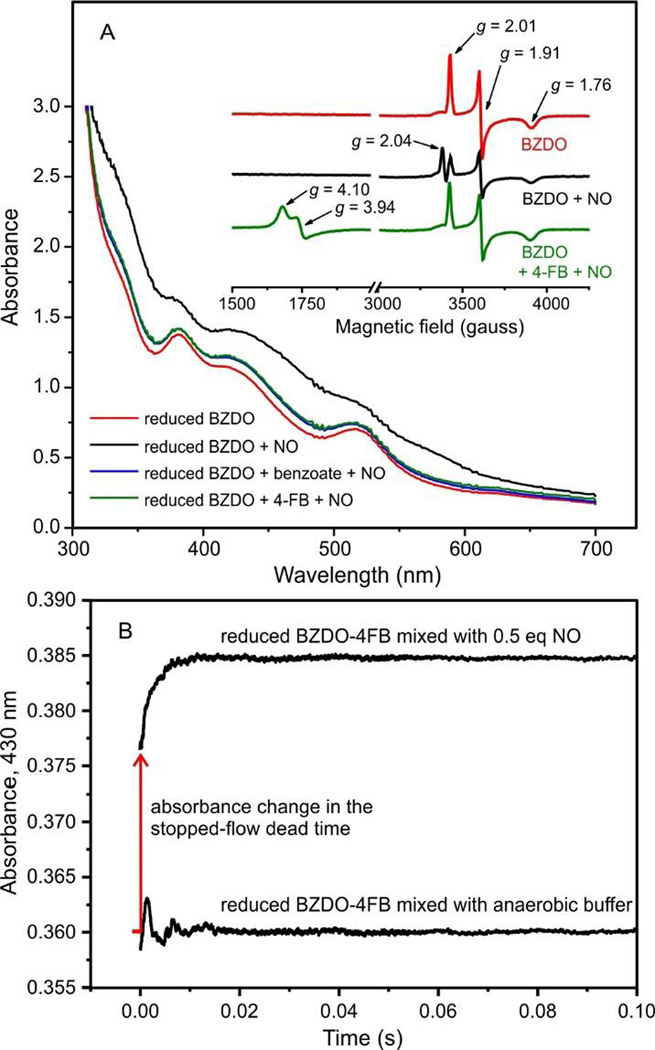
Figure 5.
NO binds rapidly to the mononuclear Fe(II) in the substrate complex. (A) The optical spectra of reduced BZDO (250 µM) with or without benzoate (50 mM) or 4-FB (50 mM) are shown 15 min after the addition of NO (~ 0.5 equivalents relative to BZDO sites). In the absence of substrate, the large change in electronic absorption is caused by NO binding to the Rieske cluster as can be observed by the attenuation of the S = ½ (g = 2.01, 1.91, and 1.76) EPR signal (inset) from the reduced Rieske cluster and formation of a new signal at g = 2.04. In the presence of benzoate or 4-FB, the NO adduct of the mononuclear iron is formed as shown by the appearance of the characteristic S = 3/2 (g = 4.10 and 3.94) EPR signal. Conditions: microwave power, 0.2 mW; temperature, 20 K; microwave frequency, 9.64 GHz. (B) A solution of reduced BZDO (200 µM) and 4-FB (1 mM) was mixed 1:1 in a stopped-flow instrument with anaerobic reaction buffer or anaerobic buffer containing NO (~ 0.5 eq relative to BZDO). Formation of the Fe(III)-NO adduct was monitored by the increase in absorption at 430 nm. The same experiment using benzoate in place of 4-FB gave indistinguishable results.