Abstract
Objective
Primary Sjögren’s syndrome (SS) is characterized by autoimmune activation and loss of function in secretory epithelia. The present study was undertaken to investigate and characterize changes in the epithelia associated with the loss of gland function in primary SS.
Methods
To identify changes in epithelial gene expression, custom microarrays were probed with complementary RNA (cRNA) isolated from minor salivary glands (MSGs) of female patients with primary SS who had low focus scores and low salivary flow rates, and the results were compared with those obtained using cRNA from the MSGs of sex-matched healthy volunteers. The effect of bone morphogenetic protein 6 (BMP-6) on salivary gland function was tested using adeno-associated virus–mediated gene transfer to the salivary glands of C57BL/6 mice.
Results
A significant increase in expression of BMP-6 was observed in RNA isolated from SS patients compared with healthy volunteers. Overexpression of BMP-6 locally in the salivary or lacrimal glands of mice resulted in the loss of fluid secretion as well as changes in the connective tissue of the salivary gland. Assessment of the fluid movement in either isolated acinar cells from mice overexpressing BMP-6 or a human salivary gland cell line cultured with BMP-6 revealed a loss in volume regulation in these cells. Lymphocytic infiltration in the submandibular gland of BMP-6 vector–treated mice was increased. No significant changes in the production of proinflammatory cytokines or autoantibodies associated with SS (anti-Ro/SSA and anti-La/SSB) were found after BMP-6 overexpression.
Conclusion
In addition to identifying BMP-6 expression in association with xerostomia and xerophthalmia in primary SS, the present results suggest that BMP-6–induced salivary and lacrimal gland dysfunction in primary SS is independent of the autoantibodies and immune activation associated with the disease.
A hallmark of primary Sjögren’s syndrome (SS) is the loss of function of secretory epithelia, specifically within lacrimal and salivary glands (1). The mechanism(s) driving primary SS are poorly understood and may involve a combination of environmental and genetic factors. In addition to the loss of secretory function in several epithelial cell types, autoantibodies, lymphocytic infiltrates in the secretory epithelia, increased apoptosis, and elevated levels of proinflammatory cytokines have been reported in patients with primary SS (1). Patients with more severe sicca symptoms report a significantly greater impact of the disease on many aspects of their daily life (2). Several lines of research suggest that salivary flow rate is independent of lymphocytic infiltration in primary SS (for review, see ref. 3). Seventeen percent of the patients who meet the American-European Consensus Group criteria for SS (4) have low levels of infiltrating lymphocytic foci with little evidence of acinar cell loss but with decreased salivary flow (3), suggesting an alternative mechanism for the loss of gland function.
In order to better understand changes in the secretory epithelia associated with the loss of gland function in patients with primary SS and low lymphocytic infiltration, we performed microarray analysis of RNA isolated from the minor salivary glands (MSGs) of patients with primary SS with low focus scores (≤ 2), decreased salivary flow, ocular symptoms, and positive autoantibodies, and compared the array results to those obtained with the MSGs of healthy volunteers.
MATERIALS AND METHODS
Patient selection criteria
Five female patients with primary SS fulfilling the American-European Consensus Group criteria were selected for microarray analysis, along with 6 healthy female volunteers. The study was approved by the Institutional Review Board of the National Institute of Dental and Craniofacial Research, National Institutes of Health (NIH) and is registered at www.clinicaltrials.gov. All subjects provided written informed consent prior to enrollment. The patients whose specimens were used in the present analysis were all chosen based on low lymphocytic scores (focus score ≤ 2) and low unstimulated salivary flow (<1.5 ml/15 minutes). Clinical features of the study subjects are summarized in Supplementary Table 1 (available on the Arthritis & Rheumatism web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38123/abstract). Two of the healthy volunteers had low salivary flow but were free of any disease and likely represent natural variation in salivary gland activity; they were included in the study to better identify changes in gland activity specifically associated with primary SS. Four of the 5 patients with primary SS were taking hydroxychloroquine, and 1 was taking prednisone (5 mg/day). Autoantibody status was tested at the Department of Laboratory Medicine, NIH, using a standardized enzyme-linked immunosorbent assay (ELISA).
Microarray studies
MSGs were obtained from study participants and stored in RNAlater (Qiagen) until RNA extraction. Samples were homogenized with a Bullet-Blender (Next Advance) or an Omni TH (Omni International). Total RNA was extracted with an RNeasy Mini kit according to the instructions of the manufacturer (Qiagen). The quality of RNA was measured using a 2100 Bioanalyzer (Agilent). Only RNA samples with a 28S/18S ribosomal RNA ratio of 1.7 and an RNA integrity number of ≥ 6.5 were used for the arrays. Total RNA from both patient and healthy volunteer samples was amplified and labeled with a low RNA input linear amplification kit (Agilent). A total of 500 ng of RNA was labeled with Cy3-CTP, and the quality and yield of complementary RNA (cRNA) were then analyzed using a NanoDrop ND-1000 UV-VIS Spectrophotometer (version 3.2.1). Only cRNA with a total yield of >1.65 μg and a specific avidity of > 9.0 pmoles Cy3/μg cRNA was used in the hybridization step.
Gene expression analysis involved the use of 4 × 44K microarrays (Agilent) containing ~41K human oligo probes. Microarrays were hybridized according to the manufacturer’s recommendations for One-Color Microarray-Based Gene Expression Analysis. Quality control criteria were established based on the results of previously published experiments (5). Microarrays that met ≥ 9 of 12 quality control criteria were deemed suitable for statistical analysis.
Statistical Analysis of Microarrays
GeneSpring GX 11 (Agilent) was used to normalize and filter the data obtained in this study. The gene expression arrays were subjected to a quantile normalization without a baseline transformation (an algorithm similar to robust multiarray average normalization techniques widely used in Affymetrix microarrays [6–8]).
After normalization, the probes with values below the 20th percentile in >80% of the study samples were removed. The resulting filtered sets were compared for genes that were differentially expressed (2-fold above or below the median in normal volunteers). An unpaired, asymptotic t-test with Benjamini and Hochberg’s false discovery rate (FDR) correction was used to identify genes that were statistically significantly up- or down-regulated (corrected P < 0.05).
The gene list was analyzed for additional pathway information using IPA software. IPA’s Molecular Network Analysis algorithm was used to generate candidate gene networks as previously described (7). Similarly, biomarker analyses were used for filtering salivary gland–specific genes. FDR-corrected P values were used to generate significant cutoffs throughout all of the analytical results obtained with IPA.
Construction of complementary DNA (cDNA) libraries
Purified RNA from the patients was reverse-transcribed using a SuperScript Vilo First-Strand cDNA synthesis kit (Invitrogen) for 2-step quantitative reverse transcription–polymerase chain reaction (RT-PCR). Gene expression is represented by the fold change, which was calculated, as 2− Δ ΔCt, according to the instructions of the manufacturer (Applied Biosystems).
Animals
Female C57BL/6 mice (6–8 weeks old) were obtained from The Jackson Laboratory. Animals were housed in a pathogen-free facility. All procedures involving live animals were performed in an accredited vivarium according to institutional guidelines and standard operating procedures and were in compliance with the NIH Guide for the Care and Use of Laboratory Animals.
Administration of recombinant adeno-associated virus type 5 (rAAV5) vector
The construction of rAAV5 encoding green fluorescent protein, luciferase, or bone morphogenetic protein 6 (BMP-6) vector (AAV5-GFP, AAV5-Luc, or AAV5-Bmp6, respectively), has been described previously (9,10). Vectors were delivered into the submandibular gland by retrograde instillation and into the lacrimal gland by injection as previously described (10,11). Briefly, AAV5-GFP or AAV5-Bmp6 (1011 particles/mouse [5 × 1010 particles/gland] in 100 μl) was delivered into the submandibular gland of 6–8-week-old C57BL/6J mice. A lower dose of AAV5-Luc (109 particles/ gland) was coadministered to enable monitoring of the expression of the AAV5 vectors by Xenogeny live imaging of the animals (10) (see Supplementary Figure 1, on the Arthritis & Rheumatism web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38123/abstract). To transduce the lacrimal gland, AAV5-Bmp6 or AAV5-Luc vector was injected at 109 particles/gland/mouse directly into the lacrimal gland of 6–8-week-old C57BL/6J mice (10). The vector dose was chosen based on previously published results, which showed detectable transgene activity with administration of >109 particles/ gland (12,13).
Saliva and tear collection, and assessment of saliva ion concentrations and serum autoantibody status
Pilocarpine-stimulated salivary flow rate and tear flow rate were determined (10,14). In mice that were administered AAV5-Bmp6 or AAV5-GFP into the lacrimal gland, unstimulated tear volume was measured (10). Serum was collected at the end of the study as described previously (15), and potassium and sodium ion concentrations were measured (16). Autoantibodies against Ro/SSA (multiantigenic peptide Ro 273) and La/SSB were detected as described previously (15).
Histopathologic analysis
After the mice were killed, whole salivary glands were surgically removed. Hematoxylin and eosin (H&E)–stained sections were obtained, and lymphocytic infiltration was assessed and a focus score assigned (15). Unstained sections were used for immunofluorescence and immunochemistry staining as previously described (14).
Immunofluorescence staining
Aquaporin 5 (AQP-5) was detected as previously described (13). BMP-6 expression in human tissue was determined by blocking with 10% donkey serum in 0.5% bovine serum albumin (BSA) in phosphate buffered saline (PBS) diluent for 30 minutes at room temperature in a humidity chamber, followed by incubation overnight at 4°C with 100 μl of 10 μg/ml Mouse Monoclonal Anti–BMP-6 Primary Antibody (Abcam) in 0.5% BSA in PBS. Control specimens were incubated overnight at 4°C with 100 μl of 10 μg/ml ChromPure Mouse IgG, Whole Molecule (Jackson ImmunoResearch) in 0.5% BSA in PBS. Slides were washed in 5 changes of PBS for 5 minutes each and then incubated with a 1:100 dilution of 2 mg/ml Alexa Fluor 488 Goat Anti-Mouse IgG Secondary Antibody (Invitrogen) for 1 hour at room temperature in the dark, followed by washing in 5 changes of PBS for 5 minutes each and counterstaining with DAPI mounting medium.
BMP-6 expression in mouse tissue was detected by direct labeling of anti–BMP-6 antibody using a Zenon labeling kit (Invitrogen) and counterstaining with DAPI mounting medium. Control specimens were incubated with 100 μl of 10 μg/ml ChromPure Mouse IgG, Whole Molecule in similarly conjugated PBS. Confocal images were acquired using a FluoView 1000 (Olympus) with a 40× objective. Fluorescence intensity was quantified using Volocity software (PerkinElmer) and a pixel intensity range between 1,500 and 4,095.
Detection of cytokine and chemokine production
Serum and salivary gland homogenates were prepared from whole blood as previously described (15). Cytokines and chemokines were assayed in the local (submandibular gland draining lymph node cell culture) and systemic (splenocyte culture and serum) immune systems. BMP-6 expression in serum was measured using a Duo-kit ELISA (R&D Systems) and detected on a plate reader according to the instructions of the manufacturer (Meso Scale Discovery).
Regulated volume decrease (RVD) measurement
Salivary gland cells from mice transduced with either AAV5-GFP or AAV5-Bmp6 administered to the salivary gland were isolated and RVD measured as described previously (17). RVD was induced by application of hypotonic media of 150 mOsm, and relative cell volume was measured before and after hypotonic stress stimulation.
RESULTS
Transcriptome of MSGs from SS patients is distinct from that of MSGs from healthy volunteers
In order to identify the candidate genes responsible for impaired salivary gland function in patients with primary SS and low levels of inflammation, RNA samples were collected from MSGs of 5 female patients who had primary SS that met the American-European Consensus Group criteria and had low focus scores (≥ 2), positive autoantibodies, and impaired salivary flow. Four of the 5 patients also reported ocular symptoms. MSGs from 6 sex-matched healthy volunteers who were free of lymphocytic foci and autoantibodies were also studied. Four of the 6 healthy volunteers had normal salivary flow (Supplementary Table 1, http://onlinelibrary.wiley.com/doi/10.1002/art.38123/abstract).
Prior to analysis, signal intensity was adjusted between samples using a quantile normalization protocol. Genes that were differentially expressed between patients with primary SS and healthy volunteers were identified based on statistical significance (P < 0.05 after adjustment by Benjamini and Hochberg’s FDR correction). By unsupervised cluster analysis, patients with primary SS and healthy volunteers segregated into 2 distinct groups (Supplementary Figure 2, http://onlinelibrary.wiley.com/doi/10.1002/art.38123/abstract). Genes shown to be differentially expressed (Supplementary Table 2) included several that have been previously reported to be associated with SS, including CCR5, IRF5, GZMK, and MMP9 (18–21).
Two-step quantitative RT-PCR was performed on a subset of genes representative of the microarray signature. Validation studies were carried out using template cDNA prepared from biopsy samples from the patients with primary SS in the microarray study. A total of 14 transcript changes were verified by quantitative PCR (Supplementary Figure 3), and all were in accordance with the microarray gene expression data.
In order to focus on salivary gland–specific genes, a biomarker filter (GeneSpring 9.0) was used to narrow the results to genes that were previously reported to be expressed in normal salivary gland epithelia. Among these, the gene for BMP-6 was highly up-regulated (Table 1). Expression of BMP-6 was also confirmed by hybridization of additional microarrays with RNA isolated from additional samples from healthy volunteers and SS patients with low gland function and low focus scores (Supplementary Figure 4).
Table 1.
Salivary gland–specific genes that were differentially expressed, as identified using a biomarker filter
| Gene | Entrez gene name | Fold change | P |
|---|---|---|---|
| BMP6 | Bone morphogenetic protein 6 | 4.465 | 0.004 |
| ARSJ | Arylsulfatase family, member J | 2.103 | 0.032 |
| ND5 | NADH dehydrogenase, subunit 5 (complex I) | −2.007 | 0.005 |
| SLC22A17 | Solute carrier family 22, member 17 | −2.008 | 0.001 |
| STAC2 | SH3 and cysteine-rich domain 2 | −2.134 | 0.02 |
| CRISP3 | Cysteine-rich secretory protein 3 | −2.179 | 0.039 |
| AQP5 | Aquaporin 5 | −2.19 | 0.043 |
| PITX1 | Paired-like homeodomain 1 | −2.215 | 0.006 |
| TEAD3 | TEA domain family member 3 | −2.464 | 0.023 |
| CLDN3 | Claudin 3 | −2.605 | 0.01 |
| METRN | Meteorin, glial cell differentiation regulator | −2.781 | 0.019 |
| ARTN | Artemin | −4.809 | 0.03 |
Overexpression of BMP-6 in the MSGs of SS patients and NOD mice
The elevated BMP-6 RNA in patients was reflected by an increase in protein levels, as detected by confocal imaging. Relative fluorescence intensity assessment across a 3-dimensional stack of images showed an increase in BMP-6 protein in representative glands from SS patients (n = 2) compared to a healthy volunteer (Figure 1A). This increase in BMP-6 expression appeared to be independent of the focus score, with 5 of the 6 patients with scores of > 2 also exhibiting a significant increase in BMP-6 protein within the gland (Supplementary Figure 5, http://onlinelibrary.wiley.com/doi/10.1002/art.38123/abstract). Slides from the 1 patient who did not have an increase in BMP-6 had minimal epithelial tissue remaining (focus score 7) compared with the slides used from the healthy volunteers, likely affecting the level of BMP-6 detected (data not shown). To determine whether the increased expression in the salivary gland reflects a general overexpression of BMP-6 in the serum of SS patients, we developed an ELISA with a sensitivity for detection of serum BMP-6 levels as low as 50 pg/ml. In serum samples from a randomly selected population of patients with primary SS (n = 20) or healthy volunteers (n = 2), BMP-6 levels above the 50 ng/ml level of sensitivity of the ELISA were not observed, suggesting that BMP-6 may remain localized at the site of expression as previously reported (22).
Figure 1.
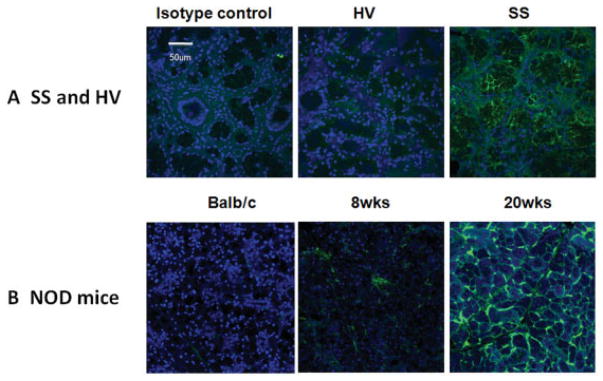
Increased expression of bone morphogenetic protein 6 (BMP-6) in the salivary glands of patients with primary Sjögren’s syndrome (SS) and NOD mice. A, Confocal images demonstrating expression of BMP-6 in the salivary glands of a representative patient and healthy volunteer (HV). As a control, the patient slide was stained with isotype control antibody. B, Expression of BMP-6 in the salivary glands of a representative BALB/c mouse and of representative NOD mice before (8 weeks) and after (20 weeks) onset of the SS-like phenotype. Samples were stained with anti–BMP-6 antibody. Original magnification ×40.
Autoimmune-prone NOD mice spontaneously develop a primary SS–like phenotype and are often used as a model for studying SS (23). Female NOD mice develop salivary gland focal infiltration starting at 8 weeks of age and show a decline in salivary gland function by 20 weeks (16,24). Investigation for BMP-6 expression revealed elevated levels of BMP-6 in salivary glands from 8-week-old NOD mice compared with BALB/c mice (Figure 1B). By 20 weeks, BMP-6 expression had continued to increase, suggesting that BMP-6 expression is associated with an SS-like phenotype in mice as well as humans.
Local overexpression of BMP-6 induces salivary and lacrimal gland dysfunction in mice
The effect of elevated BMP-6 expression on salivary gland function was tested by infusing AAV5 vectors encoding BMP-6 into the salivary glands of female C57BL/6J mice. AAV vectors were chosen because of their ability to direct long-term expression following localized delivery to the salivary glands of mice, with minimal host response to the vector (9). Previous work from our group has established that >90% of the infused vector remains localized to the gland and AAV5 is able to transduce 50% of the striated ductal cells within the gland (12). Four-to-six weeks post–vector delivery, expression of luciferase in the region of the salivary gland suggested successful infusion of the AAV5 vectors (Supplementary Figure 1A, http://onlinelibrary.wiley.com/doi/10.1002/art.38123/abstract). BMP-6 protein was detected in the ducts (Supplementary Figure 1B), and this was further confirmed by RT-PCR (results not shown). We then examined salivary gland and lacrimal gland function in the mice that received AAV-Bmp6.
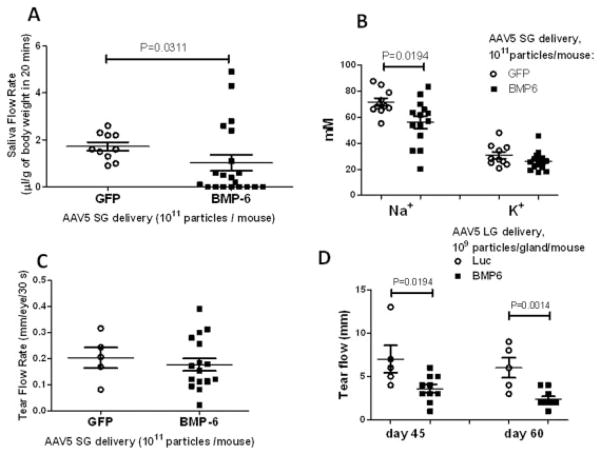
Measurement of pilocarpine-stimulated salivary flow rate showed a statistically significant decrease in the AAV5-Bmp6–treated group compared with control AAV5-GFP vector–treated mice (mean ± SEM 1.03 ± 0.3 μl/gm body weight/20 minutes and 1.72 ± 0.18 μl/gm body weight/20 minutes, respectively; P = 0.0311) (Figure 2A). Of the 20 mice cannulated with AAV5-Bmp6, 8 had no detectable saliva flow. The expression of BMP-6 in the subset of AAV5-Bmp6–treated mice with normal salivary flow rates was 10–100-fold lower than the level of BMP-6 expression in mice with low salivary flow activity (Supplementary Figure 6). In parallel with the loss of saliva flow, the ion composition of the saliva was also changed as a result of BMP-6 expression. The sodium level in the saliva of mice administered AAV5-Bmp6 into the salivary gland was significantly reduced (mean 56.1 mM, versus 72.0 mM in mice treated with AAV5-GFP; P = 0.0194). Potassium was also decreased in the saliva of AAV5-Bmp6–treated mice, but the decrease was not statistically significant (26.1 mM versus 31.41 mM; P = 0.0822) (Figure 2B).
Figure 2.
Salivary and lacrimal gland function in mice treated with adeno-associated virus type 5 encoding bone morphogenetic protein 6 (AAV5-Bmp6) and AAV5 encoding green fluorescent protein (AAV-GFP) (control). Saliva and tear flow and ion concentration were measured as described in Materials and Methods. A, Mice cannulated with AAV5-Bmp6 vector (n = 20) exhibited a significant decrease in salivary flow rate compared with control mice (n = 10). B, The level of sodium was significantly decreased in saliva from mice cannulated with AAV5-Bmp6 vector (n = 14) compared with control mice (n = 10). The potassium level was also decreased in the AAV5-Bmp6–treated mice, but the difference from controls was not statistically significant. C, Delivery of vector into the salivary gland (SG) did not result in a systemic effect, as determined by a lack of change (P = 0.5308) in lacrimal gland (LG) tear flow rate (n = 5 in the AAV5-GFP–treated group, n = 17 in the AAV5-Bmp6–treated group). D, Delivery of AAV5-Bmp6 (n = 10) to the lacrimal gland reduced the tear flow rate compared with that in control mice administered AAV5-luciferase (Luc) (n = 5). Bars show the mean ± SEM. P values were determined by Mann-Whitney U test (A and D) or by Student’s unpaired t-test (B and C).
The effect of BMP-6 is reported to be very cell-specific and localized (25). To investigate if local delivery of Bmp6 could affect secretory function in distal tissue, lacrimal gland function was tested by measuring pilocarpine-stimulated tear flow after administration of treatment to the salivary gland. In this assay, no statistically significant difference was detected between the AAV5-GFP–treated and AAV5-Bmp6–treated mice (mean ± SEM 0.20 ± 0.04/30 seconds and 0.17 ± 0.02 mm/30 seconds, respectively) (Figure 2C). To confirm that lacrimal glands could be responsive to BMP-6, we transduced the lacrimal glands of mice by direct injection of either AAV5-Bmp6 or AAV5-Luc and monitored lacrimal gland function over time (10). A statistically significant decrease in tear flow was detected at 45 days post–vector delivery and persisted for at least 60 days (mean ± SEM 7.0 ± 1.6 mm versus 3.6 ± 0.48 mm in the AAV5-Luc– and AAV5-Bmp6–treated groups, respectively, on day 45 and 6.0 ± 1.14 mm versus 2.4 ± 0.31 mm, respectively, on day 60; P = 0.0194 on day 45 and P = 0.0014 on day 60) (Figure 2D).
Minimal effect of BMP-6 expression on immune activation
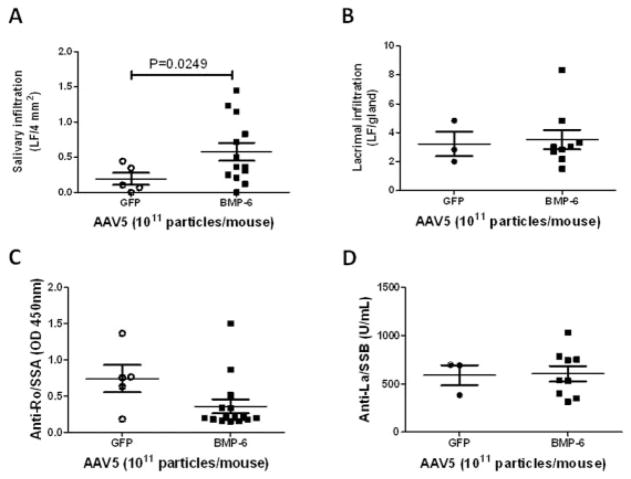
A hallmark of primary SS is lymphocytic infiltration in the exocrine glands, especially the salivary and lacrimal glands. To determine the effect of elevated BMP-6 on the local immune environment of the salivary gland, foci of lymphocytic infiltrates were quantified in both the salivary and lacrimal glands 20 weeks after cannulation. In addition to the decrease in gland function, with AAV5-Bmp6 treatment a statistically significant increase in focus score was observed in the salivary gland (mean ± SEM 0.58 ± 0.1, versus 0.19 ± 0.1 with AAV5-GFP treatment) (Figure 3A) but not the lacrimal gland (3.51 ± 0.7 and 3.22 ± 0.8, respectively) (Figure 3B). No increase in the focus score was observed in mice in which AAV5-Bmp6 was administered directly into the lacrimal gland. Furthermore, there were no significant changes in the serum levels of anti-Ro/SSA or anti-La/ SSB in the animals injected in the salivary gland with AAV5-Bmp6 compared with those injected with AAV5-GFP (mean ± SEM optical density at 450 nm 0.35 ± 0.1 and 0.74 ± 0.2, respectively [anti-Ro/SSA] and 605.5 ± 79.8 and 589.6 ± 103.5, respectively [anti-La/SSB]) (Figures 3C and D). Cytokine analysis of salivary gland homogenates indicated minimal change between AAV5-Bmp6–treated mice and control AAV5-GFP–treated mice (Supplementary Table 3, http://onlinelibrary.wiley.com/doi/10.1002/art.38123/abstract). Taken together, these data suggest that BMP-6 overexpression in the salivary gland has minimal effect on local and systemic immune activation.
Figure 3.
Lymphocytic infiltration and autoantibody production in AAV5-Bmp6– and AAV5-GFP–treated mice. Lymphocytic infiltrates were determined as described in Materials and Methods, and a focus score of lymphocytic foci (LF) per 4 mm2 was assigned. A, The focus score in salivary glands from AAV5-Bmp6–treated mice (n = 13) was significantly increased compared with that in salivary glands from AAV5-GFP–treated mice (n = 5). B, After delivery of vector to the salivary glands, the lacrimal gland focus score did not differ significantly (P = 0.8215) between the AAV5-Bmp6–treated group (n = 9) and the AAV5-GFP–treated group (n = 3). C and D, Serum levels of anti-Ro/SSA (C) and anti-La/SSB (D), as determined by enzyme-linked immunosorbent assay, did not differ significantly (P = 0.0685 for anti-Ro/SSA, P = 0.0987 for anti-La/SSB) between groups (n = 5 in the AAV5-GFP–treated group, n = 15 in the AAV5-Bmp6–treated group in C; n = 3 in the AAV5-GFP–treated group, n = 9 in the AAV5-Bmp6–treated group in D). Bars show the mean ± SEM. P values were determined by Student’s unpaired t-test. See Figure 2 for other definitions.
Histologic and morphologic changes in protein expression following BMP-6 expression in the salivary glands of mice
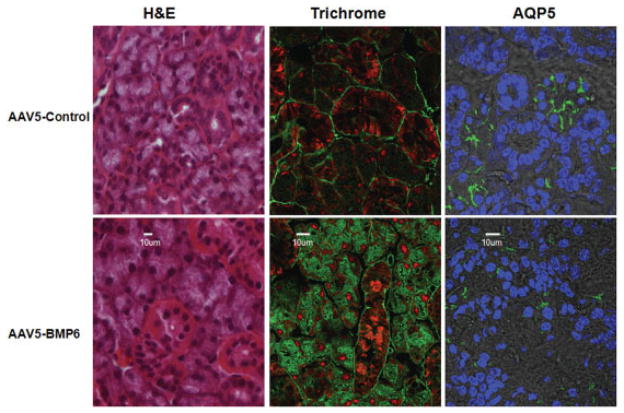
To better understand the mechanism associated with the loss of salivary gland function induced by increased BMP-6 expression, the overall structure of the salivary glands as well as the distribution of specific salivary gland proteins were investigated (Figure 4). Although no gross morphologic changes were observed by H&E staining, there appeared to be a redistribution of the extracellular matrix in mice that received AAV5-Bmp6 compared with the AAV5-GFP control vector–treated mice. This change in extracellular matrix was confirmed by staining with trichrome and fluorescent imaging. In addition to the altered extracellular matrix in the AAV5-Bmp6–treated mice, detection of the acinar-specific protein AQP-5 appeared to be less well defined on the apical surfaces in the AAV5-Bmp6–treated mice compared with controls, suggesting alterations in protein distribution on the cell surface or in acinar organization. Decreased AQP-5 messenger RNA expression was also detected on the microarray and confirmed by quantitative PCR.
Figure 4.
Morphologic changes in the salivary glands of AAV5-Bmp6–treated mice. Changes in morphology and in protein expression or distribution were assessed by hematoxylin and eosin (H&E) or trichrome staining or immunofluorescence confocal imaging for aquaporin 5 (AQP-5). Each image is representative of 4 samples tested. No gross changes in morphology were observed with H&E staining. Trichrome staining revealed a redistribution of the extracellular matrix material in AAV5-Bmp6–treated mice compared with AAV5-GFP–treated controls. AQP-5 appeared to be less well defined on the apical surfaces in the AAV5-Bmp6–treated mice compared with the AAV5-GFP–treated mice. Original magnification × 40 (H&E) or × 100 (trichrome and AQP-5). See Figure 2 for other definitions.
Loss of regulated volume decrease function in salivary gland cells induced by BMP-6 overexpression both in vivo and in vitro
Cell volume changes are induced by anisosmotic conditions. For example, hypo-osmotic solutions cause hypotonic stress, which causes cell volume to increase. The cells trigger an RVD mechanism to recover their original volume. All of these changes in cell volume are initiated by ion and water fluxes (26). AQP-5 is the main water channel for salivary fluid secretion. Mice deficient in AQP-5 secrete a lower volume of saliva after pilocarpine stimulation, and acini isolated from these mice display attenuations in the cell RVD response to hypotonic stress (17,26).
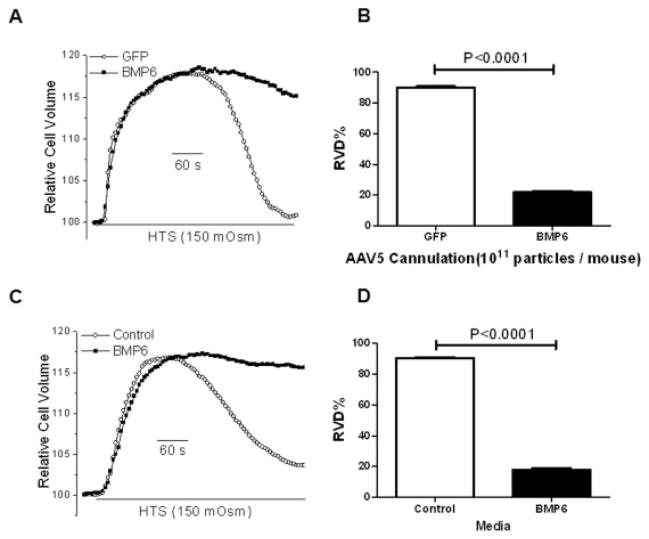
Comparison of isolated salivary gland cells showed a significant decrease in cell volume recovery following hypotonic stress in cells from AAV5-Bmp6–treated mice compared with those from the AAV5-GFP–treated control group (mean ± SEM RVD 21.84 ± 1.0% and 89.33 ± 0.8%, respectively) (Figure 5B). These data are consistent with the loss of salivary gland fluid secretion and the change in the localization of AQP-5 following BMP-6 expression in mouse salivary glands.
Figure 5.
Regulated volume decrease (RVD) following treatment with bone morphogenetic protein 6 (BMP-6) in vivo and in vitro. Salivary glands from AAV5-Bmp6–treated mice (n = 2) and AAV5-GFP–treated controls (n = 2) were collected, and cells were isolated. A, Relative cell volume was measured before and after hypotonic stress (HTS) stimulation in isolated primary salivary cells from AAV5-Bmp6–treated mice and AAV5-GFP–treated controls. B, The maximal percent RVD was determined, with a significant decrease observed in isolated primary salivary gland cells from mice treated with AAV5-Bmp6 compared with AAV5-GFP–treated controls. C, Relative cell volume was measured before and after hypotonic stress stimulation in HSG cells treated with recombinant BMP-6 in vitro or cultured with media alone (control). D, The maximal percent RVD was determined, with a significant decrease observed in HSG cells treated with recombinant BMP-6 in vitro compared with HSG cells cultured with media alone. Values in B and D are the mean ± SEM. P values were determined by Student’s unpaired t-test. See Figure 2 for other definitions.
To more directly test for the effect of BMP-6 on RVD, HSG cells (a human salivary gland cell line) were treated with 6 ng/ml of recombinant BMP-6 for 4 days and then assayed for RVD activity (Figure 5C). Cell division and viability were unaffected by BMP-6 at this dose. As was seen in primary salivary gland cells isolated from the AAV5-Bmp6–transduced mice, a loss of RVD was detected following treatment of HSG cells with BMP-6 (mean ± SEM RVD 17.95 ± 1.0% and 90.45 ± 0.6% in HSG cells cultured in media with BMP-6 and without BMP-6, respectively; P < 0.0001) (Figure 5D). This strongly suggests that BMP-6 can directly affect fluid movement in salivary gland cells, which could account for the substantial loss of salivary secretion in patients with primary SS and in mice.
DISCUSSION
A critical question in primary SS is the connection between the immune activation and the loss of gland activity. Herein we report a novel link between an increase in BMP-6 expression and the development of xerostomia in primary SS. Furthermore, in vivo experiments using AAV-mediated expression of BMP-6 in mouse salivary glands resulted in xerostomia accompanied by moderate lymphocytic infiltration, but with no evidence of an increase in levels of proinflammatory cytokines or autoantibodies, indicating little immune activation, either within the gland or systemically. Similarly, overexpression of BMP-6 in lacrimal glands reduced tear flow in mice, but this was not accompanied by lymphocytic infiltration. Thus, we have identified BMP-6 as a novel, nonimmunologic factor related to primary SS. More broadly, our findings suggest that the loss of salivary gland function in SS patients may not be directly related to the overt immune activation observed in the disease, but may result from changes in epithelial function induced by BMP-6 expression.
To date, little is known about the role of BMPs in primary SS, and the present report is, to our knowledge, the first to describe an association. A likely mechanism for the loss of gland function is through changes in AQP-5 expression, but this will require further study. Elevated BMP-6 expression in the skin of mice has been reported to induce a psoriasis-like condition, suggesting that this cytokine may be involved in the development of other autoimmune diseases depending on the site of expression (25). Decreased expression has been linked with renal fibrosis, iron overload, and some forms of lymphoma (27,28). BMP-6 has emerged as a key regulator of iron metabolism in the liver through the expression of hepcidin. Bmp6-null mice develop massive iron overload in the liver, much like that seen in the severe childhood-onset forms of human hemochromatosis. Although the effect of elevated BMP-6 expression on iron metabolism has not been reported, elevated expression of hepcidin resulting from overexpression of BMP-6 could lead to microcytic hypochromic anemia (29). Of interest, 51% of patients with primary SS have been reported to have low iron levels, with anemia reported in 21% (30).
Extensive changes in the extracellular matrix of BMP-6–expressing mice were observed in our study, and these may be related to the fibrosis associated with primary SS. During fetal development, high levels of BMP-6 expression coincide with active proliferation in the epidermis, stratification, and wound healing. Low levels of expression are seen in normal adult epidermis. A direct correlation between BMP-6 expression and acanthosis, as well as localization to hypertrophic cartilage, has also been reported (31). In keratinocytes, strong expression of BMP-6 in the suprabasal layers of the epidermis results in severe repression of cell proliferation, whereas weak and patchy expression results in strong hyperproliferation as well as parahyperkeratosis in adult epidermis. An increased rate of apoptosis among keratinocytes, as well as a down-regulation of the activator protein 1 family of transcription factors, was detectable in BMP-6–transgenic mice (31).
Changes in cell volume are involved in several cellular processes including gene transcription and proliferation, as well as driving fluid secretion in exocrine cells (32,33). Experimentally, cell volume regulation can be induced by hypotonic swelling, triggering a rise in calcium entry that is critical for the activation of water channels and the ion flux necessary for restoring cell volume (32,34–36). Although the mechanisms responsible for sensing the change in cell volume are not completely understood, transient receptor potential vanilloid channel 4 (TRPV-4) and AQP-5 are reported to play important roles in this process, with TRPV-4 activation following swelling being dependent on the presence of functional AQP-5 channels (17). Although some AQP-5 protein is detected in the acinar cells of mice treated with BMP-6, it is possible that the protein lacks a posttranslational modification necessary for function. An alternative explanation, based on our confocal imaging results, is that the dysfunction is related to the missorting of AQP-5 channels, as has been previously observed in biopsy specimens from patients with primary SS (37).
One of the most intriguing of our findings was the lack of immune activation despite the induction of severe xerostomia in animals receiving local AAV-Bmp6. Although we monitored the mice for 20 weeks post–vector delivery and observed little change in immune activation, in many other mouse models of SS the mice are at least 9–13 months of age before markers of systemic immune activation develop (38,39). Our microarray analysis did reveal increased levels of a number of proinflammatory cytokines, including CXC9, CCL19, interleukin-15, and interleukin-23, in SS patients. Although these cytokines could be activated by BMP-6 signaling, it is also possible that their expression is independent of or upstream of BMP-6 and that they are all triggered by some common insult to the gland.
An important unanswered question in this study is what factor(s) might trigger elevated BMP-6 expression. As in other autoimmune diseases, several lines of evidence point to a viral infection triggering the disease. Indeed, several viruses have been proposed as triggers of primary SS, including Tax 1 expression from human T lymphotropic virus type I, coxsackievirus, and RNA viruses (40–42). Although infection with these viruses has not been associated with elevated levels of BMP-6, reovirus infection can activate transforming growth factor β and the BMP-6 signaling pathway (43). This activation of BMP-6 has been suggested to be a protective cellular response to limit apoptosis of neuronal cells during virus infection. Our data further suggest that in epithelial cells, it could induce a loss of secretory function. Further understanding of how this signal transduction pathway interacts with other important cell signaling pathways associated with primary SS, such as interferon-induced signal transduction, may provide new potential therapeutic targets for restoring gland function.
Supplementary Material
Acknowledgments
Supported by the NIH (National Institute of Dental and Craniofacial Research intramural grants to Dr. Chiorini).
Footnotes
ClinicalTrials.gov identifier: NCT00001390
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Chiorini had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Yin, Michael, Weller, Rocha, Alevizos, Ambudkar, Chiorini.
Acquisition of data. Yin, Cabrera-Perez, Michael, Liu, Catalán, Rocha, Ismail, Rana, Di Pasquale, Alevizos, Ambudkar, Chiorini.
Analysis and interpretation of data. Yin, Cabrera-Perez, Lai, Michael, Weller, Swaim, Liu, Rocha, Afione, Rana, Di Pasquale, Alevizos, Ambudkar, Illei, Chiorini.
References
- 1.Fox RI. Sjögren’s syndrome. Lancet. 2005;366:321–31. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 2.Segal B, Bowman SJ, Fox PC, Vivino FB, Murukutla N, Brodscholl J, et al. Primary Sjögren’s syndrome: health experiences and predictors of health quality among patients in the United States. Health Qual Life Outcomes. 2009;7:46. doi: 10.1186/1477-7525-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikolov NP, Illei GG. Pathogenesis of Sjögren’s syndrome. Curr Opin Rheumatol. 2009;21:465–70. doi: 10.1097/BOR.0b013e32832eba21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. the European Study Group on Classification Criteria for Sjögren’s Syndrome. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shippy R, Fulmer-Smentek S, Jensen RV, Jones WD, Wolber PK, Johnson CD, et al. Using RNA sample titrations to assess micro-array platform performance and normalization techniques. Nat Biotechnol. 2006;24:1123–31. doi: 10.1038/nbt1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, et al. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–7. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 7.Do JH, Choi DK. Normalization of microarray data: single-labeled and dual-labeled arrays. Mol Cells. 2006;22:254–61. [PubMed] [Google Scholar]
- 8.Zahurak M, Parmigiani G, Yu W, Scharpf RB, Berman D, Schaeffer E, et al. Pre-processing Agilent microarray data. BMC Bioinformatics. 2007;8:142. doi: 10.1186/1471-2105-8-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li JZ, Li H, Hankins GR, Lieu AS, Noh E, Jacobson L, et al. Different osteogenic potentials of recombinant human BMP-6 adeno-associated virus and adenovirus in two rat strains. Tissue Eng. 2006;12:209–19. doi: 10.1089/ten.2006.12.209. [DOI] [PubMed] [Google Scholar]
- 10.Rocha EM, Di Pasquale G, Riveros PP, Quinn K, Handelman B, Chiorini JA. Transduction, tropism, and biodistribution of AAV vectors in the lacrimal gland. Invest Ophthalmol Vis Sci. 2011;52:9567–72. doi: 10.1167/iovs.11-8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vosters JL, Yin H, Roescher N, Kok MR, Tak PP, Chiorini JA. Local expression of tumor necrosis factor-receptor 1:immunoglobulin G can induce salivary gland dysfunction in a murine model of Sjögren’s syndrome. Arthritis Res Ther. 2009;11:R189. doi: 10.1186/ar2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katano H, Kok MR, Cotrim AP, Yamano S, Schmidt M, Afione S, et al. Enhanced transduction of mouse salivary glands with AAV5-based vectors. Gene Ther. 2006;13:594–601. doi: 10.1038/sj.gt.3302691. [DOI] [PubMed] [Google Scholar]
- 13.Vosters JL, Landek-Salgado MA, Yin H, Swaim WD, Kimura H, Tak PP, et al. Interleukin-12 induces salivary gland dysfunction in transgenic mice, providing a new model of Sjögren’s syndrome. Arthritis Rheum. 2009;60:3633–41. doi: 10.1002/art.24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin H, Vosters JL, Roescher N, D’Souza A, Kurien BT, Tak PP, et al. Location of immunization and interferon-γ are central to induction of salivary gland dysfunction in Ro60 peptide immunized model of Sjögren’s syndrome. PLoS One. 2011;6:e18003. doi: 10.1371/journal.pone.0018003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin H, Nguyen CQ, Samuni Y, Uede T, Peck AB, Chiorini JA. Local delivery of AAV2-CTLA4IgG decreases sialadenitis and improves gland function in the C57BL/6. NOD-Aec1Aec2 mouse model of Sjögren’s syndrome. Arthritis Res Ther. 2012;14:R40. doi: 10.1186/ar3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roescher N, Lodde BM, Vosters JL, Tak PP, Catalan MA, Illei GG, et al. Temporal changes in salivary glands of non-obese diabetic mice as a model for Sjögren’s syndrome. Oral Dis. 2012;18:96–106. doi: 10.1111/j.1601-0825.2011.01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Bandyopadhyay BC, Nakamoto T, Singh B, Liedtke W, Melvin JE, et al. A role for AQP5 in activation of TRPV4 by hypotonicity: concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J Biol Chem. 2006;281:15485–95. doi: 10.1074/jbc.M600549200. [DOI] [PubMed] [Google Scholar]
- 18.Hjelmervik TO, Petersen K, Jonassen I, Jonsson R, Bolstad AI. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjögren’s syndrome patients from healthy control subjects. Arthritis Rheum. 2005;52:1534–44. doi: 10.1002/art.21006. [DOI] [PubMed] [Google Scholar]
- 19.Hulkkonen J, Pertovaara M, Antonen J, Pasternack A, Hurme M, Pollanen P, et al. Matrix metalloproteinase 9 (MMP-9) gene polymorphism and MMP-9 plasma levels in primary Sjögren’s syndrome. Rheumatology (Oxford) 2004;43:1476–9. doi: 10.1093/rheumatology/keh369. [DOI] [PubMed] [Google Scholar]
- 20.Petrek M, Cermakova Z, Hutyrova B, Micekova D, Drabek J, Rovensky J, et al. CC chemokine receptor 5 and interleukin-1 receptor antagonist gene polymorphisms in patients with primary Sjögren’s syndrome. Clin Exp Rheumatol. 2002;20:701–3. [PubMed] [Google Scholar]
- 21.Miceli-Richard C, Gestermann N, Ittah M, Comets E, Loiseau P, Puechal X, et al. The CGGGG insertion/deletion polymorphism of the IRF5 promoter is a strong risk factor for primary Sjögren’s syndrome. Arthritis Rheum. 2009;60:1991–7. doi: 10.1002/art.24662. [DOI] [PubMed] [Google Scholar]
- 22.Blessing M, Schirmacher P, Kaiser S. Overexpression of bone morphogenetic protein-6 (BMP-6) in the epidermis of transgenic mice: inhibition or stimulation of proliferation depending on the pattern of transgene expression and formation of psoriatic lesions. J Cell Biol. 1996;135:227–39. doi: 10.1083/jcb.135.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiorini JA, Cihakova D, Ouellette CE, Caturegli P. Sjögren syndrome: advances in the pathogenesis from animal models. J Autoimmun. 2009;33:190–6. doi: 10.1016/j.jaut.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humphreys-Beher MG, Peck AB. New concepts for the development of autoimmune exocrinopathy derived from studies with the NOD mouse model. Arch Oral Biol. 1999;44 (Suppl 1):S21–5. doi: 10.1016/s0003-9969(99)00045-x. [DOI] [PubMed] [Google Scholar]
- 25.Blessing M, Nanney LB, King LE, Hogan BL. Chemical skin carcinogenesis is prevented in mice by the induced expression of a TGF-β related transgene. Teratog Carcinog Mutagen. 1995;15:11–21. doi: 10.1002/tcm.1770150103. [DOI] [PubMed] [Google Scholar]
- 26.Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274:20071–4. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 27.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41:478–81. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 28.Andriopoulos B, Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482–7. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci U S A. 2002;99:4596–601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundstrom IM, Lindstrom FD. Iron and vitamin deficiencies, endocrine and immune status in patients with primary Sjögren’s syndrome. Oral Dis. 2001;7:144–9. doi: 10.1034/j.1601-0825.2001.0070302.x. [DOI] [PubMed] [Google Scholar]
- 31.Wach S, Schirmacher P, Protschka M, Blessing M. Overexpression of bone morphogenetic protein-6 (BMP-6) in murine epidermis suppresses skin tumor formation by induction of apoptosis and downregulation of fos/jun family members. Oncogene. 2001;20:7761–9. doi: 10.1038/sj.onc.1204962. [DOI] [PubMed] [Google Scholar]
- 32.Okada Y, Maeno E. Apoptosis, cell volume regulation and volume-regulatory chloride channels. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:377–83. doi: 10.1016/s1095-6433(01)00424-x. [DOI] [PubMed] [Google Scholar]
- 33.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–69. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- 34.Langelueddecke C, Jakab M, Ketterl N, Lehner L, Hufnagl C, Schmidt S, et al. Effect of the AMP-kinase modulators AICAR, metformin and compound C on insulin secretion of INS-1E rat insulinoma cells under standard cell culture conditions. Cell Physiol Biochem. 2012;29:75–86. doi: 10.1159/000337589. [DOI] [PubMed] [Google Scholar]
- 35.Strange K, Phillips JE, Quamme GA. Mechanisms of CO2 transport in rectal salt gland of Aedes. II. Site of Cl−-HCO3− exchange. Am J Physiol. 1984;246:R735–40. doi: 10.1152/ajpregu.1984.246.5.R735. [DOI] [PubMed] [Google Scholar]
- 36.Tinel H, Kinne-Saffran E, Kinne RK. Calcium signalling during RVD of kidney cells. Cell Physiol Biochem. 2000;10:297–302. doi: 10.1159/000016375. [DOI] [PubMed] [Google Scholar]
- 37.Steinfeld S, Cogan E, King LS, Agre P, Kiss R, Delporte C. Abnormal distribution of aquaporin-5 water channel protein in salivary glands from Sjögren’s syndrome patients. Lab Invest. 2001;81:143–8. doi: 10.1038/labinvest.3780221. [DOI] [PubMed] [Google Scholar]
- 38.Shen GQ, Ojo-Amaize EA, Agopian MS, Peter JB. Silicate antibodies in women with silicone breast implants: development of an assay for detection of humoral immunity. Clin Diagn Lab Immunol. 1996;3:162–6. doi: 10.1128/cdli.3.2.162-166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren’s syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green JE, Hinrichs SH, Vogel J, Jay G. Exocrinopathy resembling Sjögren’s syndrome in HTLV-1 tax transgenic mice. Nature. 1989;341:72–4. doi: 10.1038/341072a0. [DOI] [PubMed] [Google Scholar]
- 41.Ittah M, Miceli-Richard C, Gottenberg JE, Sellam J, Lepajolec C, Mariette X. B-cell-activating factor expressions in salivary epithelial cells after dsRNA virus infection depends on RNA-activated protein kinase activation. Eur J Immunol. 2009;39:1271–9. doi: 10.1002/eji.200839086. [DOI] [PubMed] [Google Scholar]
- 42.Triantafyllopoulou A, Moutsopoulos HM. Autoimmunity and coxsackievirus infection in primary Sjögren’s syndrome. Ann N Y Acad Sci. 2005;1050:389–96. doi: 10.1196/annals.1313.090. [DOI] [PubMed] [Google Scholar]
- 43.Beckham JD, Tuttle K, Tyler KL. Reovirus activates transforming growth factor β and bone morphogenetic protein signaling pathways in the central nervous system that contribute to neuronal survival following infection. J Virol. 2009;83:5035–45. doi: 10.1128/JVI.02433-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.