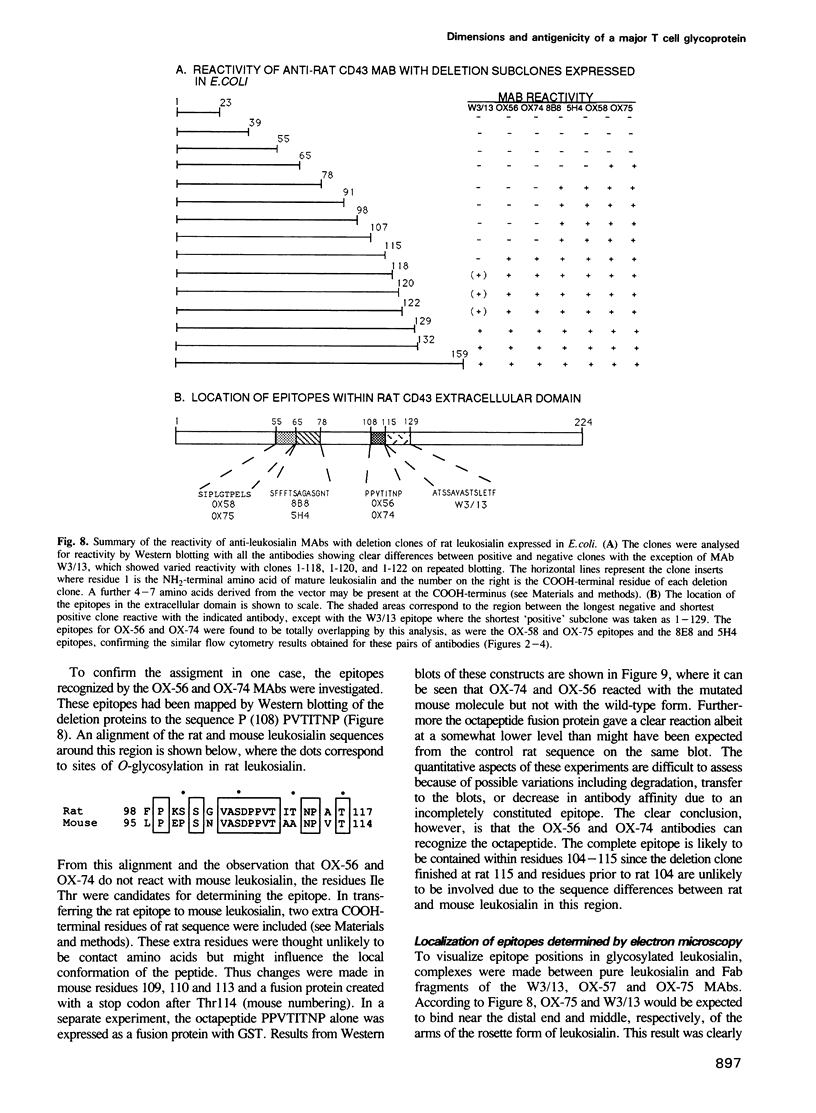

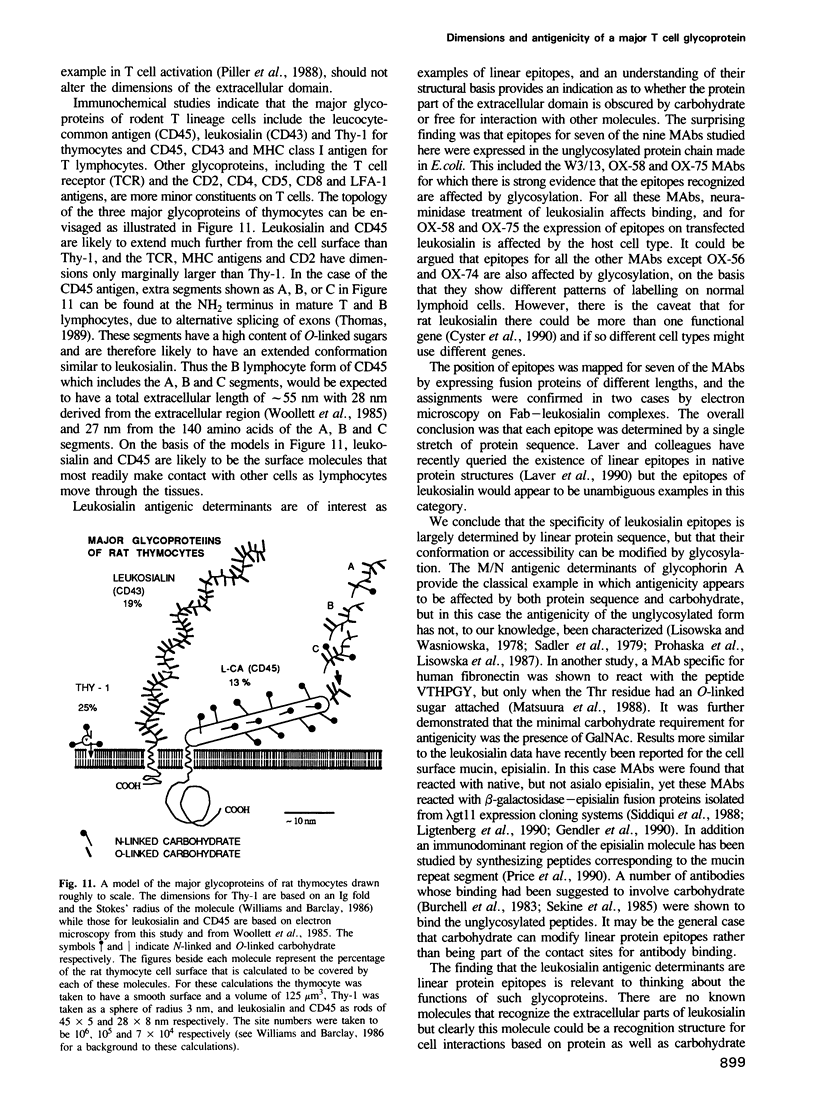



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardman B., Sikorski M. A., Settles M., Staunton D. E. Human immunodeficiency virus type 1-infected individuals make autoantibodies that bind to CD43 on normal thymic lymphocytes. J Exp Med. 1990 Oct 1;172(4):1151–1158. doi: 10.1084/jem.172.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson B., Youseffi-Etemad R., Hammarström S., Perlmann P. Induction of aggregation and enhancement of proliferation and IL-2 secretion in human T cells by antibodies to CD43. J Immunol. 1988 Nov 1;141(9):2912–2917. [PubMed] [Google Scholar]
- Bettaieb A., Farace F., Mitjavila M. T., Mishal Z., Dokhelar M. C., Tursz T., Breton-Gorius J., Vainchenker W., Kieffer N. Use of a monoclonal antibody (GA3) to demonstrate lineage restricted O-glycosylation on leukosialin during terminal erythroid differentiation. Blood. 1988 May;71(5):1226–1233. [PubMed] [Google Scholar]
- Bramwell M. E., Wiseman G., Shotton D. M. Electron-microscopic studies of the CA antigen, epitectin. J Cell Sci. 1986 Dec;86:249–261. doi: 10.1242/jcs.86.1.249. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Barclay A. N., Sunderland C. A., Williams A. F. Identification of a glycophorin-like molecule at the cell surface of rat thymocytes. Nature. 1981 Feb 5;289(5797):456–460. doi: 10.1038/289456a0. [DOI] [PubMed] [Google Scholar]
- Burchell J., Durbin H., Taylor-Papadimitriou J. Complexity of expression of antigenic determinants, recognized by monoclonal antibodies HMFG-1 and HMFG-2, in normal and malignant human mammary epithelial cells. J Immunol. 1983 Jul;131(1):508–513. [PubMed] [Google Scholar]
- Carlsson S. R., Fukuda M. Isolation and characterization of leukosialin, a major sialoglycoprotein on human leukocytes. J Biol Chem. 1986 Sep 25;261(27):12779–12786. [PubMed] [Google Scholar]
- Carlsson S. R., Sasaki H., Fukuda M. Structural variations of O-linked oligosaccharides present in leukosialin isolated from erythroid, myeloid, and T-lymphoid cell lines. J Biol Chem. 1986 Sep 25;261(27):12787–12795. [PubMed] [Google Scholar]
- Chang H. L., Zaroukian M. H., Esselman W. J. T200 alternate exon use in murine lymphoid cells determined by reverse transcription-polymerase chain reaction. J Immunol. 1989 Jul 1;143(1):315–321. [PubMed] [Google Scholar]
- Cyster J., Somoza C., Killeen N., Williams A. F. Protein sequence and gene structure for mouse leukosialin (CD43), a T lymphocyte mucin without introns in the coding sequence. Eur J Immunol. 1990 Apr;20(4):875–881. doi: 10.1002/eji.1830200424. [DOI] [PubMed] [Google Scholar]
- Davis S. J., Brady R. L., Barclay A. N., Harlos K., Dodson G. G., Williams A. F. Crystallization of a soluble form of the rat T-cell surface glycoprotein CD4 complexed with Fab from the W3/25 monoclonal antibody. J Mol Biol. 1990 May 5;213(1):7–10. doi: 10.1016/S0022-2836(05)80116-0. [DOI] [PubMed] [Google Scholar]
- Dyer M. J., Hunt S. V. Committed T lymphocyte stem cells of rats. Characterization by surface W3/13 antigen and radiosensitivity. J Exp Med. 1981 Oct 1;154(4):1164–1177. doi: 10.1084/jem.154.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrick D. A., Sambhara S. R., Ballhausen W., Iwamoto A., Pircher H., Walker C. L., Yokoyama W. M., Miller R. G., Mak T. W. T cell function and expression are dramatically altered in T cell receptor V gamma 1.1J gamma 4C gamma 4 transgenic mice. Cell. 1989 May 5;57(3):483–492. doi: 10.1016/0092-8674(89)90923-9. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Carlsson S. R., Klock J. C., Dell A. Structures of O-linked oligosaccharides isolated from normal granulocytes, chronic myelogenous leukemia cells, and acute myelogenous leukemia cells. J Biol Chem. 1986 Sep 25;261(27):12796–12806. [PubMed] [Google Scholar]
- Fukuda M., Carlsson S. R. Leukosialin, a major sialoglycoprotein on human leukocytes as differentiation antigens. Med Biol. 1986;64(6):335–343. [PubMed] [Google Scholar]
- Gahmberg C. G., Häyry P., Andersson L. C. Characterization of surface glycoproteins of mouse lymphoid cells. J Cell Biol. 1976 Mar;68(3):642–653. doi: 10.1083/jcb.68.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendler S. J., Lancaster C. A., Taylor-Papadimitriou J., Duhig T., Peat N., Burchell J., Pemberton L., Lalani E. N., Wilson D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990 Sep 5;265(25):15286–15293. [PubMed] [Google Scholar]
- Gerken T. A., Butenhof K. J., Shogren R. Effects of glycosylation on the conformation and dynamics of O-linked glycoproteins: carbon-13 NMR studies of ovine submaxillary mucin. Biochemistry. 1989 Jun 27;28(13):5536–5543. doi: 10.1021/bi00439a030. [DOI] [PubMed] [Google Scholar]
- Gulley M. L., Ogata L. C., Thorson J. A., Dailey M. O., Kemp J. D. Identification of a murine pan-T cell antigen which is also expressed during the terminal phases of B cell differentiation. J Immunol. 1988 Jun 1;140(11):3751–3757. [PubMed] [Google Scholar]
- He Q., Beyers A. D., Barclay A. N., Williams A. F. A role in transmembrane signaling for the cytoplasmic domain of the CD2 T lymphocyte surface antigen. Cell. 1988 Sep 23;54(7):979–984. doi: 10.1016/0092-8674(88)90112-2. [DOI] [PubMed] [Google Scholar]
- Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990 Aug;15(8):291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- Killeen N., Barclay A. N., Willis A. C., Williams A. F. The sequence of rat leukosialin (W3/13 antigen) reveals a molecule with O-linked glycosylation of one third of its extracellular amino acids. EMBO J. 1987 Dec 20;6(13):4029–4034. doi: 10.1002/j.1460-2075.1987.tb02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G., Smith-Gill S. J. Epitopes on protein antigens: misconceptions and realities. Cell. 1990 May 18;61(4):553–556. doi: 10.1016/0092-8674(90)90464-p. [DOI] [PubMed] [Google Scholar]
- Ligtenberg M. J., Vos H. L., Gennissen A. M., Hilkens J. Episialin, a carcinoma-associated mucin, is generated by a polymorphic gene encoding splice variants with alternative amino termini. J Biol Chem. 1990 Apr 5;265(10):5573–5578. [PubMed] [Google Scholar]
- Lisowska E., Messeter L., Duk M., Czerwiński M., Lundblad A. A monoclonal anti-glycophorin A antibody recognizing the blood group M determinant: studies on the subspecificity. Mol Immunol. 1987 Jun;24(6):605–613. doi: 10.1016/0161-5890(87)90041-1. [DOI] [PubMed] [Google Scholar]
- Lisowska E., Waśniowska K. Immunochemical characterization of cyanogen bromide degradation products of M and N blood-group glycopeptides. Eur J Biochem. 1978 Jul 17;88(1):247–252. doi: 10.1111/j.1432-1033.1978.tb12444.x. [DOI] [PubMed] [Google Scholar]
- Mallett S., Fossum S., Barclay A. N. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes--a molecule related to nerve growth factor receptor. EMBO J. 1990 Apr;9(4):1063–1068. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura H., Takio K., Titani K., Greene T., Levery S. B., Salyan M. E., Hakomori S. The oncofetal structure of human fibronectin defined by monoclonal antibody FDC-6. Unique structural requirement for the antigenic specificity provided by a glycosylhexapeptide. J Biol Chem. 1988 Mar 5;263(7):3314–3322. [PubMed] [Google Scholar]
- Pallant A., Eskenazi A., Mattei M. G., Fournier R. E., Carlsson S. R., Fukuda M., Frelinger J. G. Characterization of cDNAs encoding human leukosialin and localization of the leukosialin gene to chromosome 16. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1328–1332. doi: 10.1073/pnas.86.4.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piller F., Piller V., Fox R. I., Fukuda M. Human T-lymphocyte activation is associated with changes in O-glycan biosynthesis. J Biol Chem. 1988 Oct 15;263(29):15146–15150. [PubMed] [Google Scholar]
- Price M. R., Hudecz F., O'Sullivan C., Baldwin R. W., Edwards P. M., Tendler S. J. Immunological and structural features of the protein core of human polymorphic epithelial mucin. Mol Immunol. 1990 Aug;27(8):795–802. doi: 10.1016/0161-5890(90)90089-i. [DOI] [PubMed] [Google Scholar]
- Prohaska R., Koerner T. A., Jr, Armitage I. M., Furthmayr H. Chemical and carbon-13 nuclear magnetic resonance studies of the blood group M and N active sialoglycopeptides from human glycophorin A. J Biol Chem. 1981 Jun 10;256(11):5781–5791. [PubMed] [Google Scholar]
- Remold-O'Donnell E., Kenney D., Rosen F. S. Biosynthesis of human sialophorins and analysis of the polypeptide core. Biochemistry. 1987 Jun 30;26(13):3908–3913. doi: 10.1021/bi00387a025. [DOI] [PubMed] [Google Scholar]
- Rose M. C., Voter W. A., Sage H., Brown C. F., Kaufman B. Effects of deglycosylation on the architecture of ovine submaxillary mucin glycoprotein. J Biol Chem. 1984 Mar 10;259(5):3167–3172. [PubMed] [Google Scholar]
- Sadler J. E., Paulson J. C., Hill R. L. The role of sialic acid in the expression of human MN blood group antigens. J Biol Chem. 1979 Mar 25;254(6):2112–2119. [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Sekine H., Ohno T., Kufe D. W. Purification and characterization of a high molecular weight glycoprotein detectable in human milk and breast carcinomas. J Immunol. 1985 Nov;135(5):3610–3615. [PubMed] [Google Scholar]
- Shelley C. S., Remold-O'Donnell E., Davis A. E., 3rd, Bruns G. A., Rosen F. S., Carroll M. C., Whitehead A. S. Molecular characterization of sialophorin (CD43), the lymphocyte surface sialoglycoprotein defective in Wiskott-Aldrich syndrome. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2819–2823. doi: 10.1073/pnas.86.8.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley C. S., Remold-O'Donnell E., Rosen F. S., Whitehead A. S. Structure of the human sialophorin (CD43) gene. Identification of features atypical of genes encoding integral membrane proteins. Biochem J. 1990 Sep 15;270(3):569–576. doi: 10.1042/bj2700569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shogren R. L., Jamieson A. M., Blackwell J., Jentoft N. Conformation of mucous glycoproteins in aqueous solvents. Biopolymers. 1986 Aug;25(8):1505–1517. doi: 10.1002/bip.360250809. [DOI] [PubMed] [Google Scholar]
- Shogren R., Gerken T. A., Jentoft N. Role of glycosylation on the conformation and chain dimensions of O-linked glycoproteins: light-scattering studies of ovine submaxillary mucin. Biochemistry. 1989 Jun 27;28(13):5525–5536. doi: 10.1021/bi00439a029. [DOI] [PubMed] [Google Scholar]
- Shotton D. M., Burke B. E., Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol. 1979 Jun 25;131(2):303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- Siddiqui J., Abe M., Hayes D., Shani E., Yunis E., Kufe D. Isolation and sequencing of a cDNA coding for the human DF3 breast carcinoma-associated antigen. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2320–2323. doi: 10.1073/pnas.85.7.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Sportsman J. R., Park M. M., Cheresh D. A., Fukuda M., Elder J. H., Fox R. I. Characterization of a membrane surface glycoprotein associated with T-cell activation. J Immunol. 1985 Jul;135(1):158–164. [PubMed] [Google Scholar]
- Standring R., McMaster W. R., Sunderland C. A., Williams A. F. The predominant heavily glycosylated glycoproteins at the surface of rat lymphoid cells are differentiation antigens. Eur J Immunol. 1978 Dec;8(12):832–839. doi: 10.1002/eji.1830081203. [DOI] [PubMed] [Google Scholar]
- Thomas M. L. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:339–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- Uchida Y., Tsukada Y., Sugimori T. Enzymatic properties of neuraminidases from Arthrobacter ureafaciens. J Biochem. 1979 Nov;86(5):1573–1585. doi: 10.1093/oxfordjournals.jbchem.a132675. [DOI] [PubMed] [Google Scholar]
- Wikén M., Björck P., Axelsson B., Perlmann P. Induction of CD43 expression during activation and terminal differentiation of human B cells. Scand J Immunol. 1988 Oct;28(4):457–464. doi: 10.1111/j.1365-3083.1988.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Winship P. R. An improved method for directly sequencing PCR amplified material using dimethyl sulphoxide. Nucleic Acids Res. 1989 Feb 11;17(3):1266–1266. doi: 10.1093/nar/17.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett G. R., Williams A. F., Shotton D. M. Visualisation by low-angle shadowing of the leucocyte-common antigen. A major cell surface glycoprotein of lymphocytes. EMBO J. 1985 Nov;4(11):2827–2830. doi: 10.1002/j.1460-2075.1985.tb04010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]