Abstract
l-Tryptophan (l-Trp) is an essential amino acid that possesses diverse metabolic, neurological, and immunological roles spanning from the synthesis of proteins, neurotransmitter serotonin, and neurohormone melatonin, to its degradation into immunosuppressive catabolites by indoleamine-2, 3-dioxygenase (IDO) in the kynurenine pathway (KP). Trp catabolites, by activating aryl hydrocarbon receptor (AhR), play an important role in antimicrobial defense and immune regulation. IDO/AhR acts as a double-edged sword by both depleting l-Trp to starve the invaders and by contributing to the state of immunosuppression with microorganisms that were not cleared during acute infection. Pathogens experiencing Trp deprivation by IDO-mediated degradation include certain bacteria, parasites, and less likely viruses. However, chronic viral infections highjack the host immune response to create a state of disease tolerance via kynurenine catabolites. This review covers the latest data involving chronic viral infections such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), herpes, and cytomegalovirus (CMV) and their cellular interplay with Trp catabolites. Strategies developed by viruses to escape immune control also represent new avenues for therapeutic interventions based on Trp metabolism.
Keywords: tryptophan metabolism, IDO, AhR, HIV, CMV, herpes, viral hepatitis
Introduction
l-Tryptophan (l-Trp) is one of the nine essential amino acids and is the least abundant of all 21 dietary amino acids in human beings. The distinguishing structural characteristic of Trp is that it contains an indole functional group. The l-stereoisomer of Trp is used in protein synthesis and in the generation of products such as aminergic neurotransmitter serotonin (5-hydroxytryptamine [5-HT]), the neurohormone melatonin, kynuramine metabolites, amine tryptamine, and importantly products of the kynurenine pathway (KP)1,2 (Fig. 1). Trp and its catabolites are well known for their immunosuppressive functions, disease tolerance, and contribution to immune privileged sites such as eyes, brain, placenta, and testes.1,2 The KP represents >95% of Trp-catabolizing pathways and is now established as a key regulator of innate and adaptive immunity through its involvement in cancer, autoimmunity, and infection. Infection-induced inflammation triggers catabolism of Trp in several bacterial, protozoan, and viral infections such as Chlamydia psittaci, Toxoplasma gondii, Leishmania donovani, and herpes simplex virus (HSV)-2.3–7 Trp is mainly catabolized through the enzymatic activity of indoleamine-2,3-dioxygenase (IDO) 1 and 2, which are expressed widely in human tissues, and induced by interferon gamma (IFN-γ).8 Immune dysfunction during human immunodeficiency virus (HIV) infection is also associated with increased Trp catabolism by IDO.9
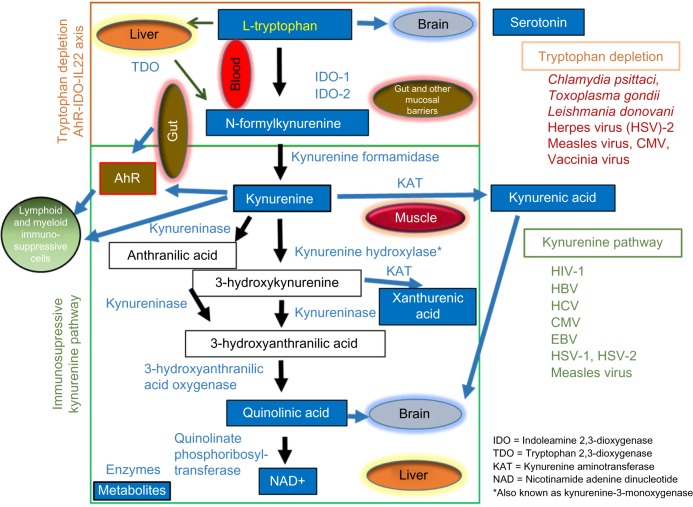
Figure 1.
Schematic representation of key enzymes and metabolites in the Trp/Kyn catabolic pathway, depicting frequent pathogens and the tissues/organs involved. Dietary Trp can be catabolized in the digestive tract by AhR-IDO-IL22 axis or by immunosuppressive Kyn pathway involving multiple enzymes, metabolites, and body organs. TDO, IDO-1, and IDO-2 are the first enzymes implicated in the Trp catabolism involving gut, immune cells, and liver. In muscles, exercise leads to the depletion of Kyn by inducing its catabolism into the nonbrain penetrating kynurenic acid. Overproduction of Kyn and its ligation to AhR leads to the induction of immunosuppressive cells. Trp metabolites like serotonin, Quin, and kynurenic acid have important implications in the brain function and mood disorders. NAD, an important cellular cofactor, is also produced by the liver during Trp catabolism. The list of microbial pathogens for which cell growth is reduced with Trp depletion or contributes to a state of immunosuppression/tolerance via KP is also depicted.
KP can be considered to have equivocal roles, as IDO is known to induce inflammation, while it is also reported to be involved in the control of acute and chronic infections.10,11 The metabolic immune regulation of IDO involves the protection of the host from overreactive immune responses via the induction of systemic immune tolerance.12 Another mechanism of IDO activity is through interaction with ligand-dependent transcription factor aryl hydrocarbon receptor (AhR), a dioxin receptor that induces detoxifying enzymes and modulates immune cell differentiation after sensing environmental toxins and endogenous ligands.13 AhR is also known to regulate chronic gut inflammation. The Trp catabolites that act as AhR ligands include kynurenine (Kyn), kynurenic acid, and tryptamine. A recent review has summarized several studies reporting the mechanisms by which IDO activity activates AhR leading to inhibition of colonization and induction of tolerance at the host–microbe mucosal interface.14
IDO is strongly induced by IFN-γ, which catalyzes the conversion of Trp into N-formylkynurenine.8 IDO-associated Trp depletion is implicated in growth inhibition of certain bacteria,5 parasites,16 and is also associated with antiviral properties against several viruses to a lesser extent, including measles,17 herpes simplex type 1 and 2,6,18 and vaccinia virus.19 In addition to this role in infection, altered Trp metabolism has an impact on immune responses such as during maternal tolerance toward the fetus, immune-escape of cancer cells, and neurocognition.20,21 By inducing serotonin depletion, the KP has become recognized as a key player in the pathogenesis of several major neuroinflammatory brain conditions associated with chronic viral infections such as HIV, cytomegalovirus (CMV), and HSV.6,22–25 Recently, neuroinflammation has been reported to be regulated by the muscular enzymes kynurenine transaminases (KATs), which metabolize Kyn to nonbrain penetrating kynurenic acid.26 Herein, we have reviewed recent studies reporting modulation of Trp metabolism in the context of chronic viral infections.
Trp Metabolism in Health and Disease: a Complex Interplay Between Microbiota, Muscle, Brain, and the Immune System
Trp is metabolized into several downstream physiologically active substances, including serotonin, melatonin, nicotinic acid, and nicotinamide adenine dinucleotide (NAD).1 The KP is the major Trp catabolizing pathway, regulated in human beings by three distinct enzymes: IDO-1 and IDO-2 inducible in many tissues and tryptophan 2,3-dioxygenase (TDO) expressed in liver, brain, and cancer cells.1,2 In physiological conditions, TDO is the main enzyme degrading Trp, while in the context of infection, IDO-1 is induced and becomes the most important intracellular enzyme. Based on the health condition, IDO or TDO leads to the production of Kyn, an immunosuppressive derivative of Trp.1 (Fig. 1). Antigen-presenting cells such as macrophages, dendritic cells (DCs), and B-cells, as well as epithelial cells, deplete Trp by producing IDO-1, IDO-2, and TDO, resulting in a mechanism of defense against certain microorganisms.5 In contrast, the KP induces immunosuppression through induction of T-cell exhaustion and expansion of Tregs.27,28 Increased activity of the KP as measured by the ratio of Kyn to Trp in plasma (KT ratio) has also been associated with progressive AIDS9 and liver cirrhosis in hepatitis C virus (HCV) infection.29
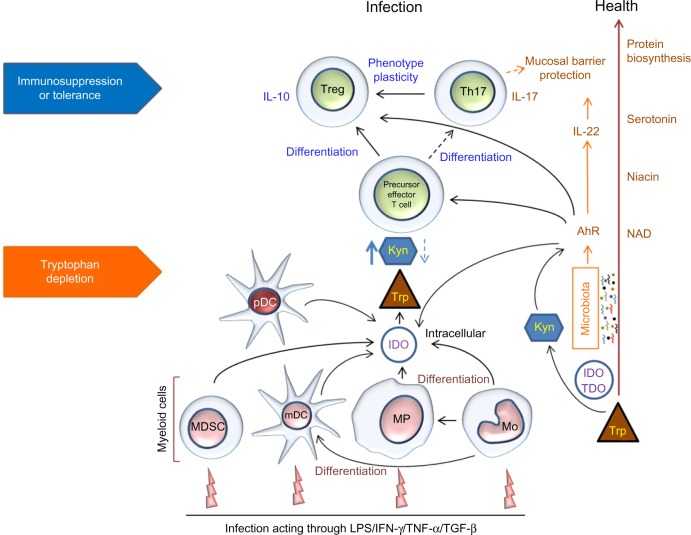
Recent studies on gut microbiota have found an important link between Trp metabolism and the mucosal/barrier interphase via microbial/toxin sensor AhR, a ligand-activated cytosolic transcription factor.13,30,31 AhR was found to create a positive feedback loop with IDO and Kyn to maintain a state of immune tolerance between commensal microbiota and the host.13,32 Nguyen et al reported that AhR induced IDO expression in DCs and that the expression of AhR was enhanced by stimulating the DCs with bacterial lipopolysaccharides (LPS).33 AhR contributes to immune homeostasis by having an antimicrobial role through induction of interleukin-22 (IL-22) transcription, and an anti-inflammatory role through mediating IDO-dependent differentiation of Tregs.14 IDO can also be induced by IFN-γ in response to Toll-like receptor (TLR) and/or caspase inflammatory signals. Furthermore, IDO is the rate-limiting enzyme of the KP producing several metabolites, which are also AhR ligands.34 Kyn is one such catabolite that regulates immune homeostasis by acting as an AhR ligand, allowing for the generation of immunosuppressive Tregs (Fig. 2).35,36
Figure 2.
Schematic representation of Trp metabolism and immune cell interactions in health and infection.
Trp Catabolism in HIV Infection: Dealing with a Dangerous Enemy
CD4 T-cell depletion and chronic immune activation are hallmarks of HIV infection. Persistent immune activation despite suppressive antiretroviral therapy (ART) is associated with an increased risk of AIDS and non-AIDS related events, including cardiovascular, liver and kidney diseases, cancers, and alteration of neurocognition.37 HIV is additionally capable of altering the gastrointestinal environment leading to changes in gut microbiota and mucosal permeability, which results in microbial translocation contributing to systemic immune activation.38 We and others have identified several factors implicated in HIV immune dysfunction, including programmed death-1 (PD-1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4),39–41 and, more recently, Trp catabolites.27,42–45 Trp degrading bacteria present in the intestinal flora have been associated with the dysfunction of gut mucosal CD4 Th17/Th22 cells, leading to the creation of a systemic KP activation cycle. This self-sustaining loop between microbiota and IDO-expressing myeloid cells has harmful effects on disease progression and neurocognitive impairment in HIV-infected patients.22,24
In 1998, Huengsberg et al first reported an increased IDO activity, which was measured by the elevation of KT ratio in HIV-infected patients vs healthy subjects, thus suggesting the link between increased KP activity and HIV immune dysfunction.9 Microbial products and types I and II interferons (IFNs) induce IDO-1 in the context of HIV infection. Two Trp metabolites, Kyn and quinolinic acid (Quin), can be detected in the cerebrospinal fluid (CSF) of HIV-infected patients and are correlated with the severity of HIV-associated neurocognitive disorder (HAND) and infection of myeloid-derived cells in the brain.22,46 Drewes et al recently demonstrated that Quin and Trp ratios are capable of predicting neurological disease in the CSF of SIV-infected macaques even under effective ART.47 In addition, HIV proteins Tat, Nef, and gp41 have been reported to directly activate the KP through the production of neurotoxic Quin in macrophages.48 A dose-dependent elevation of KT ratio in patients has been associated with severity of depression, and ART can partially revert this elevation leading to improvement in neurocognition.49
Several studies of Austrian, Ugandan, and Chinese cohorts of HIV-infected patients showed that 6–12 months of successful ART can reduce KT ratios by two folds,44,50,51 while our group has shown a complete recovery after a decade of successful ART.27 In addition, we also showed that patients treated in their early phase of HIV infection, normalized KT ratio in <12 months of therapy. Importantly, the KT ratio was significantly associated with other soluble and cellular markers such as IL-6, IP10, IL-18, and tumor necrosis factor alpha (TNF-α and CD8+ T-cell activation even during the very early phase of HIV infection.42 Collectively, these findings indicate that the KT ratio represents an independent marker of disease progression linked to CD4 T-cell counts, level of T-cell activation, inflammatory markers, and viral load.27,42,44
Trp Metabolism in Viral Hepatitis: Damaging the Firewall for Immune Activation
IDO induction in chronic viral infections is considered to be the main cause of the decreased serum Trp levels. Cozzi et al studied patients chronically infected by HCV or hepatitis B virus (HBV) who were found to have lower serum Trp concentrations than healthy volunteers.45 Furthermore, Comai et al confirmed the decrease of Trp in HCV-infected patients as well as a decline of serotonin pathway, contributing to the development of depressive symptoms in HCV patients undergoing IFN-α therapy.52 Using primary human hepatocytes, Lepiller et al showed that HCV infection stimulates IDO expression and concurred with the expression of types I and III IFNs and IFN-stimulated genes.53 These study findings showed that HCV infection directly induced IDO and IFN expression.
Like in HIV, ineffective cytotoxic T-lymphocyte (CTL) responses have been reported in chronic HBV and HCV infections.54–56 However, in vitro, IDO activity, when induced by IFN-γ, was not found to modify HCV replication in Huh7 cells, which are a hepatocellular carcinoma cell line.54 It was speculated that IDO activity suppresses an overactive immune response triggered by TNF-α-producing NK-cells and macrophages infiltrating the liver. Following the concept of reestablishing immunocompetent CTLs, wild-type and IDO knockout (KO) mice were immunized with a combination of α-GalCer and HBsAg.55 IDO KO mice showed an increased expression of IL-2 and IL-12 after immunization, leading to the induction of HBsAg-specific CTLs. An increase in the number of IDO-expressing CD11b+ Ly6G+ myeloid-derived suppressor cells was observed postimmunization in spleen, which was associated with suppression of CTL. Another study determined the role of IFN-induced genes in vitro and identified IDO as the major mediator of the IFN-γ-induced antiviral response in HBV infection.57
High plasma KT ratios have been reported in association with increased IDO expression in hepatocytes and DCs infected with HBV and HCV.56,58 Higashitani et al demonstrated that Kyn levels correlated with advanced liver conditions such as fibrosis.58 Systemic effects resulting from induction of KP have been reported in monocytes isolated from PBMCs obtained from HCV-positive patients. When activated with LPS or INF-γ, these cells were shown to differentiate into IDO-expressing DCs capable of a more potent Treg induction (Fig. 2).58 We also recently reported that in ART-treated HIV/HCV coinfected patients, elevated plasma levels of KT ratio were present only for those presenting with liver fibrosis.59 The liver is now considered to serve as a “firewall” to filter gut microbial products, such as LPS that egress to systemic vascular circuits in patients with fibrosis.60,61 The phagocytic Kupffer and stellate cells are activated via an exaggerated LPS/TLR-4 interaction and in turn induce the KP.60,62 Fibrosis can be linked with a major dysfunction of the liver TLR-4-AhR-TDO-IDO microbial model, contributing to the breakdown of endotoxin and disease tolerance defense.
The Pitfalls of Trp Degradation in Herpesviridae Infections
Herpes viruses
Human herpes simplex virus type 1 (HSV-1) and HSV-2 are members of the Herpesviridae family, which establish latency in neural ganglia. HSV-2 is the primary cause of genital herpes lesions and establishes a lifelong latent infection in the neurons of the sacral ganglia, which can be reactivated depending on the host immune response.63,64 IFN-γ production remains a key element of defense against HSV infection, capable of inhibiting virus replication.65 Adams et al demonstrated using HeLa and astrocytoma cell lines that IFN-γ-induced IDO activity acts as a potent antiviral effector mechanism against HSV-2 infection. They further reported that excess Trp is capable of abrogating the antiviral effect of IFN-γ.6 In a mouse model of HSV infection, increased activity of IDO and Kyn hydroxylase were reported.66 Both of these enzymes are required for the formation of the neurotoxin Quin.
Cytomegalo virus
Human infection with CMV, another member of the Herpesviridae family, also persists for life by counteracting IFN-mediated antiviral defense.67–69 CMV infection remains latent within the body and can be reactivated by severe immunosuppressive states like HIV infection, cancers, and following an organ transplant. Bodaghi et al revealed that IFN-γ-induced IDO activity inhibited the replication of CMV in human retinal pigment epithelial cells and that supplementation of Trp blocked the antiviral effect.70,71 Additionally, IDO was proposed to represent the prime effector restricting CMV growth in cells downstream from IFN-γ induction.70 The IFN-γ-dependent iNOS pathway was reported as being blocked by CMV infection, further strengthening the belief that a selective IFN-γ induction of IDO is modified by CMV. An increase in IDO activity in vivo has been described during infection, as well as in patients receiving IFN-γ therapy.72 However, it has also been reported that IDO induction in vivo results in an inhibition of T-cell activation and proliferation.35 Given that T-cells are the main producers of IFN-γ and that their activation is necessary to maintain defense against viruses, IDO activity would be expected to have a negative effect on the activation of an antiviral defense. However, a recent report indicated that CMV infection itself might induce IDO expression through an IFN-γ like transcriptional response mediated by the viral immediate early 1/pp72 protein.73
Zimmermann et al have recently demonstrated that CMV rigorously controls the IFN-γ-dependent induction of IDO at the level of IDO mRNA transcription in epithelial cells and fibroblasts.67 CMV infection abrogated IDO-mediated immunosuppressive properties of human fibroblasts in coculture with activated T-cells.74 In addition, Sadeghi et al investigated the clinical relevance of plasma Trp and its metabolites (Kyn and Quin) in kidney transplant recipients with CMV or polyomavirus BK (BKV) infection.23 Both Kyn and Quin levels were increased in CMV infection and associated with the severity of infection, highlighting their role as biomarkers for disease progression. Human mesenchymal stromal cells (MSCs) have potential as a novel cellular immunosuppressant to control steroid-refractory acute graft versus host disease (GvHD) because of their increased IDO activity that would lead to immunosuppressive and antimicrobial effects. However, Meisel et al recently reported that CMV is a major negative regulator of IDO activity in human MSCs, and therefore undermines the clinical efficacy of MSC treatment in stem cell transplant recipients.75
Epstein–Barr virus
Infectious mononucleosis is the most common clinical manifestation of infection with Epstein–Barr virus (EBV), another widely spread herpesvirus family member that is also associated with malignancies such as Burkitt’s lymphoma and nasopharyngeal carcinoma in human beings.76 EBV is known for its epithelial and B-cell tropism and also infects monocytes/macrophages, intraepithelial macrophages, and Langerhans cells. EBV-infected monocytes demonstrate a suppression of phagocytic activity and potent antiviral activity,77 further leading to apoptosis and an inhibition of their differentiation into DCs.78 Song et al reported a role for EBV infection in the modulation of Trp metabolism through increased expression of IDO in B-cells, translating into decreased NK-cell cytotoxicity.79 Liu et al found that macrophages in tumor stroma express significantly higher amounts of IDO in comparison to tumor cells induced by infection with EBV.80 They also showed that EBV-induced IDO expression of macrophages suppressed T-cell proliferation, impaired the cytotoxic activity of CD8 T-cells, and was dependent on TNF-α and IL-6 secretion. IDO induction during chronic active EBV infection is also associated with decreased serotonin levels leading to symptoms, including mood disturbances.52 All these observations point to the contribution of the KP on disease tolerance and may have a major impact in HIV-infected patients or transplant recipients who have concomitant chronic viral infections in the context of severe immunosuppression.
Therapeutic Options for Modulation of the KP to Improve Patient Outcomes
Interventions to normalize KP should include direct IDO/TDO inhibitors as well as modulation of factors contributing to its induction such as gut microbiota composition and gut epithelial damage. A competitive inhibitor of IDO, 1-methyl-tryptophan (1-MT), induced transitory neurological protection after LPS challenge in CX3CL1−/− mice.81 In another mouse model, 1-MT was able to reduce by 90% the number of HIV-infected macrophages in the brain.82 However, disappointing results were reported with 1-MT used in SIV-infected rhesus macaques on ART.83,84 New IDO inhibitors are under development as anticancer agents, and some are currently under assessment in clinical trials, including INCB024360 and indoximod (D-1-methyl-tryptophan), combined with chemotherapy.85–87 Another emerging area is the combination of immune therapies involving the simultaneous blockade of PD-1, CTLA-4 nonredundant pathways, and IDO expression for myeloid/T-cell interactions that may significantly revert the immune system, as has been reported in mouse cancer models.88 In cancer patients, a recent study reported IDO-specific T-cells to influence adaptive immune reactions, while vaccination with IDO-derived epitope in a phase I clinical trial showed long-lasting disease stabilization without toxicity.89,90 An alternative Trp-degrading enzyme TDO, which is not inhibited by 1-MT and is mainly expressed in the liver or brain, requires a specific inhibitor to normalize KP activity. TDO inhibition research is just starting and is limited to mouse cancer animal models.91 These studies will result in novel therapeutic options for treating patients with chronic viral infections because of multiple similarities between cancer and infection as both induce immune activation and inflammation.92
The general findings indicate that the Trp catabolic pathway is an important link between microbiome and systemic immune activation, which further worsens in chronic viral infections. The microbiota can also influence the immune system by stimulating the AhR through Trp catabolites.
A recent study on a mouse colitis model showed 6-formylindolo-(3,2-b)-carbazole (Ficz), which is a Trp catab-olite and an AhR ligand, to suppress epithelial IL-7 secretion improving the gut inflammation.93 Ficz was also associated with a decrease in the percentage of activated CD4 and CD8 T-cells. These findings suggest AhR inhibitors as promising therapeutic interventions to be considered in a variety of conditions, including chronic viral infections.
Natural products such as curcumin, green tea, resveratrol, and rosemary have been found to downregulate IDO expression via JAK/STAT kinase pathways.94–97 Interestingly, Gostner et al98 recently reported a dose-dependent suppression of Trp breakdown by extracts of coffee and decaffeinated coffee in PBMCs stimulated with mitogen.98 Translational research, bridging fundamental research with clinical investigations in the field of Infectious Diseases, Oncology, and Inflammation, will be needed to provide effective KP-based therapy.
Conclusion
Trp starvation plays a limited role in mechanisms of host resistance to viral infection when compared to its more extensive protective contribution in certain bacterial or parasitic infections.99 However, in the absence of viral clearance, IDO activation could pose an acceptable compromise for the host to prevent overwhelming tissue destruction at the expense of inhibition of antiviral T-cell responses and expansion of Tregs. IDO overexpression by antigen-presenting cells contributes to withdrawal of the virus from immune surveillance, leading to disease tolerance. The studies outlined herein indicate the important role of Trp metabolism via IDO/AhR in the host response to chronic viral infection, and its major role in infections such as HIV, HBV, and HCV where systemic immune activation is most elevated. The identification of the “environmental/microbial” sensor AhR, that induces IDO expression,33 represents an important finding, which may pave the way for targeted therapeutic interventions. New directions include further examination into Trp immune-metabolic pathway inhibitors, as well as the possibility of combination therapy with nonredundant immune checkpoint inhibitors such as those targeting the PD-1, TIM-3, and CTLA4 pathways.87,92 Such immunological approach in chronic viral infections using immune check point inhibitors and/or IL-7 may result in different toxicities as compared to cancer patients.7,100 Future studies on Trp metabolism will be fruitful for enhancing vaccine responses and designing therapeutic approaches to prevent and potentially cure chronic viral infections as well as other debilitating conditions such as autoimmune disorders and cancer.
Acknowledgments
The authors acknowledge Angie Massicotte for coordination and assistance and Kishanda Vyboh and Alexandra Averback for a critical reading of the manuscript.
Abbreviations
- Trp
tryptophan
- KP
kynurenine pathway
- IDO
indoleamine-2,3-dioxygenase
- IFN-γ
interferon gamma
- AhR
aryl hydrocarbon receptor
- HIV
human immunodeficiency virus
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- CMV
cytomegalovirus
- HSV
herpes simplex virus
- KAT
kynurenine transaminase
- NAD
nicotinamide adenine dinucleotide
- TDO
tryptophan-2,3-dioxygenase
- Kyn
kynurenine
- DCs
dendritic cells
- TLR
Toll-like receptor
- ART
antiretroviral therapy
- PD-1
programmed death-1
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- Quin
Quinolinic acid
- HAND
HIV-associated neurocognitive disorder
- TNF-α
tumor necrosis factor alpha
- EBV
Epstein-Barr virus
Footnotes
ACADEMIC EDITOR: Gilles Guillemin, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 991 words, excluding any confidential comments to the academic editor.
FUNDING: This study was supported by the Canadian Institutes of Health Research (grant MOP #103230 and CTN #257), Fonds de la Recherche Québec-Santé (FRQ-S): Thérapiecellulaire, Réseau SIDA/Maladies infectieuses, Québec, Canada, and by The Canadian HIV Cure Enterprise Team Grant HIG-133050 (JPR) from the CIHR in partnership with CANFAR and IAS. Vikram Mehraj is supported by FRQ-S postdoctoral fellowship award. Dr Jean-Pierre Routy is the holder of Louis Lowenstein Chair in Hematology and Oncology, McGill University. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the review: VM, JPR. Wrote the first draft of the manuscript: VM. Contributed to the writing of the manuscript: VM, JPR. Jointly developed the structure and arguments for the paper: VM, JPR. Made critical revisions and approved the final version: JPR. Both the authors reviewed and approved the final manuscript.
REFERENCES
- 1.Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003;81(4):247–65. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- 2.Thackray SJ, Mowat CG, Chapman SK. Exploring the mechanism of trypto-phan 2,3-dioxygenase. Biochem Soc Trans. 2008;36(pt 6):1120–3. doi: 10.1042/BST0361120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlin JM, Borden EC, Byrne GI. Interferon-induced indoleamine 2,3-dioxygenase activity inhibits Chlamydia psittacireplication in human macrophages. J Interferon Res. 1989;9(3):329–7. doi: 10.1089/jir.1989.9.329. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz JL, Carlin JM, Borden EC, Byrne GI. Beta interferon inhibits Toxoplasma gondiigrowth in human monocyte-derived macrophages. Infect Immun. 1989;57(10):3254–6. doi: 10.1128/iai.57.10.3254-3256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt SV, Schultze JL. New insights into IDO biology in bacterial and viral infections. Front Immunol. 2014;5:384. doi: 10.3389/fimmu.2014.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams O, Besken K, Oberdorfer C, MacKenzie CR, Russing D, Daubener W. Inhibition of human herpes simplex virus type 2 by interferon gamma and tumor necrosis factor alpha is mediated by indoleamine 2,3-dioxygenase. Microbes Infect. 2004;6(9):806–12. doi: 10.1016/j.micinf.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Vaccari M, Boasso A, Fenizia C, et al. Fatal pancreatitis in simian immunodeficiency virus SIV(mac251)-infected macaques treated with 2’,3’-dideoxyinos-ine and stavudine following cytotoxic-T-lymphocyte-associated antigen 4 and indoleamine 2,3-dioxygenase blockade. J Virol. 2012;86(1):108–3. doi: 10.1128/JVI.05609-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werner ER, Bitterlich G, Fuchs D, et al. Human macrophages degrade tryptophan upon induction by interferon-gamma. Life Sci. 1987;41(3):273–80. doi: 10.1016/0024-3205(87)90149-4. [DOI] [PubMed] [Google Scholar]
- 9.Huengsberg M, Winer JB, Gompels M, Round R, Ross J, Shahmanesh M. Serum kynurenine-to-tryptophan ratio increases with progressive disease in HIV-infected patients. Clin Chem. 1998;44(4):858–62. [PubMed] [Google Scholar]
- 10.Xu H, Zhang GX, Ciric B, Rostami A. IDO: a double-edged sword for T(H)1/T(H)2 regulation. Immunol Lett. 2008;121(1):1–6. doi: 10.1016/j.imlet.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwidzinski E, Bechmann I. IDO expression in the brain: a double-edged sword. J Mol Med (Berl) 2007;85(12):1351–9. doi: 10.1007/s00109-007-0229-7. [DOI] [PubMed] [Google Scholar]
- 12.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34(3):137–43. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romani L, Zelante T, De Luca A, et al. Microbiota control of a tryptophan-AhR pathway in disease tolerance to fungi. Eur J Immunol. 2014;44(11):3192–200. doi: 10.1002/eji.201344406. [DOI] [PubMed] [Google Scholar]
- 14.Zelante T, Iannitti RG, Fallarino F, et al. Tryptophan feeding of the IDO1-AhR axis in host-microbial symbiosis. Front Immunol. 2014;5:640. doi: 10.3389/fimmu.2014.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacKenzie CR, Worku D, Daubener W. Regulation of IDO-mediated bacteriostasis in macrophages: role of antibiotics and anti-inflammatory agents. Adv Exp Med Biol. 2003;527:67–76. doi: 10.1007/978-1-4615-0135-0_7. [DOI] [PubMed] [Google Scholar]
- 16.Däubener W, Spors B, Hucke C, et al. Restriction of Toxoplasma gondiigrowth in human brain microvascular endothelial cells by activation of indoleamine 2,3-dioxygenase. Infect Immun. 2001;69(10):6527–31. doi: 10.1128/IAI.69.10.6527-6531.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obojes K, Andres O, Kim KS, Daubener W, Schneider-Schaulies J. Indoleamine 2,3-dioxygenase mediates cell type-specific anti-measles virus activity of gamma interferon. J Virol. 2005;79(12):7768–6. doi: 10.1128/JVI.79.12.7768-7776.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams O, Besken K, Oberdorfer C, MacKenzie CR, Takikawa O, Daubener W. Role of indoleamine-2,3-dioxygenase in alpha/beta and gamma interferon-mediated antiviral effects against herpes simplex virus infections. J Virol. 2004;78(5):2632–6. doi: 10.1128/JVI.78.5.2632-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terajima M, Leporati AM. Role of indoleamine 2,3-dioxygenase in antiviral activity of interferon-gamma against vaccinia virus. Viral Immunol. 2005;18(4):722–9. doi: 10.1089/vim.2005.18.722. [DOI] [PubMed] [Google Scholar]
- 20.Prendergast GC, Smith C, Thomas S, et al. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014;63(7):721–35. doi: 10.1007/s00262-014-1549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abumaree MH, Chamley LW, Badri M, El-Muzaini MF. Trophoblast debris modulates the expression of immune proteins in macrophages: a key to maternal tolerance of the fetal allograft? J Reprod Immunol. 2012;94(2):131–41. doi: 10.1016/j.jri.2012.03.488. [DOI] [PubMed] [Google Scholar]
- 22.Kandanearatchi A, Brew BJ. The kynurenine pathway and quinolinic acid: pivotal roles in HIV associated neurocognitive disorders. FEBS J. 2012;279(8):1366–74. doi: 10.1111/j.1742-4658.2012.08500.x. [DOI] [PubMed] [Google Scholar]
- 23.Sadeghi M, Lahdou I, Daniel V, et al. Strong association of phenylalanine and tryptophan metabolites with activated cytomegalovirus infection in kidney transplant recipients. Hum Immunol. 2012;73(2):186–92. doi: 10.1016/j.humimm.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Davies NW, Guillemin G, Brew BJ. Tryptophan, neurodegeneration and HIV-associated neurocognitive disorder. Int J Tryptophan Res. 2010;3:121–40. doi: 10.4137/ijtr.s4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillemin GJ, Kerr SJ, Brew BJ. Involvement of quinolinic acid in AIDS dementia complex. Neurotox Res. 2005;7(1–2):103–23. doi: 10.1007/BF03033781. [DOI] [PubMed] [Google Scholar]
- 26.Agudelo LZ, Femenía T, Orhan F, et al. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159(1):33–45. doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 27.Jenabian MA, Patel M, Kema I, et al. Distinct tryptophan catabolism and Th17/Treg balance in HIV progressors and elite controllers. PLoS One. 2013;8(10):e78146. doi: 10.1371/journal.pone.0078146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Favre D, Mold J, Hunt PW, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2(32):32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asghar K, Ashiq MT, Zulfiqar B, Mahroo A, Nasir K, Murad S. Indoleamine 2,3-dioxygenase expression and activity in patients with hepatitis C virus-induced liver cirrhosis. Exp Ther Med. 2015;9(3):901–4. doi: 10.3892/etm.2014.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–85. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Noakes R. The aryl hydrocarbon receptor: a review of its role in the physiology and pathology of the integument and its relationship to the tryptophan metabolism. Int J Tryptophan Res. 2015;8:7–18. doi: 10.4137/IJTR.S19985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bessede A, Gargaro M, Pallotta MT, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511(7508):184–90. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen NT, Kimura A, Nakahama T, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A. 2010;107(46):19961–6. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soliman H, Mediavilla-Varela M, Antonia S. Indoleamine 2,3-dioxygenase: is it an immune suppressor? Cancer J. 2010;16(4):354–9. doi: 10.1097/PPO.0b013e3181eb3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189(9):1363–72. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185(6):3190–8. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210(8):1248–59. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunt PW, Sinclair E, Rodriguez B, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis. 2014;210(8):1228–38. doi: 10.1093/infdis/jiu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12(10):1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 40.Kaufmann DE, Kavanagh DG, Pereyra F, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8(11):1246–54. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 41.Mehraj V, Jenabian MA, Vyboh K, Routy JP. Immune suppression by myeloid cells in HIV infection: new targets for immunotherapy. Open AIDS J. 2014;8:66–78. doi: 10.2174/1874613601408010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenabian MA, El-Far M, Vyboh K. For the Montreal Primary infection and Slow Progressor Study Groups. Immunosuppressive tryptophan catabolism and gut mucosal dysfunction following early HIV infection. J Infect Dis. 2015 Aug 1;212(3):355–66. doi: 10.1093/infdis/jiv037. [DOI] [PubMed] [Google Scholar]
- 43.Serrano-Villar S, Sainz T, Lee SA, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014;10(5):e1004078. doi: 10.1371/journal.ppat.1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Byakwaga H, Boum Y, II, Huang Y, et al. The kynurenine pathway of tryptophan catabolism, CD4+ T-cell recovery, and mortality among HIV-infected Ugandans initiating antiretroviral therapy. J Infect Dis. 2014;210(3):383–91. doi: 10.1093/infdis/jiu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cozzi A, Zignego AL, Carpendo R, et al. Low serum tryptophan levels, reduced macrophage IDO activity and high frequency of psychopathology in HCV patients. J Viral Hepat. 2006;13(6):402–8. doi: 10.1111/j.1365-2893.2005.00706.x. [DOI] [PubMed] [Google Scholar]
- 46.Atlas A, Gisslen M, Nordin C, Lindstrom L, Schwieler L. Acute psychotic symptoms in HIV-1 infected patients are associated with increased levels of kynurenic acid in cerebrospinal fluid. Brain Behav Immun. 2007;21(1):86–91. doi: 10.1016/j.bbi.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Drewes JL, Meulendyke KA, Liao Z, et al. Quinolinic acid/tryptophan ratios predict neurological disease in SIV-infected macaques and remain elevated in the brain under cART. J Neurovirol. 2015 doi: 10.1007/s13365-015-0334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith DG, Guillemin GJ, Pemberton L, et al. Quinolinic acid is produced by macrophages stimulated by platelet activating factor, Nef and Tat. J Neurovirol. 2001;7(1):56–60. doi: 10.1080/135502801300069692. [DOI] [PubMed] [Google Scholar]
- 49.Martinez P, Tsai AC, Muzoora C, et al. Reversal of the kynurenine pathway of tryptophan catabolism may improve depression in ART-treated HIV-infected Ugandans. J Acquir Immune Defic Syndr. 2014;65(4):456–62. doi: 10.1097/QAI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zangerle R, Widner B, Quirchmair G, Neurauter G, Sarcletti M, Fuchs D. Effective antiretroviral therapy reduces degradation of tryptophan in patients with HIV-1 infection. Clin Immunol. 2002;104(3):242–7. doi: 10.1006/clim.2002.5231. [DOI] [PubMed] [Google Scholar]
- 51.Chen J, Shao J, Cai R, et al. Anti-retroviral therapy decreases but does not normalize indoleamine 2,3-dioxygenase activity in HIV-infected patients. PLoS One. 2014;9(7):e100446. doi: 10.1371/journal.pone.0100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Comai S, Cavalletto L, Chemello L, et al. Effects of PEG-interferon alpha plus ribavirin on tryptophan metabolism in patients with chronic hepatitis C. Pharmacol Res. 2011;63(1):85–92. doi: 10.1016/j.phrs.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 53.Lepiller Q, Soulier E, Li Q, et al. Antiviral and immunoregulatory effects of indoleamine-2,3-dioxygenase in hepatitis C virus infection. J Innate Immun. 2015 doi: 10.1159/000375161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Larrea E, Riezu-Boj JI, Gil-Guerrero L, et al. Upregulation of indoleamine 2,3-dioxygenase in hepatitis C virus infection. J Virol. 2007;81(7):3662–6. doi: 10.1128/JVI.02248-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ito H, Ando T, Ando K, et al. Induction of hepatitis B virus surface antigen-specific cytotoxic T lymphocytes can be up-regulated by the inhibition of indoleamine 2, 3-dioxygenase activity. Immunology. 2014;142(4):614–23. doi: 10.1111/imm.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iwamoto N, Ito H, Ando K, et al. Upregulation of indoleamine 2,3-dioxygenase in hepatocyte during acute hepatitis caused by hepatitis B virus-specific cytotoxic T lymphocytes in vivo. Liver Int. 2009;29(2):277–83. doi: 10.1111/j.1478-3231.2008.01748.x. [DOI] [PubMed] [Google Scholar]
- 57.Mao R, Zhang J, Jiang D, et al. Indoleamine 2,3-dioxygenase mediates the antiviral effect of gamma interferon against hepatitis B virus in human hepatocyte-derived cells. J Virol. 2011;85(2):1048–57. doi: 10.1128/JVI.01998-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Higashitani K, Kanto T, Kuroda S, et al. Association of enhanced activity of indoleamine 2,3-dioxygenase in dendritic cells with the induction of regulatory T cells in chronic hepatitis C infection. J Gastroenterol. 2013;48(5):660–70. doi: 10.1007/s00535-012-0667-z. [DOI] [PubMed] [Google Scholar]
- 59.Jenabian M-A, Kema I, Ramirez R, et al. Liver fibrosis is strongly associated with an enhanced level of immunosuppressive tryptophan catabolism independently of HCV viremia in ART-treated HIV/HCV co-infected patients. BMC Infect Dis. 2014;14(suppl 2):O16. [Google Scholar]
- 60.Vyboh K, Jenabian MA, Mehraj V, Routy JP. HIV and the gut microbiota, partners in crime: breaking the vicious cycle to unearth new therapeutic targets. J Immunol Res. 2015;2015:614127. doi: 10.1155/2015/614127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balmer ML, Slack E, de Gottardi A, et al. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med. 2014;6(237):237ra266. doi: 10.1126/scitranslmed.3008618. [DOI] [PubMed] [Google Scholar]
- 62.Yan ML, Wang YD, Tian YF, Lai ZD, Yan LN. Inhibition of allogeneic T-cell response by Kupffer cells expressing indoleamine 2,3-dioxygenase. World J Gastroenterol. 2010;16(5):636–40. doi: 10.3748/wjg.v16.i5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rinaldo CR, Jr, Torpey DJ., III Cell-mediated immunity and immunosuppression in herpes simplex virus infection. Immunodeficiency. 1993;5(1):33–90. [PubMed] [Google Scholar]
- 64.Becker Y. Herpes simplex virus evolved to use the human defense mechanisms to establish a lifelong infection in neurons – a review and hypothesis. Virus Genes. 2002;24(2):187–96. doi: 10.1023/a:1014532919088. [DOI] [PubMed] [Google Scholar]
- 65.Katze MG, He Y, Gale M., Jr Viruses and interferon: a fight for supremacy. Nat Rev Immunol. 2002;2(9):675–87. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 66.Reinhard JF., Jr Altered tryptophan metabolism in mice with herpes simplex virus encephalitis: increases in spinal cord quinolinic acid. Neurochem Res. 1998;23(5):661–5. doi: 10.1023/a:1022438822023. [DOI] [PubMed] [Google Scholar]
- 67.Zimmermann A, Hauka S, Maywald M, et al. Checks and balances between human cytomegalovirus replication and indoleamine-2,3-dioxygenase. J Gen Virol. 2014;95(pt 3):659–70. doi: 10.1099/vir.0.061994-0. [DOI] [PubMed] [Google Scholar]
- 68.Marshall EE, Geballe AP. Multifaceted evasion of the interferon response by cytomegalovirus. J Interferon Cytokine Res. 2009;29(9):609–19. doi: 10.1089/jir.2009.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trilling M, Le VT, Hengel H. Interplay between CMVs and interferon signaling: implications for pathogenesis and therapeutic intervention. Future Microbiol. 2012;7(11):1269–82. doi: 10.2217/fmb.12.109. [DOI] [PubMed] [Google Scholar]
- 70.Bodaghi B, Goureau O, Zipeto D, Laurent L, Virelizier JL, Michelson S. Role of IFN-gamma-induced indoleamine 2,3 dioxygenase and inducible nitric oxide synthase in the replication of human cytomegalovirus in retinal pigment epithelial cells. J Immunol. 1999;162(2):957–64. [PubMed] [Google Scholar]
- 71.Bodaghi B, Slobbe-van Drunen ME, Topilko A, et al. Entry of human cytomegalovirus into retinal pigment epithelial and endothelial cells by endocytosis. Invest Ophthalmol Vis Sci. 1999;40(11):2598–607. [PubMed] [Google Scholar]
- 72.Byrne GI, Lehmann LK, Kirschbaum JG, Borden EC, Lee CM, Brown RR. Induction of tryptophan degradation in vitro and in vivo: a gamma-interferon-stimulated activity. J Interferon Res. 1986;6(4):389–96. doi: 10.1089/jir.1986.6.389. [DOI] [PubMed] [Google Scholar]
- 73.Knoblach T, Grandel B, Seiler J, Nevels M, Paulus C. Human cytomegalovirus IE1 protein elicits a type II interferon-like host cell response that depends on activated STAT1 but not interferon-gamma. PLoS Pathog. 2011;7(4):e1002016. doi: 10.1371/journal.ppat.1002016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heseler K, Schmidt SK, Spekker K, et al. Cytomegalovirus impairs the induction of indoleamine 2,3-dioxygenase mediated antimicrobial and immunoregu-latory effects in human fibroblasts. PLoS One. 2013;8(5):e64442. doi: 10.1371/journal.pone.0064442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meisel R, Heseler K, Nau J, et al. Cytomegalovirus infection impairs immuno-suppressive and antimicrobial effector functions of human multipotent mesenchymal stromal cells. Mediators Inflamm. 2014;2014:898630. doi: 10.1155/2014/898630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tao Q, Young LS, Woodman CB, Murray PG. Epstein-Barr virus (EBV) and its associated human cancers – genetics, epigenetics, pathobiology and novel therapeutics. Front Biosci. 2006;11:2672–713. doi: 10.2741/2000. [DOI] [PubMed] [Google Scholar]
- 77.Savard M, Belanger C, Tardif M, Gourde P, Flamand L, Gosselin J. Infection of primary human monocytes by Epstein-Barr virus. J Virol. 2000;74(6):2612–9. doi: 10.1128/jvi.74.6.2612-2619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li L, Liu D, Hutt-Fletcher L, Morgan A, Masucci MG, Levitsky V. Epstein-Barr virus inhibits the development of dendritic cells by promoting apoptosis of their monocyte precursors in the presence of granulocyte macrophage-colony-stimulating factor and interleukin-4. Blood. 2002;99(10):3725–34. doi: 10.1182/blood.v99.10.3725. [DOI] [PubMed] [Google Scholar]
- 79.Song H, Park H, Kim J, et al. IDO metabolite produced by EBV-transformed B cells inhibits surface expression of NKG2D in NK cells via the c-Jun N-terminal kinase (JNK) pathway. Immunol Lett. 2011;136(2):187–93. doi: 10.1016/j.imlet.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 80.Liu WL, Lin YH, Xiao H, et al. Epstein-Barr virus infection induces indoleamine 2,3-dioxygenase expression in human monocyte-derived macrophages through p38/mitogen-activated protein kinase and NF-kappaB pathways: impairment in T cell functions. J Virol. 2014;88(12):6660–71. doi: 10.1128/JVI.03678-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Corona AW, Norden DM, Skendelas JP, et al. Indoleamine 2,3-dioxygenase inhibition attenuates lipopolysaccharide induced persistent microglial activation and depressive-like complications in fractalkine receptor (CX(3)CR1)-deficient mice. Brain Behav Immun. 2013;31:134–42. doi: 10.1016/j.bbi.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Potula R, Poluektova L, Knipe B, et al. Inhibition of indoleamine 2,3-dioxygenase (IDO) enhances elimination of virus-infected macrophages in an animal model of HIV-1 encephalitis. Blood. 2005;106(7):2382–90. doi: 10.1182/blood-2005-04-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boasso A, Vaccari M, Fuchs D, et al. Combined effect of antiretroviral therapy and blockade of IDO in SIV-infected rhesus macaques. J Immunol. 2009;182(7):4313–20. doi: 10.4049/jimmunol.0803314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dunham RM, Gordon SN, Vaccari M, et al. Preclinical evaluation of HIV eradication strategies in the simian immunodeficiency virus-infected rhesus macaque: a pilot study testing inhibition of indoleamine 2,3-dioxygenase. AIDS Res Hum Retroviruses. 2013;29(2):207–14. doi: 10.1089/aid.2012.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Munn DH. Blocking IDO activity to enhance anti-tumor immunity. Front Biosci (Elite Ed) 2012;4:734–45. doi: 10.2741/e414. [DOI] [PubMed] [Google Scholar]
- 86.Soliman HH, Jackson E, Neuger T, et al. A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients with metastatic solid tumors. Oncotarget. 2014;5(18):8136–46. doi: 10.18632/oncotarget.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vacchelli E, Aranda F, Eggermont A, et al. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2014;3(10):e957994. doi: 10.4161/21624011.2014.957994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20(20):5290–301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andersen MH, Svane IM. Indoleamine 2,3-dioxygenase vaccination. Oncoimmunology. 2015;4(1):e983770. doi: 10.4161/2162402X.2014.983770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iversen TZ, Engell-Noerregaard L, Ellebaek E, et al. Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase. Clin Cancer Res. 2014;20(1):221–32. doi: 10.1158/1078-0432.CCR-13-1560. [DOI] [PubMed] [Google Scholar]
- 91.Pilotte L, Larrieu P, Stroobant V, et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci USA. 2012;109(7):2497–502. doi: 10.1073/pnas.1113873109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hotchkiss RS, Moldawer LL. Parallels between cancer and infectious disease. N Engl J Med. 2014;371(4):380–3. doi: 10.1056/NEJMcibr1404664. [DOI] [PubMed] [Google Scholar]
- 93.Ji T, Xu C, Sun L, et al. Aryl Hydrocarbon Receptor Activation Down-Regulates IL-7 and Reduces Inflammation in a Mouse Model of DSS-Induced Colitis. Dig Dis Sci. 2015 Jul;60(7):1958–66. doi: 10.1007/s10620-015-3632-x. [DOI] [PubMed] [Google Scholar]
- 94.Jeong YI, Kim SW, Jung ID, et al. Curcumin suppresses the induction of indoleamine 2,3-dioxygenase by blocking the Janus-activated kinase-protein kinase Cdelta-STAT1 signaling pathway in interferon-gamma-stimulated murine dendritic cells. J Biol Chem. 2009;284(6):3700–8. doi: 10.1074/jbc.M807328200. [DOI] [PubMed] [Google Scholar]
- 95.Cheng CW, Shieh PC, Lin YC, et al. Indoleamine 2,3-dioxygenase, an immunomodulatory protein, is suppressed by (-)-epigallocatechin-3-gallate via blocking of gamma-interferon-induced JAK-PKC-delta-STAT1 signaling in human oral cancer cells. J Agric Food Chem. 2010;58(2):887–94. doi: 10.1021/jf903377e. [DOI] [PubMed] [Google Scholar]
- 96.Noh KT, Chae SH, Chun SH, Jung ID, Kang HK, Park YM. Resveratrol suppresses tumor progression via the regulation of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2013;431(2):348–53. doi: 10.1016/j.bbrc.2012.12.093. [DOI] [PubMed] [Google Scholar]
- 97.Lee HJ, Jeong YI, Lee TH, et al. Rosmarinic acid inhibits indoleamine 2,3-dioxygenase expression in murine dendritic cells. Biochem Pharmacol. 2007;73(9):1412–21. doi: 10.1016/j.bcp.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 98.Gostner JM, Schroecksnadel S, Jenny M, et al. Coffee extracts suppress tryptophan breakdown in mitogen-stimulated peripheral blood mononuclear cells. J Am Coll Nutr. 2015;34(3):21–23. doi: 10.1080/07315724.2014.907756. [DOI] [PubMed] [Google Scholar]
- 99.Zelante T, Fallarino F, Bistoni F, Puccetti P, Romani L. Indoleamine 2,3-dioxygenase in infection: the paradox of an evasive strategy that benefits the host. Microbes Infect. 2009;11(1):133–141. doi: 10.1016/j.micinf.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 100.Sereti I, Estes JD, Thompson WL, et al. Decreases in colonic and systemic inflammation in chronic HIV infection after IL-7 administration. PLoS Pathog. 2014;10(1):e1003890. doi: 10.1371/journal.ppat.1003890. [DOI] [PMC free article] [PubMed] [Google Scholar]