Abstract
Adding platinum drugs to anthracycline/taxane (ANC-Tax)-based neoadjuvant chemotherapy (NAC) improves pathological complete response (pCR) rates in triple-negative breast cancer (TNBC). Copper transporter 1 (CTR1) and organic cation transporter 2 (OCT2) critically affect the uptake and cytotoxicity of platinum drugs. We immunohistochemically determined CTR1 and OCT2 levels in pre-chemotherapy biopsies from 105 patients with HER2-negative breast cancer treated with ANC-Tax-based NAC. In the TNBC group, Ki-67high [pathological good response (pGR), P = 0.04] was associated with response, whereas CTR1high (non-pGR, P = 0.03), OCT2high (non-pGR, P = 0.01; non-pCR, P = 0.03), and combined CTR1high and/or OCT2high (non-pGR, P = 0.005; non-pCR, P = 0.003) were associated with non-response. In multivariate analysis, Ki-67high was an independent factor for pGR and CTR1 for non-pGR. Combined CTR1/OCT2 was a strong independent factor for non-pGR. However, no variables were associated with response in luminal BC. These results indicate that platinum uptake transporters are predominantly expressed in ANC-Tax-resistant TNBCs, which implies that advantage associated with adding platinum drugs may depend on high drug uptake.
Keywords: copper transporter 1, organic cation transporter 2, platinum drug, neoadjuvant chemotherapy, triple-negative breast cancer, immunohistochemistry
Introduction
Triple-negative breast cancer (TNBC) is defined by the lack of expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). TNBCs derive no benefit from targeted treatments such as endocrine therapy or anti-HER2 therapy, and thus cytotoxic chemotherapy is the only systemic therapy available for TNBC patients.1 Anthracycline/taxane (ANC-Tax)-based chemotherapy remains the standard treatment for TNBC patients. However, some TNBC patients fail to respond to the chemotherapy and show worse prognosis due to an increased risk of recurrence.2,3 Thus, there is a major need to identify non-cross-resistant drugs and more appropriate regimens for this aggressive type of breast cancer (BC).
Platinum drugs, including cisplatin, carboplatin, and oxaliplatin, exert their cytotoxic effect through direct damage to DNA. As TNBC may be highly susceptible to DNA damage,4,5 the effectiveness of platinum drugs as neoadjuvant chemotherapy (NAC) for TNBC has become more widely studied; and several clinical trials have concluded that pathological complete response (pCR) rates in TNBC increased significantly with the addition of platinum drugs to ANC-Tax-based regimens.6–11 However, the mechanisms that underlie these results have not been fully elucidated.
As platinum drugs are highly polar, they do not readily diffuse across cell membranes.12 Influx of drug molecules via solute carrier (SLC) transporters is an important determinant of intracellular drug concentrations, and uptake mechanisms into tumor cells may greatly affect susceptibility to anticancer drugs including platinum.13 Copper transporter 1 (CTR1), also called SLC31A1, has been shown to control intracellular accumulation and cytotoxicity of the platinum drugs cisplatin and carboplatin.12,14,15 This is supported by several studies using clinical tissue samples. Lee et al16 reported that high levels of CTR1 mRNA were significantly associated with longer disease-free survival (DFS) in patients with ovarian cancer treated with adjuvant carboplatin-containing chemotherapy. Chen et al17 found that high levels of CTR1 were associated with better tumor response, longer DFS, and overall survival (OS) in non-small-cell lung cancer treated with cisplatin- or carboplatin-containing first-line chemotherapy. Furthermore, our recent study showed that high CTR1 expression was significantly associated with longer OS of patients with endometrial cancer treated with adjuvant paclitaxel/carboplatin chemotherapy.18 Conversely, organic cation transporter 2 (OCT2), also known as SLC22A2, is a critical determinant in the uptake and consequent cytotoxicity of cisplatin and oxaliplatin.19–21 Recently, we found that a high OCT2 expression was significantly correlated with longer progression-free survival in patients with metastatic colorectal cancer treated with first-line 5-fluorouracil/leucovorin/oxaliplatin chemotherapy.22 Taken together, the expression levels of CTR1 and OCT2 may critically affect an individual’s susceptibility to platinum-containing chemotherapy. Therefore, we explored the association of CTR1 and OCT2 expression with pathological response to ANC-Tax-based NAC in TNBCs and the underlying mechanisms that mediate the effectiveness of adding platinum drugs to the conventional regimens.
Materials and Methods
Patients and NAC
We studied 105 patients with primary BCs. The eligible criteria were invasive, HER2-negative BCs with stages II–III disease that were histopathologically diagnosed. Each patient received one of the following regimens of ANC-Tax-based NAC (FEC-T, EC-T, or AC-T) at the Kanagawa Cancer Center Hospital during 2004–2012: a median of four cycles of fluorouracil/epirubicin/cyclophosphamide at 500/100/500 mg/m2 every 3 weeks; a median of four cycles of epirubicin/cyclophosphamide at 90/600 mg/m2 every 3 weeks; or a median of four cycles of doxorubicin/cyclophosphamide at 60/600 mg/m2 every 3 weeks, followed by a median of 12 cycles of paclitaxel at 80 mg/m2 weekly or a median of four cycles of docetaxel at 75 mg/m2 every 3 weeks. The percentages of patients receiving FEC-T, EC-T, and AC-T were 91.4, 4.8, and 3.8, respectively.
Each patient underwent a pre-chemotherapy core needle biopsy of her tumor. The present study was approved by the ethics committees of Kobe University Graduate School of Health Sciences and Kanagawa Cancer Center Hospital. All the experiments were performed under the current laws of Japan. Written informed consent for the core needle biopsy and experimental use of tumor samples was obtained from all patients. The study was conducted according to the principles of the Declaration of Helsinki.
Tumor specimens and histopathological assessment
Biopsy specimens were fixed in 10% formalin and embedded into paraffin wax, and 3-μm-thick sections were prepared. Histological grade was assessed using the Nottingham modification of the Bloom and Richardson system.23 HER2-negative status was defined as a score of 0 or 1+ by immunohistochemistry or HER2 gene/centromere 17 ratio of ≤2.2 by fluorescence in situ hybridization. ER, PR, and Ki-67 status prior to NAC were determined immunohistochemically. Tumors were classified as positive for ER and PR when nuclear staining was visible in >10% of tumor cells. For Ki-67, nuclear staining in ≥14% of the tumor cells was considered to indicate Ki-67high level. Of the 105 patients, 41 (39%) were identified as TNBCs, 29 (27%) as luminal A, and 35 (33%) as luminal B.
After NAC, all patients underwent breast-conserving surgery or mastectomy. Sentinel lymph node biopsy and axillary node dissection were performed in 8% and 90% of the patients, respectively. Pathological response to NAC was evaluated using the surgical specimens. A pCR was defined as the absence of residual invasive tumor cells in both the breast and lymph nodes. Minimal residual tumor was defined as invasive tumors that showed marked changes with isolated cancer cells or tumor clusters of fewer than 10 cancer cells.24 pCR plus minimal residual tumor was defined as a pathological good response (pGR).
Expression of CTR1 and OCT2
Sections of biopsy specimens were deparaffinized in xylene and rehydrated with a graded alcohol series. Endogenous peroxidase was blocked by incubating sections in 0.3% hydrogen peroxide in methanol for 30 minutes. The antigenicities of CTR1 and OCT2 were retrieved by protease-induced antigen retrieval (PIAR) and heat-induced antigen retrieval (HIAR) methods, respectively. PIAR was performed using 0.16 U/g pronase solution (Nichirei Bioscience) for four minutes at room temperature (RT). HIAR was applied using a pressure cooker for 10 minutes at 120°C in 0.001 mol/L EDTA, pH 8.0. After pressure-cooking, the sections were allowed to cool to RT in the soaking solution for 30 minutes. Sections were then rinsed in tap water followed by 0.01 mol/L phosphate-buffered saline (PBS; pH 7.2).
After treatment with a protein-blocking solution (Dako) for 5 minutes at RT, sections were incubated overnight at RT with rabbit polyclonal anti-human CTR1 antibody (1:100 dilution; Abnova) or rabbit polyclonal anti-human OCT2 antibody (1:400 dilution; Sigma-Aldrich). Sections were then rinsed in PBS, followed by incubation with the Histofine Simple Stain MAX-PO (Nichirei Bioscience) for one hour at RT. Reaction products were developed using diaminobenzidine solution (Nichirei Bioscience). A negative control was included in each run by omitting the primary antibody. Sections of serous ovarian cancer for CTR1 and normal kidney for OCT2 were used as positive controls.
Evaluation of CTR1 and OCT2 levels
Three investigators (RT, AN, and SK) with no previous knowledge of patients’ clinicopathological characteristics evaluated the immunostained sections. Differences in scores were adjudicated by consensus among the three investigators. Staining was regarded as positive when tumor cells showed membrane staining. Expression levels of CTR1 and OCT2 were semiquantitatively assessed by scoring the staining intensity and the percentage of stained tumor cells. Briefly, staining intensity scores were: 0, no staining; 1, weakly positive; 2, moderately positive; and 3, strongly positive; and positive percentage of tumor cell scores were: 0, none; 1, 1–10%; 2, 11–50%; 3, >50%. These two scores were added for a composite score (0–6). Receiver operating characteristic curve analysis showed the best sensitivity and specificity cut-off values of 5 for CTR1 and 4 for OCT2: 0–4, CTR1low; 0–3, OCT2low; 5–6, CTR1high; and 4–6, OCT2high. Because CTR1 and OCT2 share common ground in terms of cisplatin uptake, as described in the Introduction, we also analyzed data from CTR1 and OCT2 in combination.
Statistical analysis
All statistical analyses were performed using SPSS 21.0 software (SPSS Inc.). Statistical significance was defined as P < 0.05. Spearman rank correlation test was used to calculate correlations between CTR1 and OCT2 expression levels. Fisher’s exact test was used to assess associations of CTR1 and OCT2 levels with patient age, clinical tumor stage and node status, histological grade and type, Ki-67 level, intrinsic subtype, and pathological response. To identify variables independently associated with pathological response, multivariate analysis was performed. Variables with a P-value <0.10 in the univariate analysis were included in a logistic regression model.
Results
Association of CTR1 and OCT2 levels with clinicopathological parameters
Clinicopathological characteristics of patients are shown in Table 1. The patients’ median age was 54 years (range: 32–73 years). Median tumor size was 4.3 cm (range: 1.0–16.3 cm). Eight percent of patients had T1 tumors, 70% had T2 tumors, 7% had T3 tumors, and 16% had T4 tumors. Clinically positive lymph nodes were found in 75% of the patients. Histological types were invasive ductal carcinoma in 87%, invasive lobular carcinoma in 7%, and other types (mucinous, apocrine) in 7% of tumors.
Table 1.
Correlation of CTR1 and OCT2 levels with clinicopathological characteristics in the HER2-negative BC patients.
| VARIABLES | NO | CTR1 | OCT2 | ||
|---|---|---|---|---|---|
| HIGH (%) | P-VALUE | HIGH (%) | P-VALUE | ||
| Age (years) | |||||
| ≥50 | 68 | 18 (26) | 0.19 | 40 (59) | 0.41 |
| <50 | 37 | 15 (41) | 25 (68) | ||
| Clinical tumor stage | |||||
| T3 + T4 | 24 | 9 (38) | 0.47 | 14 (58) | 0.81 |
| T1 + T2 | 81 | 24 (30) | 51 (63) | ||
| Clinical lymph node status | |||||
| N1–N3 | 79 | 24 (30) | 0.81 | 48 (61) | 0.82 |
| N0 | 26 | 9 (35) | 17 (65) | ||
| Histological grade | |||||
| 3 | 17 | 6 (35) | 0.78 | 8 (47) | 0.18 |
| 1 + 2 | 88 | 27 (31) | 57 (65) | ||
| Histological type | |||||
| Invasive ductal carcinoma | 91 | 28 (31) | 0.90 | 53 (58) | 0.07 |
| Invasive lobular carcinoma | 7 | 2 (29) | 7 (100) | ||
| Others | 7 | 3 (43) | 5 (71) | ||
| Ki-67 | |||||
| High | 64 | 19 (30) | 0.67 | 33 (52) | 0.01a |
| Low | 41 | 14 (34) | 32 (78) | ||
| Intrinsic subtype | |||||
| TNBC | 41 | 15 (37) | 0.39 | 17 (41) | <0.001a |
| Luminal BC | 64 | 18 (28) | 48 (75) | ||
| Luminal A | 29 | 11 (38) | 25 (86) | ||
| Luminal B | 35 | 7 (20) | 23 (66) | ||
Notes:
Statistically significant.
Abbreviations: CTR1, copper transporter 1; OCT2, organic cation transporter 2; HER2, human epidermal growth factor receptor type 2; BC, breast cancer; TNBC, triple negative BC.
Representative staining patterns of CTR1 and OCT2 are shown in Figure 1. CTR1 and OCT2 were expressed in cell membranes and/or cytoplasm of tumor cells. We found CTR1high and OCT2high expression in 33 (31%) and 65 (62%) tumors, respectively, but CTR1 and OCT2 levels were not correlated (r = 0.11, P = 0.27). Seventy-five tumors (71%) showed high levels of at least one transporter (CTR1high and/or OCT2high), whereas 30 tumors (29%) had low levels of both CTR1 and OCT2 (CTR1low and OCT2low).
Figure 1.
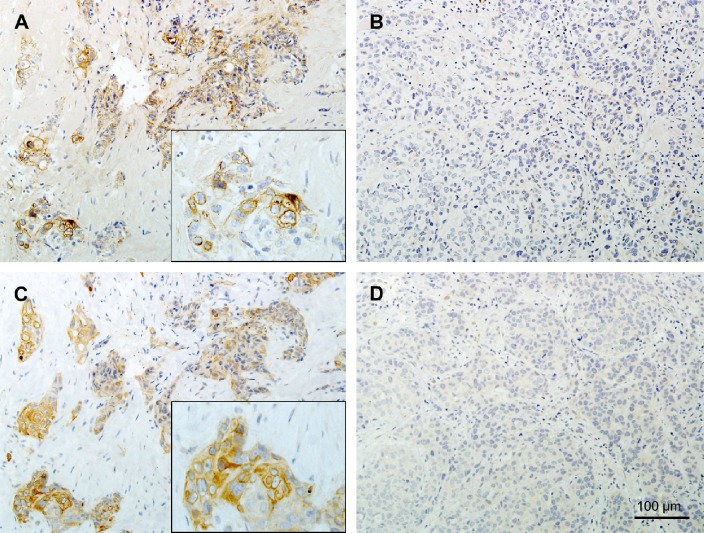
Representative immunostaining patterns in pre-chemotherapy biopsies of triple negative breast cancers according to pathological response. (A, B) Copper transporter 1 (CTR1). (C, D) Organic cation transporter 2 (OCT2). (A, C) Immunohistochemical results of a tumor from a patient with no pathological response, showing high expression levels of CTR1 and OCT2. Insets show high-power views of positive cells. (B, D) Immunohistochemical staining of a tumor from a patient with pathological complete response, showing low expression levels of CTR1 and OCT2.
Table 1 shows the association of CTR1 and OCT2 levels with clinicopathological findings. OCT2high level was significantly more prevalent in Ki-67low tumors than in Ki-67high tumors (78% vs 52%; P = 0.01). Additionally, the rate of OCT2high tumors was significantly higher in luminal BCs than in TNBCs (75% vs 41%; P < 0.001). However, OCT2 level was not associated with other clinicopathological parameters. No association was found between CTR1 level and any of the clinicopathological parameters.
Association of CTR1 and OCT2 levels with pathological response in the HER2-negative BCs
pGR and pCR were achieved in 23 (22%) and 11 (10%) patients, respectively. In the univariate analysis of the entire cohort (Table 2), patients with Ki-67high tumors had significantly higher pGR rates than patients with Ki-67low tumors (30% vs 10%, P = 0.02). The pGR rate in patients with TNBCs was significantly higher than that in patients with luminal BCs (44% vs 8%, P < 0.001). The pCR rate in patients with TNBCs was 27%, but no patients with luminal BCs achieved pCR (P < 0.001).
Table 2.
Univariate analysis of the correlation of CTR1 and OCT2 levels or clinicopathological parameters with pGR or pCR in the HER2-negative BC patients.
| VARIABLES | NO. | pGR (%) | NON-pGR (%) | P-VALUE | pCR (%) | NON-pCR (%) | P-VALUE |
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
| ≥50 | 68 | 17 (25) | 51 (75) | 0.34 | 9 (13) | 59 (87) | 0.32 |
| <50 | 37 | 6 (16) | 31 (84) | 2 (5) | 35 (95) | ||
| Clinical tumor stage | |||||||
| T3 + T4 | 24 | 6 (25) | 18 (75) | 0.78 | 2 (8) | 22 (92) | 1.00 |
| T1 + T2 | 81 | 17 (21) | 64 (79) | 9 (11) | 72 (89) | ||
| Clinical lymph node status | |||||||
| N1–N3 | 79 | 19 (24) | 60 (76) | 0.42 | 9 (11) | 70 (89) | 0.73 |
| N0 | 26 | 4 (15) | 22 (85) | 2 (8) | 24 (92) | ||
| Histological grade | |||||||
| 3 | 17 | 7 (41) | 10 (59) | 0.05 | 3 (18) | 14 (82) | 0.38 |
| 1 + 2 | 88 | 16 (18) | 72 (82) | 8 (9) | 80 (91) | ||
| Histological type | |||||||
| Invasive ductal carcinoma | 91 | 20 (22) | 71 (78) | 0.17 | 11 (12) | 80 (88) | 1.00 |
| Invasive lobular carcinoma | 7 | 3 (43) | 4 (57) | 0 (0) | 7 (100) | ||
| Others | 7 | 0 (0) | 7 (100) | 0 (0) | 7 (100) | ||
| Ki-67 | |||||||
| High | 64 | 19 (30) | 45 (70) | 0.02a | 10 (16) | 54 (84) | 0.05 |
| Low | 41 | 4 (10) | 37 (90) | 1 (2) | 40 (98) | ||
| Intrinsic subtype | |||||||
| TNBC | 41 | 18 (44) | 23 (56) | <0.001a | 11 (27) | 30 (73) | <0.001a |
| Luminal BC | 64 | 5 (8) | 59 (92) | 0 (0) | 64 (100) | ||
| Luminal A | 29 | 2 (7) | 27 (93) | 0 (0) | 29 (100) | ||
| Luminal B | 35 | 3 (9) | 32 (91) | 0 (0) | 35 (100) | ||
| CTR1 | |||||||
| High | 33 | 5 (15) | 28 (85) | 0.32 | 2 (6) | 31 (94) | 0.50 |
| Low | 72 | 18 (25) | 54 (75) | 9 (12) | 63 (88) | ||
| OCT2 | |||||||
| High | 65 | 8 (12) | 57 (88) | 0.003a | 1 (2) | 64 (98) | <0.001a |
| Low | 40 | 15 (37) | 25 (63) | 10 (25) | 30 (75) | ||
| Combined CTR1/OCT2 | |||||||
| CTR1-high and/or OCT2-high | 75 | 10 (13) | 65 (87) | 0.002a | 2 (3) | 73 (97) | <0.001a |
| CTR1-low and OCT2-low | 30 | 13 (43) | 17 (57) | 9 (30) | 21 (70) | ||
Notes:
Statistically significant.
Abbreviations: CTR1, copper transporter 1; OCT2, organic cation transporter 2; pGR, pathological good response; pCR, pathological complete response; HER2, human epidermal growth factor receptor type 2; BC, breast cancer; TNBC, triple negative BC.
In contrast, pGR and pCR rates were significantly lower in patients with OCT2high tumors than in patients with OCT2low tumors (pGR, 12% vs 38%, P = 0.003; pCR, 2% vs 25%, P < 0.001). However, no significant association between CTR1 level and pathological response was detected. When analyzing CTR1 and OCT2 in combination, pGR and pCR rates in patients with CTR1high and/or OCT2high tumors were significantly lower in patients with CTR1low and OCT2low tumors (pGR, 13% vs 43%, P = 0.002; pCR, 3% vs 30%, P < 0.001). However, other parameters showed no significant association with pathological response.
Multivariate analysis in HER2-negative BCs
Table 3 shows the results of multivariate analysis of the entire cohort. We first included the histological grade, Ki-67 level, intrinsic subtype, and OCT2 level (Model 1). TNBC subtype was an independent factor for pGR [odds ratio (OR), 6.79; 95% confidence interval (CI), 2.03–22.74; P = 0.002). Unfortunately, the OR for pCR in intrinsic subtype could not be calculated because the data contained zero: no patients with luminal BCs achieved pCR. Conversely, OCT2high level was identified as an independent factor for non-pCR (OR, 0.10; 95% CI, 0.01–0.92; P = 0.04).
Table 3.
Multivariate logistic regression analysis of predictive factors for pGR or pCR in the HER2-negative BC patients.
| VARIABLES | pGR | pCR | ||
|---|---|---|---|---|
| OR (95% CI) | P-VALUE | OR (95% CI) | P-VALUE | |
| Model 1 | ||||
| Histological grade | 1.13 (0.32–4.08) | 0.85 | NA | NA |
| Ki-67 | 2.81 (0.78–10.13) | 0.11 | 4.49 (0.46–44.02) | 0.20 |
| Intrinsic subtype | 6.79 (2.03–22.74) | 0.002a | – | – |
| OCT2 | 0.46 (0.15–1.40) | 0.17 | 0.10 (0.01–0.92) | 0.04a |
| Model 2 | ||||
| Histological grade | 1.01 (0.28–3.73) | 0.98 | NA | NA |
| Ki-67 | 2.78 (0.76–10.16) | 0.12 | 5.57 (0.54–57.08) | 0.15 |
| Intrinsic subtype | 7.40 (2.22–24.63) | 0.001a | – | – |
| Combined CTR1/OCT2 | 0.34 (0.11–1.01) | 0.05 | 0.10 (0.02–0.57) | 0.01a |
Notes:
Statistically significant. OR for pCR in the intrinsic subtype could not be calculated because the data contained zero.
Abbreviations: pGR, pathological good response; pCR, pathological complete response; HER2, human epidermal growth factor receptor type 2; BC, breast cancer; OR, odds ratio; CI, confidence interval; NA, not applicable; OCT2, organic cation transporter 2; CTR1, copper transporter 1.
Next, we included combined CTR1 and OCT2 status instead of OCT2 level (Model 2). The TNBC subtype remained an independent factor for pGR (OR, 7.40; 95% CI, 2.22–24.63; P = 0.001). Combined CTR1/OCT2 was identified as an independent factor for non-pCR (OR, 0.10; 95% CI, 0.02–0.57; P = 0.01). However, histological grade and Ki-67 level were not significant in Model 1 or Model 2.
Association of CTR1 and OCT2 levels with pathological response according to intrinsic subtype
To confirm the significance of CTR1 and OCT2 levels in TNBCs, the TNBC and luminal BC groups were separately analyzed (Table 4). In the TNBC group, pGR was observed in 55% of patients with Ki-67high tumors and in 17% of patients with Ki-67low tumors (P = 0.04). In contrast, a significantly lower pGR rate was seen in patients with CTR1high tumors than in patients with CTR1low tumors (20% vs 58%, P = 0.03). However, Ki-67 and CTR1 levels were not associated with the pCR rate. pGR and pCR rates were significantly lower in patients with OCT2high tumors than in patients with OCT2low tumors (pGR: 19% vs 60%, P = 0.01; pCR: 6% vs 40%, P = 0.03). In the combined analysis, pGR and pCR rates in patients with CTR1high and/or OCT2high tumors were significantly lower than in patients with CTR1low and OCT2low tumors (pGR: 25% vs 71%, P = 0.005; pCR: 8% vs 53%, P = 0.003; Fig. 1), which implies greater statistical significance for the combination than for OCT2 alone. However, no significant association with pathological response was detected for other parameters in the TNBC group or for any parameters in the luminal BC group.
Table 4.
Univariate analysis of the correlation of CTR1 and OCT2 levels or clinicopathological parameters with pGR or pCR according to intrinsic subtype.
| VARIABLES | TNBC | LUMINAL BC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NO. | pGR (%) | NON-pGR (%) | P-VALUE | pCR (%) | NON-pCR (%) | P-VALUE | NO. | pGR (%) | NON-pGR (%) | P-VALUE | |
| Clinical tumor stage | |||||||||||
| T3 + T4 | 14 | 5 (36) | 9 (64) | 0.52 | 2 (14) | 12 (86) | 0.28 | 10 | 1 (10) | 9 (90) | 1.00 |
| T1 + T2 | 27 | 13 (48) | 14 (52) | 9 (33) | 18 (67) | 54 | 4 (7) | 50 (93) | |||
| Clinical lymph node status | |||||||||||
| N1–N3 | 34 | 15 (44) | 19 (56) | 1.00 | 9 (26) | 25 (74) | 1.00 | 45 | 4 (9) | 41 (91) | 1.00 |
| N0 | 7 | 3 (43) | 4 (57) | 2 (29) | 5 (71) | 19 | 1 (5) | 18 (95) | |||
| Histological grade | |||||||||||
| 3 | 14 | 7 (50) | 7 (50) | 0.74 | 3 (21) | 11 (79) | 0.72 | 3 | 0 (0) | 3 (100) | 1.00 |
| 1 + 2 | 27 | 11 (41) | 16 (59) | 8 (30) | 19 (70) | 61 | 5 (8) | 56 (92) | |||
| Ki-67 | |||||||||||
| High | 29 | 16 (55) | 13 (45) | 0.04a | 10 (34) | 19 (66) | 0.13 | 35 | 3 (9) | 32 (91) | 1.00 |
| Low | 12 | 2 (17) | 10 (83) | 1 (8) | 11 (92) | 29 | 2 (7) | 27 (93) | |||
| CTR1 | |||||||||||
| High | 15 | 3 (20) | 12 (80) | 0.03a | 2 (13) | 13 (87) | 0.17 | 18 | 2 (11) | 16 (89) | 0.62 |
| Low | 26 | 15 (58) | 11 (42) | 9 (35) | 17 (65) | 46 | 3 (7) | 43 (93) | |||
| OCT2 | |||||||||||
| High | 16 | 3 (19) | 13 (81) | 0.01a | 1 (6) | 15 (94) | 0.03a | 48 | 5 (10) | 43 (90) | 0.32 |
| Low | 25 | 15 (60) | 10 (40) | 10 (40) | 15 (60) | 16 | 0 (0) | 16 (100) | |||
| Combined CTR1/OCT2 | |||||||||||
| CTR1-high and/or OCT2-high | 24 | 6 (25) | 18 (75) | 0.005a | 2 (8) | 22 (92) | 0.003a | 52 | 5 (10) | 47 (90) | 0.57 |
| CTR1-low and OCT2-low | 17 | 12 (71) | 5 (29) | 9 (53) | 8 (47) | 12 | 0 (0) | 12 (100) | |||
Notes:
Statistically significant.
Abbreviations: CTR1, copper transporter 1; OCT2, organic cation transporter 2; pGR, pathological good response; pCR, pathological complete response; TNBC, triple negative breast cancer; BC, breast cancer.
The above-mentioned trends remained unchanged even after we eliminated from our analysis the nine patients who were not treated with FEC-T alone. In the TNBC group, the pGR rate was significantly higher in the Ki-67high group than in the Ki-67low group (P = 0.01). In contrast, the pGR rate was significantly lower in the CTR1high group than in the CTR1low group (P = 0.04), and also in the OCT2high group than in the OCT2low group (P = 0.04). The groups with CTR1high and/or OCT2high tumors had significantly lower pGR (P = 0.005) and pCR rates (P = 0.01) than did the CTR1low/OCT2low group. However, no parameters were associated with pathological response in the luminal BC group.
Multivariate analysis of TNBC
Multivariate analysis was performed only for pGR in the TNBC group (Table 5) because in the univariate analysis only OCT2 and combined CTR1/OCT2 were significant factors for pCR and no significant factors for pGR were found in the luminal BC group. In Model 1, Ki-67high level was a significant independent factor for pGR (OR, 9.04; 95% CI, 1.28–63.82; P = 0.03), whereas CTR1high level was an independent factor for non-pGR (OR, 0.14; 95% CI, 0.02–0.82; P = 0.03). However, OCT2 level did not reach statistical significance.
Table 5.
Multivariate logistic regression analysis of predictive factors for pGR or pCR in the TNBC patients.
| VARIABLES | pGR | |
|---|---|---|
| OR (95% CI) | P-VALUE | |
| Model 1 | ||
| Ki-67 | 9.04 (1.28–63.82) | 0.03a |
| CTR1 | 0.14 (0.02–0.82) | 0.03a |
| OCT2 | 0.22 (0.04–1.20) | 0.08 |
| Model 2 | ||
| Ki-67 | 7.33 (1.10–48.78) | 0.04a |
| Combined CTR1/OCT2 | 0.12 (0.03–0.57) | 0.008a |
Notes:
Statistically significant.
Abbreviations: pGR, pathological good response; pCR, pathological complete response; TNBC, triple negative breast cancer; OR, odds ratio; CI, confidence interval; CTR1, copper transporter 1; OCT2, organic cation transporter 2.
In Model 2, Ki-67 remained a significant independent factor for pGR (OR, 7.33; 95% CI, 1.10–48.78; P = 0.04). Combined CTR1/OCT2 was an independent factor for non-pGR, with a higher significance level than CTR1 alone (OR, 0.12; 95% CI, 0.03–0.57; P = 0.008).
Even when only FEC-T-treated patients in the TNBC group were analyzed, Ki-67high level remained a significant independent predictor for pGR in Model 1 (P = 0.009) and Model 2 (P = 0.03), as did CTR1high for non-pGR in Model 1 (P = 0.02) and combined CTR1/OCT2 for non-pGR in Model 2 (P = 0.02).
Discussion
ANC-Tax-based regimens are most commonly used to treat TNBC, but have pCR rates of only ≤30%.25,26 Early identification of non-cross-resistant drugs could improve TNBC responsiveness. Platinum drugs are another cytotoxic drug type that is often effective for TNBC.4,5 Torrisi et al6 evaluated epirubicin/cisplatin/5-fluorouracil followed by paclitaxel as NAC, which resulted in a pCR rate of 40%. Frasci et al7 assessed a weekly NAC regimen of cisplatin/epirubicin/paclitaxel supported by granulocyte colony-stimulating factor in which 62% of patients showed pCRs.
The addition of carboplatin to ANC-Tax-based NAC for TNBC improved pCR rates in two recent randomized studies. In a Phase II study by Ando et al,9 carboplatin and weekly paclitaxel followed by cyclophosphamide/epirubicin/5-fluorouracil showed a significantly higher pCR rate than the regimen without carboplatin (61% vs 26%). Von Minckwitz et al11 assessed adding carboplatin to paclitaxel/liposomal doxorubicin/bevacizumab and obtained a pCR rate of 53%. Unfortunately, these regimens were associated with high incidences of adverse events and increased discontinuation rates. Furthermore, Alba et al27 found no improvement of the pCR rate by adding carboplatin to epirubicin/cyclophosphamide followed by docetaxel. Hurley et al8 found ANC-Tax-based NAC with cisplatin to be superior to that with carboplatin, with significantly improved OS for cisplatin-treated patients.
Relatively few studies of the effectiveness of oxaliplatin-containing regimens in BC have been reported. Phase II studies have shown the combination of oxaliplatin with 5-fluorouracil/leucovorin to be effective and safe.28,29 However, to the best of our knowledge, the effect of adding oxaliplatin to ANC-Tax-based NAC for TNBC has not been studied. We therefore considered that a better understanding of the underlying characteristics of TNBC is needed to identify any specific subgroup that might benefit from the addition of platinum drugs, particularly cisplatin.
Because intracellular uptake of platinum drugs depends on facilitated transport systems, expression levels of platinum uptake transporters may predict the sensitivity of tumors to platinum-based chemotherapy.30 CTR1 is a critical determinant of the uptake and consequent cytotoxicity of cisplatin and carboplatin,12,14,15 as OCT2 is of cisplatin and oxaliplatin.19–21 In the present study, therefore, we immunohistochemically investigated the expression levels of CTR1 and OCT2 in pre-chemotherapy biopsies from HER2-negative BC patients, with or without pathological responses to ANC-Tax-based NAC. In a univariate analysis of the entire cohort, Ki-67high level (pGR, P = 0.02) and TNBC subtype (pGR, P< 0.001; pCR, P < 0.001) were associated with response, whereas OCT2high level was associated with non-response (non-pGR, P = 0.003; non-pCR, P < 0.001). Because CTR1 and OCT2 both function as cisplatin uptake transporters, we also analyzed data from CTR1 and OCT2 in combination. CTR1high and/or OCT2high status were significantly associated with non-response (non-pGR, P = 0.002; non-pCR, P < 0.001). Two separate multivariate analyses showed the TNBC subtype to be a powerful independent factor for pGR (P = 0.002 in Model 1 and P = 0.001 in Model 2). Several studies showed that pCR rates of patients with TNBC were significantly higher than those of patients with non-TNBC.2,26 In contrast, OCT2 (P = 0.04) and combined CTR1/OCT2 (P = 0.01) were independent factors for non-pCR but not for pGR; this may be due to the small number of pCR patients.
Because platinum drugs are proposed to be effective for TNBC,6–8 we further analyzed TNBC and luminal BC to confirm the significance of CTR1 and OCT2 levels in resistance to ANC-Tax-based NAC in TNBCs. In the TNBC group, Ki-67high was associated with response (pGR, P = 0.04), whereas CTR1high (non-pGR, P = 0.03), OCT2high (non-pGR, P = 0.01; non-pCR, P = 0.03), and combined CTR1high and/or OCT2high status (non-pGR, P = 0.005; non-pCR, P = 0.003) were associated with non-response. Multivariate analysis showed that Ki-67high status was a significant independent factor for pGR (P = 0.03 in Model 1 and P = 0.04 in Model 2). Although we could not detect a significant correlation between Ki-67 level and pCR rate, several investigators reported higher pCR rates in Ki-67high TNBC than in Ki-67low TNBC.31,32 In contrast, CTR1 was identified as an independent factor for non-pGR (P = 0.03). Combined CTR1/OCT2 was the most powerful independent factor for non-pGR (P = 0.008), with greater significance than CTR1 alone. Conversely, in the luminal BC group, no variables were associated with pathological response.
Ideally, chemoresistance evaluations should focus on patient groups that received the same chemotherapy regimen. As 91.4% of patients in this study received FEC-T, we also analyzed the FEC-T-treated patients separately. We found that the major conclusion of our study remained unchanged.
These results indicate that platinum uptake transporters are predominantly expressed in ANC-Tax-resistant TNBCs, which implies that the advantage associated with adding platinum drugs, especially cisplatin, may depend on a high level of drug uptake. However, this study has several limitations: its retrospective design, the small number of patients, and the lack of molecular subtyping based on gene profiles. Our results are thus still exploratory, and larger studies are required to confirm the feasibility of our results.
Our additional findings were that the OCT2high level was significantly more prevalent in Ki-67low tumors than in Ki-67high tumors and in luminal BCs than in TNBCs. Some of these results are most likely due to the inverse correlation between Ki-67 level and hormone receptor expression in BCs.33 However, the possible role of OCT2 in cell cycle regulation and its hormone-dependent expression have not yet been clarified.
Conclusion
Our results suggest a possible contribution of CTR1 and OCT2 to the effect of platinum drugs for ANC-Tax-resistant TNBCs. Further studies that use TNBC cell lines or TNBC-based xenograft models are needed to verify the associations between high CTR1 and OCT2 levels and ANC-Tax-resistance, ANC-Tax dose dependency, and the effectiveness of adding platinum drugs to ANC-Tax-based regimens. Whether CTR1 and OCT2 expression levels predict the prognosis of patients treated with platinum-containing chemotherapy also warrants further study.
Footnotes
ACADEMIC EDITOR: Goberdhan P. Dimri, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: The authors declare no conflicts of interest in this work.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Performed experiments and data analysis and wrote the manuscript: RT. Performed experiments: AN; Collected clinicopathologic data and tissue samples: NO, YK, KK, SS. Designed the study, guided the experiments, and edited the manuscript: SK. All authors read and approved the final manuscript.
REFERENCES
- 1.Dawson SJ, Provenzano E, Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur J Cancer. 2009;45(suppl 1):27–40. doi: 10.1016/S0959-8049(09)70013-9. [DOI] [PubMed] [Google Scholar]
- 2.Wu J, Li S, Jia W, Su F. Response and prognosis of taxanes and anthracyclines neoadjuvant chemotherapy in patients with triple-negative breast cancer. J Cancer Res Clin Oncol. 2011;137(10):1505–1510. doi: 10.1007/s00432-011-1029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 4.Telli ML, Ford JM. Novel treatment approaches for triple-negative breast cancer. Clin Breast Cancer. 2010;10(suppl 1):E16–E22. doi: 10.3816/CBC.2010.s.003. [DOI] [PubMed] [Google Scholar]
- 5.Petrelli F, Coinu A, Borgonovo K, et al. The value of platinum agents as neoadjuvant chemotherapy in triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;144(2):223–232. doi: 10.1007/s10549-014-2876-z. [DOI] [PubMed] [Google Scholar]
- 6.Torrisi R, Balduzzi A, Ghisini R, et al. Tailored preoperative treatment of locally advanced triple negative (hormone receptor negative and HER2 negative) breast cancer with epirubicin, cisplatin, and infusional fluorouracil followed by weekly paclitaxel. Cancer Chemother Pharmacol. 2008;62(4):667–672. doi: 10.1007/s00280-007-0652-z. [DOI] [PubMed] [Google Scholar]
- 7.Frasci G, Comella P, Rinaldo M, et al. Preoperative weekly cisplatin-epirubicin-paclitaxel with G-CSF support in triple-negative large operable breast cancer. Ann Oncol. 2009;20(7):1185–1192. doi: 10.1093/annonc/mdn748. [DOI] [PubMed] [Google Scholar]
- 8.Hurley J, Reis IM, Rodgers SE, et al. The use of neoadjuvant platinum-based chemotherapy in locally advanced breast cancer that is triple negative: retrospective analysis of 144 patients. Breast Cancer Res Treat. 2013;138(3):783–794. doi: 10.1007/s10549-013-2497-y. [DOI] [PubMed] [Google Scholar]
- 9.Ando M, Yamauchi H, Aogi K, et al. Randomized phase II study of weekly paclitaxel with and without carboplatin followed by cyclophosphamide/epirubicin/5-fluorouracil as neoadjuvant chemotherapy for stage II/IIIA breast cancer without HER2 overexpression. Breast Cancer Res Treat. 2014;145(2):401–409. doi: 10.1007/s10549-014-2947-1. [DOI] [PubMed] [Google Scholar]
- 10.Byrski T, Huzarski T, Dent R, et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2014;147(2):401–405. doi: 10.1007/s10549-014-3100-x. [DOI] [PubMed] [Google Scholar]
- 11.von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15(7):747–756. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 12.Howell SB, Safaei R, Larson CA, Sailor MJ. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharmacol. 2010;77(6):887–894. doi: 10.1124/mol.109.063172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sprowl JA, Mikkelsen TS, Giovinazzo H, Sparreboom A. Contribution of tumoral and host solute carriers to clinical drug response. Drug Resist Updat. 2012;15(1–2):5–20. doi: 10.1016/j.drup.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holzer AK, Manorek GH, Howell SB. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol Pharmacol. 2006;70(4):1390–1394. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- 15.Song IS, Savaraj N, Siddik ZH, et al. Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol Cancer Ther. 2004;3(12):1543–1549. [PubMed] [Google Scholar]
- 16.Lee YY, Choi CH, Do IG, et al. Prognostic value of the copper transporters, CTR1 and CTR2, in patients with ovarian carcinoma receiving platinum-based chemotherapy. Gynecol Oncol. 2011;122:361–365. doi: 10.1016/j.ygyno.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Chen HH, Yan JJ, Chen WC, et al. Predictive and prognostic value of human copper transporter 1 (hCtr1) in patients with stage III non-small-cell lung cancer receiving first-line platinum-based doublet chemotherapy. Lung Cancer. 2012;75:228–234. doi: 10.1016/j.lungcan.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogane N, Yasuda M, Kameda Y, et al. Prognostic value of organic anion transporting polypeptide 1B3 and copper transporter 1 expression in endometrial cancer patients treated with paclitaxel and carboplatin. Biomed Res. 2013;34(3):143–151. doi: 10.2220/biomedres.34.143. [DOI] [PubMed] [Google Scholar]
- 19.Yonezawa A, Masuda S, Yokoo S, Katsura T, Inui K. Cisplatin and oxaliplatin but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1-3 and multidrug and toxin extrusion family) J Pharmacol Exp Ther. 2006;319(2):879–886. doi: 10.1124/jpet.106.110346. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Lovejoy KS, Shima JE, et al. Organic cation transporters are determinants of oxaliplatin cytotoxicity. Cancer Res. 2006;66(17):8847–8857. doi: 10.1158/0008-5472.CAN-06-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burger H, Zoumaro-Djayoon A, Boersma AW, et al. Differential transport of platinum compounds by the human organic cation transporter hOCT2 (hSLC22A2) Br J Pharmacol. 2010;159(4):898–908. doi: 10.1111/j.1476-5381.2009.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tatsumi S, Matsuoka H, Hashimoto Y, Hatta K, Maeda K, Kamoshida S. Organic cation transporter 2 and tumor budding as independent prognostic factors in metastatic colorectal cancer patients treated with oxaliplatin-based chemotherapy. Int J Clin Exp Pathol. 2014;7(1):204–212. [PMC free article] [PubMed] [Google Scholar]
- 23.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto Y, Tatsumi S, Takeda R, et al. Expression of organic anion-transporting polypeptide 1A2 and organic cation transporter 6 as a predictor of pathologic response to neoadjuvant chemotherapy in triple negative breast cancer. Breast Cancer Res Treat. 2014;145(1):101–111. doi: 10.1007/s10549-014-2913-y. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 26.Huober J, von Minckwitz G, Denkert C, et al. Effect of neoadjuvant anthracycline-taxane-based chemotherapy in different biological breast cancer phenotypes: overall results from the GeparTrio study. Breast Cancer Res Treat. 2010;124(1):133–140. doi: 10.1007/s10549-010-1103-9. [DOI] [PubMed] [Google Scholar]
- 27.Alba E, Chacon JI, Lluch A, et al. A randomized phase II trial of platinum salts in basal-like breast cancer patients in the neoadjuvant seeting. Results from the GEICAM/2006-03, multicenter study. Breast Cancer Res Treat. 2012;136(2):487–493. doi: 10.1007/s10549-012-2100-y. [DOI] [PubMed] [Google Scholar]
- 28.Zelek L, Cottu P, Tubiana-Hulin M, et al. Phase II study of oxaliplatin and fluorouracil in taxane and anthracycline-pretreated breast cancer patients. J Clin Oncol. 2002;20(10):2551–2558. doi: 10.1200/JCO.2002.06.164. [DOI] [PubMed] [Google Scholar]
- 29.Pectasides D, Pectasides M, Farmakis D, et al. Oxaliplatin plus high-dose leucovorin and 5-fluorouracil in pretreated advanced breast cancer: a phase II study. Ann Oncol. 2003;14(4):537–542. doi: 10.1093/annonc/mdg172. [DOI] [PubMed] [Google Scholar]
- 30.Burger H, Loos WJ, Eechoute K, Verweij J, Mathijssen RH, Wiemer EA. Drug transporters of platinum-based anticancer agents and their clinical significance. Drug Resist Updat. 2011;14(1):22–34. doi: 10.1016/j.drup.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Darb-Esfahani S, Loibl S, Müller BM, et al. Identification of biology-based breast cancer types with distinct predictive and prognostic features: role of steroid hormone and HER2 receptor expression in patients treated with neoadjuvant anthracycline/taxane-based chemotherapy. Breast Cancer Res. 2009;11(5):R69. doi: 10.1186/bcr2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keam B, Im SA, Lee KH, et al. Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res. 2011;13(2):R22. doi: 10.1186/bcr2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosa FE, Caldeira JR, Felipes J, et al. Evaluation of estrogen receptor alpha and beta and progesterone receptor expression and correlation with clinicopathologic factors and proliferative marker Ki-67 in breast cancers. Hum Pathol. 2008;39(5):720–730. doi: 10.1016/j.humpath.2007.09.019. [DOI] [PubMed] [Google Scholar]