Abstract
Background
Primacy performance in recall has been shown to predict cognitive decline in cognitively intact elderly and conversion from mild cognitive impairment to Alzheimer's disease (AD). Delayed primacy performance, but not delayed nonprimacy performance, has been shown to be associated with hippocampal volume in cognitively intact older individuals. Because presence of neurofibrillary tangles is an early sign of AD-related pathology, we set out to test whether cerebrospinal fluid (CSF) levels of tau had an effect on delayed primacy performance, while controlling for hippocampal volume and CSF amyloid-β 1-42 levels.
Methods
Forty-seven individuals, aged 60 years or older and cognitively intact, underwent a multisession study including lumbar puncture, a magnetic resonance imaging (MRI) scan of the head, and memory testing.
Results
Our regression analyses show that CSF levels of hyperphosphorylated (P) tau are only associated with reduced delayed primacy performance when hippocampal volumes are smaller.
Conclusion
Our findings suggest that hippocampal size may play a protective role against the negative effects of P tau on memory.
Keywords: Serial position, Primacy, Memory, Amyloid β 1-42, Tau, Hippocampus, Alzheimer's disease, CSF biomarkers
1. Background
The identification of individuals at risk of Alzheimer's disease (AD) during preclinical stages is critical for the implementation of early intervention strategies [1]. Recently, episodic memory performance for primacy items (i.e., first few items on a study list) has been shown to provide predictive value for cognitive decline in both cognitively intact elderly [2] and conversion from mild cognitive impairment (MCI) to AD [3]. Primacy performance, especially in delayed memory tasks (e.g., after 20 minutes), is thought to reflect consolidation ability [4], a critical target function for prediction of subsequent neurodegeneration [5]. Importantly, consolidation is thought to rely on the hippocampal formation [6], whose integrity has also been examined in studies of AD prediction (e.g., [7], [8], [9]). Finally, we have shown that hippocampal gray matter volume predicts delayed primacy performance, but not memory performance for other regions of the study list, in cognitively intact older individuals, thus confirming the link between hippocampus and memory for early list items [10].
A key component of AD neuropathology is the presence of neurofibrillary tangles, which are typically observed in the medial temporal lobe (MTL) first, and in the hippocampus in particular [11]. The degree of neurofibrillary tangle burden has been associated with levels of total (T) and hyperphosphorylated (P) tau in clinical-postmortem comparison studies ([12], [13], [14] but see [15]), suggesting that in vivo cerebrospinal fluid (CSF) levels of T and P tau may serve as surrogate measures for the degree of hippocampal and cortical neurofibrillary pathology. CSF levels of T and P tau have been found to associate with short-term memory performance in AD [16] and to correlate negatively with hippocampal volume both in individuals with AD [17] and MCI [18]. Moreover, P tau is considered a key factor in entorhinal cortex degeneration in cognitively intact participants [19].
For the reasons mentioned previously, we set out to test whether delayed primacy performance—defined as the first four words on the study list to maintain consistency with [2]—in cognitively intact individuals is associated with CSF levels of T and P tau. In particular, we expect that higher levels of P tau, which may reflect tangle pathology [20] affecting the hippocampus and cortical brain areas, will be associated with poorer primacy performance. Moreover, we explore whether the relationship between hippocampal size and delayed primacy performance [10] is moderated by CSF tau levels, while controlling also for CSF levels of amyloid-β (Aβ) 1-42, which provide an index of amyloid pathology (e.g., [14]).
2. Methods
2.1. Subjects
Participants for the study were recruited from either the Memory Education and Research Initiative (MERI) program at the Nathan Kline Institute for Psychiatric Research (NKI) or via advertisements; recruitment was originally for a study on major depression disorder (MDD) in old age (see [21]). The study was approved by the institutional review boards of the NKI and the New York University (NYU) School of Medicine. All participants were paid up to $450.00 for their participation in the study and provided formal consent before testing. A total of 133 participants were recruited for the study, although only 51 received a lumbar puncture from which CSF could be extracted. To maintain a cognitively intact sample, we excluded participants whose Mini-Mental State Examination (MMSE) score was below 28 and/or presented magnetic resonance imaging (MRI) evidence of confluent deep or periventricular white matter hyperintensities. These exclusion criteria left us with a total of 47 participants, 28 of whom received a diagnosis of MDD from a board-certified psychiatrist (N.P.) and 19 were controls.
2.2. CSF measurements
Aβ1-42 CSF levels were analyzed with electrochemiluminescence technology using the MS6000 Human Ab Ultra-Sensitive Kit (Meso Scale Discovery, Gaithersburg, MD, USA). Both T and P tau concentrations were determined using a sandwich ELISA (Innotest hTAU-Ag, Innogenetics, Ghent, Belgium) specifically constructed for all tau isoforms, irrespective of phosphorylation status.
2.3. MRI acquisition
The acquisition was performed on a 1.5-T Siemens Vision system (Erlangen, Germany) at the NKI. Images were acquired using a sagittal magnetization prepared rapid gradient-echo sequence (repetition time [TR]/echo time [TE] = 11.4/11.9 ms, 1 excitation [NEX], matrix = 256 × 256, field of view [FOV] = 307 mm, 1.2 mm3 isotropic voxel, 172 slices, no gap). Evaluation of white matter hyperintensities was performed using a fluid-attenuated inversion recovery sequence (TR/TE = 9000/119 ms, inversion time = 2400 ms, NEX = 1, matrix 256 × 256, FOV = 240 mm, slice thickness = 4 mm, 1 mm gap).
2.4. MRI preprocessing and analysis
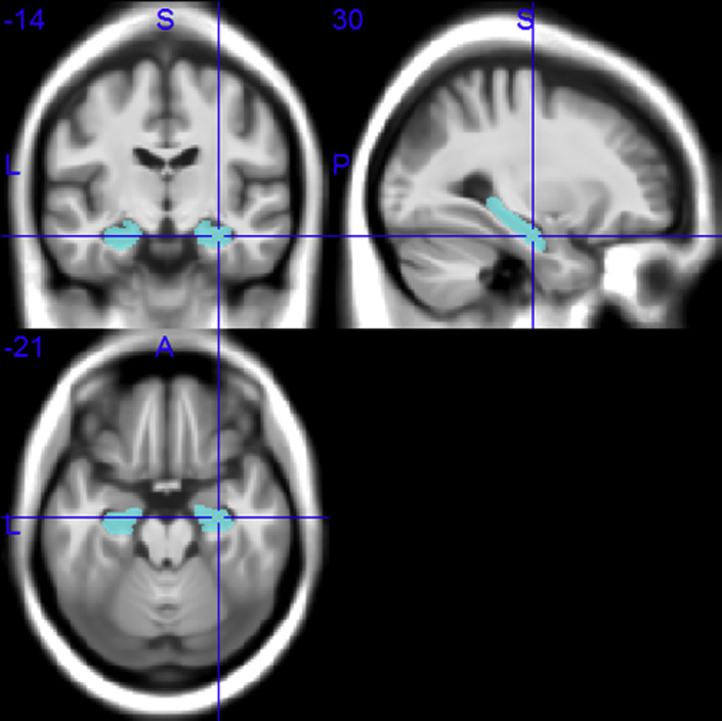
MRI data processing followed procedures described previously [22], [23]. Fig. 1 illustrates the hippocampal regions of interest. The total intracranial volume (TIV) was used in the statistical model to account for differences in head size (see Study design and analysis below) and was calculated as the sum of the total segmented gray matter, white matter, and CSF volumes in native space.
Fig. 1.
Hippocampal regions of interest in Montreal Neurological Institute space.
2.5. Procedure
The study was conducted at the NKI and at the Clinical and Translational Science Institute, NYU, over multiple visits. On the first visit, after informed consent was provided, volunteers were administered a general medical intake questionnaire and had their vital signs measured; the MMSE score and the Hamilton Depression Rating (HAM-D) score, which measures severity of current depressive symptoms, were obtained during this visit. On a second visit, participants received an MRI scan of the head. Neuropsychological testing took place on a third visit, and memory performance was assessed at this stage with the Buschke Selective Reminding Test (BSRT) [24]. This test comprises a list of 16 unrelated nouns, presented orally to the participant at a rate of 2 seconds each. After presentation, participants were asked to freely recall as many items as possible, stopping once they feel no more items can be retrieved. In the delayed trial, which is the focus of our current examination (cf., 2), the free recall task occurs roughly after a 20-minute delay from the initial presentation.
During a fourth and final session, a lumbar puncture was performed under guided fluoroscopy. Participants were asked to fast overnight, and the procedure took place between 9 am and 10 am in the morning. A total of 15 mL of clear CSF was collected in three polypropylene tubes labeled “A” (first 5 mL), “B” (second 5 mL), and “C” (third 5 mL). The tubes were immediately placed on ice for a maximum of 1 hour until the samples were centrifuged at 4°C (at 1500 rpm) for 10 minutes. Aliquots of 0.25 mL were subsequently placed into 1-mL polypropylene cryogenic vials and put into Nunc eight-cell storage boxes (Nalge Nunc International, Rochester, NY, USA) at −80°C. All Aβ and tau determinations were performed from tube C.
2.6. Study design and analysis
To test our hypothesis that P tau, either directly or via moderation with hippocampal gray matter volume, predicts delayed primacy performance, we carried out multiple linear regression analyses. The outcome variables, analyzed separately, were (1) the proportion of primacy items (first four words; cf. [2]) recalled in the delayed task of the BSRT task and (2) the proportion of nonprimacy (all words recalled minus primacy words) items recalled in the same task. Both outcome variables were normally distributed based on assessment of skewness and kurtosis [25]. The main predictor was CSF tau, either T or P in separate analyses to avoid multicollinearity (the correlation between T and P tau yielded an r coefficient of 0.968, P < .001). Moreover, we also tested the tau (both T and P) by hippocampal volume moderation term in a separate model. Age, CSF Aβ1-42, total hippocampal volume (mm3), TIV (mm3) to control for head size, and HAM-D score to control for presence of current depressive symptoms were used as control variables in a three-model procedure. Model 1 included all control variables, model 2 included the predictor, and model 3 included the moderation term. Finally, all predictors and control variables were standardized.
3. Results
Table 1 reports group demographics, CSF values, and memory performance scores. The total N for T tau was 46 because of a missing value. No issues of multicollinearity were observed (variance inflation factor ≤2.628) in the regression analyses. Considering the mixed nature of our sample (i.e., individuals with MDD and controls), we evaluated the possibility that the HAM-D scores could be highly skewed (e.g., 0 values for all controls). However, visual exploration of the scores yielded no significant outliers, and z scores for both skewness and kurtosis were within the typically accepted 1.96 threshold (≤1.80; 25).
Table 1.
Demographics, CSF values, and memory performance: age in years (mean and standard deviation); HDS score (mean and standard deviation); CSF levels of Aβ1-42, T and P tau (mean and standard deviation); and proportions of primacy and nonprimacy performance (mean and standard deviation)
| Variable | Mean (SD) |
|---|---|
| Age | 67.13 (6.23) |
| HDS | 10.55 (11.01) |
| Aβ1-42 | 269.45 (158.93) |
| T tau | 296.02 (132.38)∗ |
| P tau | 50.00 (23.78) |
| Primacy | 0.64 (0.27) |
| Nonprimacy | 0.54 (0.17) |
Abbreviations: CSF, cerebrospinal fluid; HDS, Hamilton Depression Rating score; Aβ, amyloid-β.
NOTE. Number of subjects (i.e., N) for each measurement was 47 except stated otherwise.
Because of an undetermined value, the N for T tau is 46.
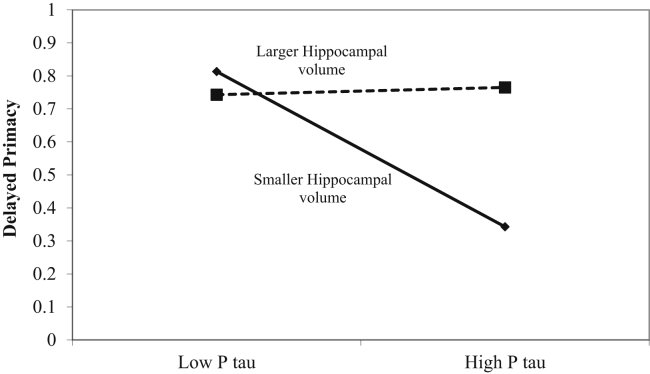
P tau was a significant predictor of delayed primacy performance (β = −0.388, P = .022), but this relationship appears to be qualified by the interaction between P tau and hippocampal size (β = 0.354, P = .035). The interaction brought explained variance (R2) from 0.191 with model 1 and 2 combined to 0.280 with model 3, for a 32% increase. Fig. 2 shows that delayed primacy performance is unaffected by changes in P tau CSF levels at larger hippocampal volumes but declines when the P tau levels are higher and the size of the hippocampus is smaller. These results suggest that hippocampal size may provide a form of protection against increases in P tau CSF levels.
Fig. 2.
Plot of cerebrospinal fluid P tau levels (x-axis) by proportion of delayed recalled primacy items (y-axis), as moderated by hippocampal gray matter volume.
In contrast, neither P tau (only 0.009 variance explained over model 1), nor the moderation term (only 0.001 variance explained over model 2), nor any of the control variables appeared to predict delayed nonprimacy performance significantly. The closest predictor in this analysis was Aβ1-42 (β = 0.280, P = .090), suggesting that higher levels of CSF Aβ1-42, which should index less amyloid pathology, may be associated with better memory performance for nonprimacy items.
Analogously to P tau, T tau showed marginal effects on delayed primacy both independently (β = −0.336, P = .078) and as part of the interaction (β = 0.315, P = .072), although neither relationship is statistically significant. Also, once again, the closest significant predictor of nonprimacy performance was CSF Aβ1-42 (β = 0.279, P = .095).
4. Discussion
Recent findings have shown that delayed free recall performance for early list words (i.e., delayed primacy performance) may be predictive of cognitive decline in older individuals who are cognitively intact at baseline [2] and that, also in cognitively healthy participants, hippocampal gray matter volume is associated with delayed primacy, but not delayed nonprimacy, performance [10]. These results are consistent with a burgeoning literature on the predictive and diagnostic value of serial position analysis, and primacy in particular (e.g., [3], [26], [27]). In this study on cognitively intact elderly individuals, we have demonstrated for the first time that delayed primacy performance is also affected by CSF levels of P tau. P tau is a marker of neurofibrillary pathology, which is considered to be one of the first neuropathological events in AD, and is thought to be initiated in the MTL, including the hippocampus. Critically, we observed in our study an interaction effect between hippocampal gray volume and CSF P tau levels on delayed primacy performance. This interaction suggests that hippocampal size may act as a protective factor against the deleterious effects of P tau on delayed primacy performance.
A limitation of our present study is that the sample was made up of both participants with a diagnosis of MDD and healthy controls. MDD has been associated with poorer memory performance (e.g., [28]) and reduced hippocampal volume (e.g., [29]), and it is therefore possible that the combination of healthy and depressed participants may have confounded our results. However, considering that we controlled for the HAM-D score in all of our analyses, we feel quite confident that depressive symptoms did not significantly alter the pattern of our results.
Another potential issue pertains to the possibility of a selection bias in CSF collection. As noted, of 133 total participants, only 51 subjects consented to the lumbar puncture. The idea of having a lumbar puncture can be frightening, and all prospective participants were informed of the possible side effects, such as bleeding and headaches. Therefore, it is not implausible that a systematic bias may be introduced in the study and that only certain participants (e.g., only highly motivated participants, or highly educated) may have consented to the lumbar puncture. However, a basic comparison between consenters and nonconsenters, showing no significant difference across groups in age, MMSE score, or years of education (P ≥ .208), provides no evidence of a bias, although this possibility cannot be categorically ruled out.
Attempts at investigating the relationship between hippocampal volume and episodic memory function in older adults have been met so far with high levels of variability across studies and little supporting evidence of a positive correlation between size and performance [30]. Our results may help clarifying this issue. First of all, if the hippocampus is primarily involved with the retrieval of early list items, as suggested by Bruno et al. [10], then it is possible that studies that ignore serial position performance and focus exclusively on total list memory will find associations between hippocampal size and memory only when, incidentally, primacy and nonprimacy outputs are highly correlated. For example, we can imagine three study participants: participant A recalls all primacy items (e.g., 4) and all nonprimacy items (e.g., 12) for a total of 16 items; participant B recalls 0 primacy items, but all nonprimacy items for a total of 12; and participant C recalls all primacy items, but 0 nonprimacy items for a total of 4. In this example, and based on our conjecture of a preferential link between hippocampus and primacy, participant C would be expected to have a larger hippocampus than participant B despite a lower total memory score; additionally, participant A would be expected to have roughly a similar-sized hippocampus as participant C, despite a much higher total score. This example illustrates how the issues of interstudy variability in examining the relationship between hippocampal volume and episodic memory ability may at least partly be related to differences in serial position effects.
An alternative solution to the issue of inconsistency across studies may be found in our current results. A look at Fig. 2 suggests that smaller hippocampal volumes are only associated with a reduction of memory performance, specifically delayed primacy, in the presence of high levels of P tau. Therefore, it may be possible that hippocampal function, and consequently memory function, is roughly comparable across a wide range of hippocampal volumes as long as these remain above a certain size that we can consider to be “normal.” In contrast, when hippocampal volumes are smaller, they are only good estimators of individual hippocampal dysfunction, and therefore poorer memory, when they are also accompanied by a neurodegenerative process, such as, for example, macrostructural hippocampal degeneration caused by neurofibrillary tangles. More research however is required to clarify these issues further.
Research in context.
-
1.
Systematic Review: Articles examining the links, in elderly individuals, between cerebrospinal fluid biomarkers of Alzheimer's disease (AD), hippocampal volume, and memory performance were identified via reference sections and/or popular search engines. Our goal was to evaluate the complex relationship between these variables, keeping in mind recent evidence suggesting that delayed primacy performance may predict cognitive decline.
-
2.
Interpretation: Our findings suggest that the relationship between hippocampal size and memory performance may be understood better if the serial position in free recall tests is examined and that hippocampal size may play a protective role against the deleterious effects of P tau on memory.
-
3.
Future directions: Future studies should investigate whether delayed primacy performance is a sensitive predictor of conversion to mild cognitive impairment and/or AD from a cognitively healthy baseline. Moreover, the relationship between brain structure and serial position performance in both cognitively intact and impaired elderly participants should be elucidated further.
Acknowledgments
The authors thank Marina Boccardi for expert advice and quality check of the traced hippocampus outlines following the EADC-ADNI harmonized hippocampus protocol, as well as Luigi Antelmi for helping with format conversion of the traced hippocampus mask. We would also like to acknowledge the help and support provided by Chelsea Reichert, Dr Antero Sarreal, and Dr Hernando Raymundo. We also wish to thank Jeremy Dawson for making available online resources to plot simple slope effects (http://www.jeremydawson.com/slopes.htm). This study was funded in part by an NIMH grant (R01 MH-080405) to N.P. The authors declare no conflicts of interest.
References
- 1.Emery V.O.B. Alzheimer disease: Are we intervening too late? J Neural Transm. 2011;118:1361–1378. doi: 10.1007/s00702-011-0663-0. [DOI] [PubMed] [Google Scholar]
- 2.Bruno D., Reiss P.T., Petkova E., Sidtis J.J., Pomara N. Decreased recall of primacy words predicts cognitive decline. Arch Clin Neuropsychol. 2013;28:95–103. doi: 10.1093/arclin/acs116. [DOI] [PubMed] [Google Scholar]
- 3.Egli S.C., Beck I.R., Berres M., Foldi N.S., Monsch A.U., Sollberger M. Serial position effects are sensitive predictors of conversion from MCI to Alzheimer's disease dementia. Alzheimers Dement. 2014;10(5 Suppl):S420–S424. doi: 10.1016/j.jalz.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 4.McGaugh J.L. Memory: A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 5.Gomar J.J., Bobes-Bascaran M.T., Conejero-Goldberg C., Davies P., Goldberg T.E. Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the Alzheimer's disease neuroimaging initiative. Arch Gen Psychiatry. 2011;68:961–969. doi: 10.1001/archgenpsychiatry.2011.96. [DOI] [PubMed] [Google Scholar]
- 6.Wixted J.T. The psychology and neuroscience of forgetting. Annu Rev Psychol. 2004;55:235–269. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]
- 7.Apostolova L.G., Mosconi L., Thompson P.M., Green A.E., Hwang K.S., Ramirez A. Subregional hippocampal atrophy predicts Alzheimer's dementia in the cognitively normal. Neurobiol Aging. 2010;31:1077–1088. doi: 10.1016/j.neurobiolaging.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Achterberg H.C., van der Lijn F., den Heijer T., Vernooij M.W., Ikram M.A., Niessen W.J. Hippocampal shape is predictive for the development of dementia in a normal, elderly population. Hum Brain Mapp. 2014;35:2359–2371. doi: 10.1002/hbm.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tondelli M., Wilcock G.K., Nichelli P., De Jager C.A., Jenkinson M., Zamboni G. Structural MRI changes detectable up to ten years before clinical Alzheimer's disease. Neurobiol Aging. 2012;33:825.e25–825.e36. doi: 10.1016/j.neurobiolaging.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Bruno D., Grothe M.J., Nierenberg J., Teipel S, Pomara N. Hippocampal size predicts delayed primacy performance in cognitively intact elderly participants. Neuropsychologia. 2015;69:1–8. doi: 10.1016/j.neuropsychologia.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 12.Buerger K., Ewers M., Pirttilä T., Zinkowski R., Alafuzoff I., Teipel S.J. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 13.Tapiola T., Overmyer M., Lehtovirta M., Helisalmi S., Ramberg J., Alafuzoff I. The level of cerebrospinal fluid tau correlates with neurofibrillary tangles in Alzheimer's disease. Neuroreport. 1997;8:3961–3963. doi: 10.1097/00001756-199712220-00022. [DOI] [PubMed] [Google Scholar]
- 14.Tapiola T., Alafuzoff I., Herukka S.K., Parkkinen L., Hartikainen P., Soininen H. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66:382–389. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 15.Engelborghs S., Sleegers K., Cras P., Brouwers N., Serneels S., De Leenheir E. No association of CSF biomarkers with APOEε4, plaque and tangle burden in definite Alzheimer's disease. Brain. 2007;130:2320–2326. doi: 10.1093/brain/awm136. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y.T., Cheng J.T., Yao Y.C., Lo Y.K., Lin C.H., Ger L.P. Increased total tau but not amyloid-β _ {42}; in cerebrospinal fluid correlates with short-term memory impairment in Alzheimer's disease. J Alzheimers Dis. 2009;18:907–918. doi: 10.3233/JAD-2009-1214. [DOI] [PubMed] [Google Scholar]
- 17.De Souza L.C., Chupin M., Lamari F., Jardel C., Leclercq D., Colliot O. CSF tau markers are correlated with hippocampal volume in Alzheimer's disease. Neurobiol Aging. 2012;33:1253–1257. doi: 10.1016/j.neurobiolaging.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Herukka S.K., Pennanen C., Soininen H., Pirttilä T. CSF Aβ42, tau and phosphorylated tau correlate with medial temporal lobe atrophy. J Alzheimers Dis. 2008;14:51–57. doi: 10.3233/jad-2008-14105. [DOI] [PubMed] [Google Scholar]
- 19.Desikan R.S., McEvoy L.K., Thompson W.K., Holland D., Roddey J.C., Blennow K. Amyloid-β associated volume loss occurs only in the presence of phospho-tau. Ann Neurol. 2011;70:657–661. doi: 10.1002/ana.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blennow K., Hampel H., Weiner M., Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 21.Pomara N., Bruno D., Sarreal A., Hernando R., Nierenberg J., Petkova E. Lower CSF amyloid beta peptides and higher F2-isoprostanes in cognitively intact elderly individuals with major depressive disorder. Am J Psychiatry. 2012;169:523–530. doi: 10.1176/appi.ajp.2011.11081153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grothe M.J., Ewers M., Krause B., Heinsen H., Teipel S.J. Basal forebrain atrophy and cortical amyloid deposition in nondemented elderly subjects. Alzheimers Dement. 2014;10(5 Suppl):S344–S353. doi: 10.1016/j.jalz.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grothe M.J., Schuster C., Bauer F., Heinsen H., Prudlo J., Teipel S.J. Atrophy of the cholinergic basal forebrain in dementia with Lewy bodies and Alzheimer's disease dementia. J Neurol. 2014;261:1939–1948. doi: 10.1007/s00415-014-7439-z. [DOI] [PubMed] [Google Scholar]
- 24.Buschke H., Fuld P.A. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 25.Kim H.Y. Statistical notes for clinical researchers: assessing normal distribution using skewness and kurtosis. Restor Dent Endod. 2013;38:52–54. doi: 10.5395/rde.2013.38.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howieson D.B., Mattek N., Seeyle A.M., Dodge H.H., Wasserman D., Zitzelberger T. Serial position effects in mild cognitive impairment. J Clin Exp Neuropsychol. 2011;33:292–299. doi: 10.1080/13803395.2010.516742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.La Rue A., Hermann B., Jones J.J., Johnson S., Ashtana S., Sager M.A. Effect of parental family history of Alzheimer's disease on serial position profiles. Alzheimers Dement. 2008;4:285–290. doi: 10.1016/j.jalz.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin M.P., Mitchell P., Goodwin G.M. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- 29.Bremner J.D., Narayan M., Anderson E.R., Staib L.H., Miller H.L., Charney D.S. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 30.Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]