Abstract
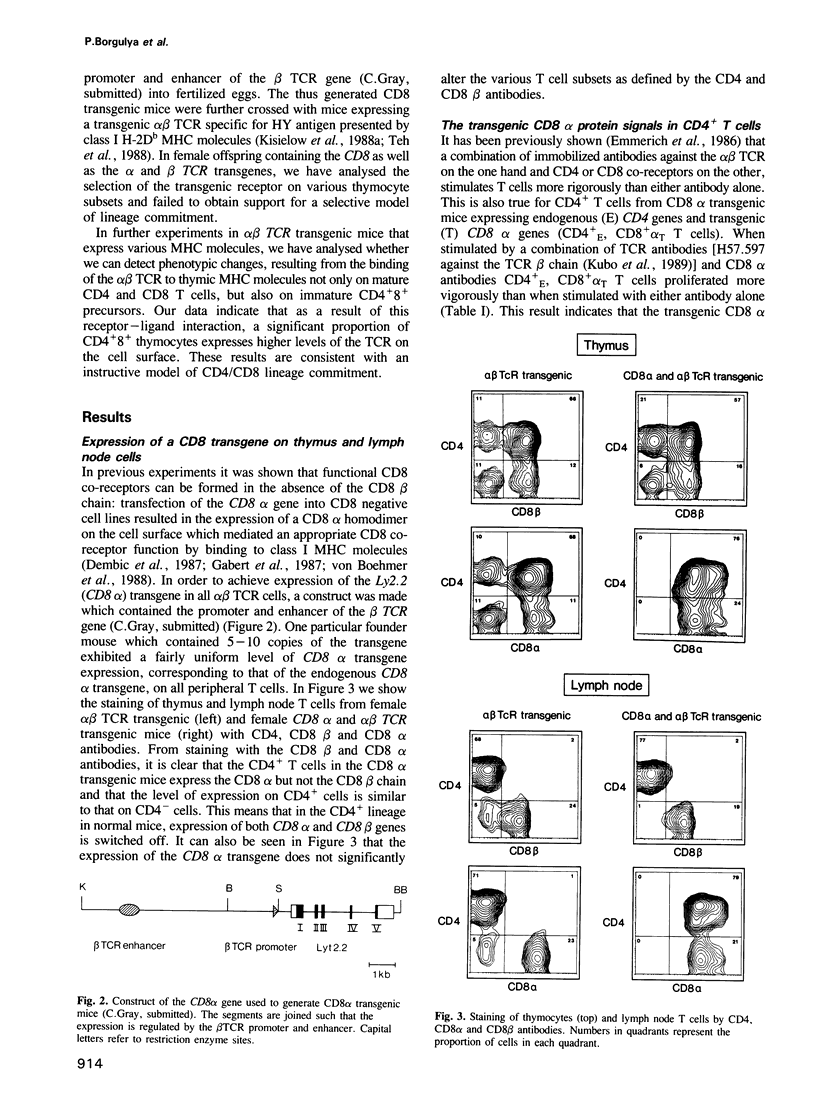
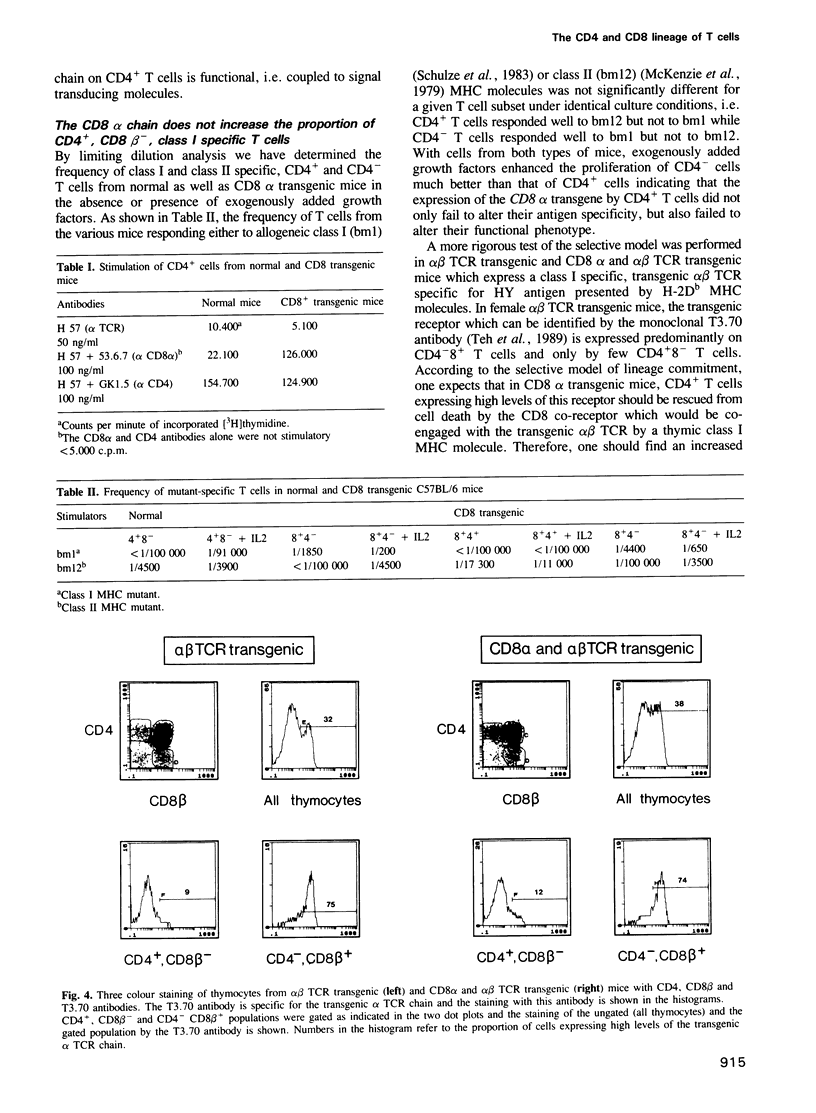
T cells bearing the alpha beta T cell receptor (TCR) can be divided into CD4+8- and CD4-8+ subsets which develop in the thymus from CD4+8+ precursors. The commitment to the CD4 and CD8 lineage depends on the binding of the alpha beta TCR to thymic major histocompatibility complex (MHC) coded class II and class I molecules, respectively. In an instructive model of lineage commitment, the binding of the alpha beta TCR, for instance to class I MHC molecules, would generate a specific signal instructing the CD4+8+ precursors to switch off the expression of the CD4 gene. In a selective model, the initial commitment, i.e. switching off the expression of either the CD4 or the CD8 gene would be a stochastic event which is then followed by a selective step rescuing only CD4+ class II and CD8+ class I specific T cells while CD4+ class I and CD8+ class II specific cells would have a very short lifespan. The selective model predicts that a CD8 transgene which is expressed in all immature and mature T cells should rescue CD4+ class I MHC specific T cells from cell death. We have performed experiments in CD8 transgenic mice which fail to support a selective model and we present data which show that the binding of the alpha beta TCR to thymic class I MHC molecules results in up-regulation of the TCR in the CD4+8+ population. Therefore, these experiments are consistent with an instructive model of lineage commitment.
Full text
PDF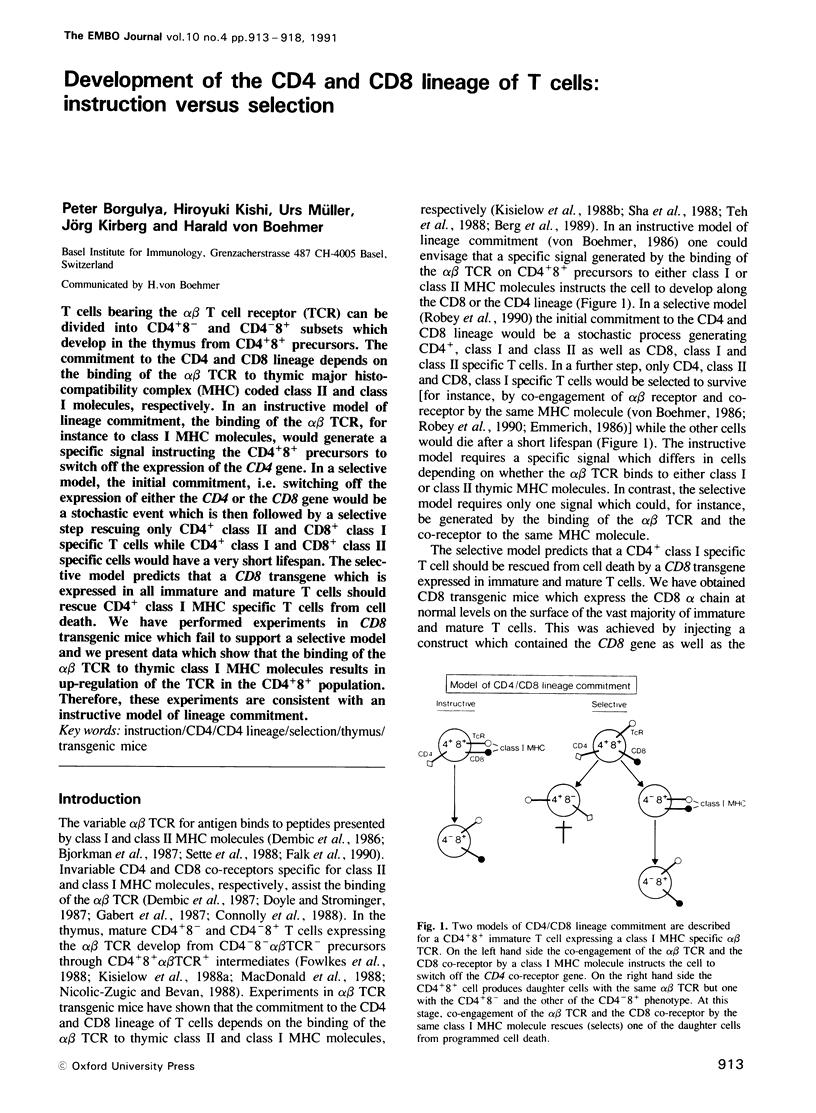



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg L. J., Pullen A. M., Fazekas de St Groth B., Mathis D., Benoist C., Davis M. M. Antigen/MHC-specific T cells are preferentially exported from the thymus in the presence of their MHC ligand. Cell. 1989 Sep 22;58(6):1035–1046. doi: 10.1016/0092-8674(89)90502-3. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Blue M. L., Daley J. F., Levine H., Craig K. A., Schlossman S. F. Identification and isolation of a T4+T8+ cell with high T3 expression in human thymus: a possible late intermediate in thymocyte differentiation. J Immunol. 1987 Aug 15;139(4):1065–1069. [PubMed] [Google Scholar]
- Connolly J. M., Potter T. A., Wormstall E. M., Hansen T. H. The Lyt-2 molecule recognizes residues in the class I alpha 3 domain in allogeneic cytotoxic T cell responses. J Exp Med. 1988 Jul 1;168(1):325–341. doi: 10.1084/jem.168.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembić Z., Haas W., Weiss S., McCubrey J., Kiefer H., von Boehmer H., Steinmetz M. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature. 1986 Mar 20;320(6059):232–238. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- Dembić Z., Haas W., Zamoyska R., Parnes J., Steinmetz M., von Boehmer H. Transfection of the CD8 gene enhances T-cell recognition. Nature. 1987 Apr 2;326(6112):510–511. doi: 10.1038/326510a0. [DOI] [PubMed] [Google Scholar]
- Doyle C., Strominger J. L. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987 Nov 19;330(6145):256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Strittmatter U., Eichmann K. Synergism in the activation of human CD8 T cells by cross-linking the T-cell receptor complex with the CD8 differentiation antigen. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8298–8302. doi: 10.1073/pnas.83.21.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Rammensee H. G. Cellular peptide composition governed by major histocompatibility complex class I molecules. Nature. 1990 Nov 15;348(6298):248–251. doi: 10.1038/348248a0. [DOI] [PubMed] [Google Scholar]
- Fowlkes B. J., Schwartz R. H., Pardoll D. M. Deletion of self-reactive thymocytes occurs at a CD4+8+ precursor stage. Nature. 1988 Aug 18;334(6183):620–623. doi: 10.1038/334620a0. [DOI] [PubMed] [Google Scholar]
- Gabert J., Langlet C., Zamoyska R., Parnes J. R., Schmitt-Verhulst A. M., Malissen B. Reconstitution of MHC class I specificity by transfer of the T cell receptor and Lyt-2 genes. Cell. 1987 Aug 14;50(4):545–554. doi: 10.1016/0092-8674(87)90027-4. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Teh H. S., Blüthmann H., von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988 Oct 20;335(6192):730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- Kubo R. T., Born W., Kappler J. W., Marrack P., Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989 Apr 15;142(8):2736–2742. [PubMed] [Google Scholar]
- Ledbetter J. A., Seaman W. E., Tsu T. T., Herzenberg L. A. Lyt-2 and lyt-3 antigens are on two different polypeptide subunits linked by disulfide bonds. Relationship of subunits to T cell cytolytic activity. J Exp Med. 1981 Jun 1;153(6):1503–1516. doi: 10.1084/jem.153.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Hengartner H., Pedrazzini T. Intrathymic deletion of self-reactive cells prevented by neonatal anti-CD4 antibody treatment. Nature. 1988 Sep 8;335(6186):174–176. doi: 10.1038/335174a0. [DOI] [PubMed] [Google Scholar]
- McKenzie I. F., Morgan G. M., Sandrin M. S., Michaelides M. M., Melvold R. W., Kohn H. I. B6.C-H-2bm12. A new H-2 mutation in the I region in the mouse. J Exp Med. 1979 Dec 1;150(6):1323–1338. doi: 10.1084/jem.150.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi P. S., Pircher H., Bürki K., Zinkernagel R. M., Hengartner H. Distinct sequence of negative or positive selection implied by thymocyte T-cell receptor densities. Nature. 1990 Aug 30;346(6287):861–863. doi: 10.1038/346861a0. [DOI] [PubMed] [Google Scholar]
- Pierres A., Naquet P., Van Agthoven A., Bekkhoucha F., Denizot F., Mishal Z., Schmitt-Verhulst A. M., Pierres M. A rat anti-mouse T4 monoclonal antibody (H129.19) inhibits the proliferation of Ia-reactive T cell clones and delineates two phenotypically distinct (T4+, Lyt-2,3-, and T4-, Lyt-2,3+) subsets among anti-Ia cytolytic T cell clones. J Immunol. 1984 Jun;132(6):2775–2782. [PubMed] [Google Scholar]
- Robey E. A., Fowlkes B. J., Gordon J. W., Kioussis D., von Boehmer H., Ramsdell F., Axel R. Thymic selection in CD8 transgenic mice supports an instructive model for commitment to a CD4 or CD8 lineage. Cell. 1991 Jan 11;64(1):99–107. doi: 10.1016/0092-8674(91)90212-h. [DOI] [PubMed] [Google Scholar]
- Robey E. A., Fowlkes B. J., Pardoll D. M. Molecular mechanisms for lineage commitment in T cell development. Semin Immunol. 1990 Jan;2(1):25–34. [PubMed] [Google Scholar]
- Schulze D. H., Pease L. R., Geier S. S., Reyes A. A., Sarmiento L. A., Wallace R. B., Nathenson S. G. Comparison of the cloned H-2Kbm1 variant gene with the H-2Kb gene shows a cluster of seven nucleotide differences. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2007–2011. doi: 10.1073/pnas.80.7.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha W. C., Nelson C. A., Newberry R. D., Kranz D. M., Russell J. H., Loh D. Y. Selective expression of an antigen receptor on CD8-bearing T lymphocytes in transgenic mice. Nature. 1988 Sep 15;335(6187):271–274. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- Teh H. S., Kishi H., Scott B., Von Boehmer H. Deletion of autospecific T cells in T cell receptor (TCR) transgenic mice spares cells with normal TCR levels and low levels of CD8 molecules. J Exp Med. 1989 Mar 1;169(3):795–806. doi: 10.1084/jem.169.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh H. S., Kisielow P., Scott B., Kishi H., Uematsu Y., Blüthmann H., von Boehmer H. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988 Sep 15;335(6187):229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Haas W. H-2 restricted cytolytic and noncytolytic T cell clones: isolation, specificity and functional analysis. Immunol Rev. 1981;54:27–56. doi: 10.1111/j.1600-065x.1981.tb00433.x. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Karjalainen K., Pelkonen J., Borgulya P., Rammensee H. G. The T-cell receptor for antigen in T-cell development and repertoire selection. Immunol Rev. 1988 Jan;101:21–37. doi: 10.1111/j.1600-065x.1988.tb00731.x. [DOI] [PubMed] [Google Scholar]




