Abstract
HER2 overexpression and amplification of the HER2/neu gene have been found in approximately 25% of invasive breast carcinomas. They are associated with a poor prognosis and resistance to therapy in breast cancer patients. Up to now, clinical evaluation of human epidermal growth factor receptor 2 (HER2) expression is based on ex vivo methods (immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH) staining of biopsied tissue). Our goal is to realize “image and treat” paradigm using targeted fluorescent probes to evaluate expression levels of cell biomarkers responsible for cancer progression and to monitor the efficacy of corresponding monoclonal antibody treatments. We used fluorescent Affibody-based probes for in vivo analysis of HER2 receptors using near-infrared optical imaging that do not interfere with binding of the therapeutic agents to these receptors. We have analyzed two types of breast carcinoma xenografts with significant differences in HER2 expression (3+ and 2+ according to classification) in the mouse model. Using our kinetic model to analyze the temporal variations of the fluorescence intensity in the tumor area after two subsequent injections allowed us to assess quantitatively the difference in HER2 expression levels for two tumor types (BT-474 and MD-MBA-361). This result was substantiated by ELISA ex vivo assays of HER2 expression in the same tumors.
Keywords: Fluorescence imaging, Near-infrared optical imaging, Targeted fluorescent probe, Affibody, Cancer treatment, Cancer diagnostics, Human epidermal growth factor receptor
Introduction
Advances in tumor biology led to development of therapies targeting specific biomarkers of cancer cells. The well-known example of such approach is application of a monoclonal antibody (mAb) trastuzumab for treatment of human epidermal growth factor receptor 2 (HER2)-positive cancers (1-3). Elevated HER2 is associated with increased proliferation and survival of cancer cells and thereby, contributes to poor therapy outcomes (4, 5). Given that the efficacy of the therapeutic mAb depends on overexpression of its target on tumor cells, development of techniques to assess the receptor expression is extremely important for patient selection and monitoring the efficacy of therapy, allowing one to optimize the treatment. Current clinical evaluation of HER2 expression is based on immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH) staining of biopsied tissue. Both methodologies are ex vivo techniques and, due to tumor heterogeneity, may deliver false-positive or -negative results (6) and can hardly be used to sequentially monitor HER2 expression in the course of therapy. On the other hand, molecular imaging using HER2-specific probes, allows assessing the status of HER2 receptors in vivo in real time before and during the treatment. In this regard, PET imaging modality is mostly discussed in the literature as a promising tool (7, 8). Applications of optical imaging are also under consideration. Compared to PET, it is a low cost modality that does not involve ionizing radiation. Optical imaging is also easier to implement due to its minimal invasiveness. Application of fluorescently-labeled cell surface markers opens new venues for in vivo assessment of biomarkers (9-11). Recently designed Affibody molecules (Affibody AB, Solna, Sweden) with their high affinity to HER2 receptors were suggested as a basis for such HER2-specific fluorescent probes (9). Affibody molecules are highly water soluble and about 20-fold smaller than antibodies. Due to their small size, they have shorter washout times from the blood circulation. As usual, the washout time is defined as the time that the free probe ligands concentration decreases to 1/e of their initial (after injection) concentration in the circulation. In addition, it was shown (9) that this molecule binds to a different epitope of the HER2 biomarker than therapeutic mAbs (i.e., trastuzumab, pertuzumab, and T-DM1) (10). The latter property makes Affibody conjugate a good candidate to realize “image and treat paradigm”, because such imaging agent would not interfere with mAbs therapy. Patient-friendly character of NIR optical imaging allows one to collect multiple images of the tumor, monitor the status of HER2 receptors during the treatment regimen and, if needed, optimize it timely.
Temporal behavior of fluorescence after the injection of specific probe reflects binding of the fluorescent molecules to the tumor receptors and washout of the free fluorescent ligands from the blood circulation. For this reason, analysis of the time series of fluorescence images can provide quantitative information on the status of the targeted receptors in the region of interest (ROI) (12-15). It should be noted that fluorescence lifetime imaging, using HER2-specific probes can also provide information on the status of HER2 receptors in the tumor. Our recent studies (3) showed that fluorescence lifetime measurements can be used to assess the binding of the probe to cancer cells qualitatively and, thereby, characterize corresponding HER2 expression.
In pre-clinical studies (small animal model) NIR imaging, using biomarker-specific probes, can be used to test different therapies/drugs to treat various cancers. To choose proper clinical applications of the fluorescence imaging, penetration depth of NIR photons into tissue should be taken into account (currently, targets deeper than several cm are not accessible).
In our previous papers (9, 12, 15) we accommodated compartmental ligand-receptor model to assess HER2 expression, using initial normalized rate of accumulation (NRA) of the HER2-targeted fluorescent probe in the tumor area. Here we discuss an alternative kinetic model approach to characterize in vivo the expression of specific receptors, using analysis of fluorescence data after two subsequent injections of the probe into the circulation, similar to what has been done in PET studies (16, 19).
This approach is suitable for the fast-kinetic probes like monomer Affibody.
Animal Model
Tumor cells (5-10 × 106 cells in 0.1 mL of 30% Matrigel (BD Biosciences, Bedford, MA) of two breast carcinoma cell lines, BT-474 and MD-MBA-361, expressing different levels of HER2 (classified as 3+ and 2+, respectively) were implanted into the right forelimbs of 6 to 8 weeks old athymic nude mice as described before (8). Imaging studies started, when the tumors grew approximately to 5-8 mm in diameter. We considered three mice in each tumor category. After the experiment had been terminated, mice were euthanized and tumors were extracted for the assessment of the HER2 expression by ELISA. All animal studies were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Animals.
Enzyme-linked Immuno Sorbent Assay (ELISA)
Ex vivo tumor tissue was homogenized in suspension buffer supplemented with proteases inhibitor mix (Complete stop Roche) and EDTA (5 mM), followed by HER2 extraction and centrifugation. HER2 ELISA assay was performed in accordance with the protocol, provided by manufacturer (Calbiochem, Gibbstown, NJ) using serial dilution of recombinant HER2 protein as standards. The measured ELISA results for all mice are presented in Table I. HER2 concentration is expressed in ng of HER2 per mg of total protein.
Table I.
Obtained ELISA values for all six mice.
| Carcinoma | Mouse | ELISA (ng/mg protein) |
|---|---|---|
| #1a | 302.1636 | |
| BT-474 | #2a | 261.8253 |
| #3a | 270.1228 | |
| #1b | 84.7655 | |
| MD-MBA-361 | #2b | 89.9211 |
| #3b | 82.6129 |
In Vivo Near-infrared Optical Imaging
Fluorescence intensity measurements were performed by a previously described NIR fluorescence small-animal imager (18). Briefly, this time-resolved system is based on an advanced time-correlated single-photon counting device, used in combination with a high-speed repetition-rate tunable laser. A photomultiplier tube is used as a detector. The scanning head (10 × 6 mm) incorporates multimode optical fibers, used to deliver light from an excitation source and an emitted fluorescence signal to detector through the optical switch. Laser source provides excitation photons (λ = 750 nm) and emission photons (λ = 780 nm) are filtered before detection. The imager scans in a raster pattern over the skin or other tissue surfaces at close distance of 1 to 2 mm to produce a real-time, two-dimensional image of the ROI. A cooled, charge-coupled device (CCD) camera is used to guide the scan to the ROI, and to measure spatial distribution of the fluorescence intensity, which helps to locate the tumor inside the tissue. The measurements with the small animal imager were performed in such a manner that the source-detectors head is scanning only over a ROI, i.e., tumor or corresponding contralateral area. A temperaturecontrolled scanning stage with an electrocardiogram and temperature monitoring device is used to hold small animals during measurements.
To analyze the target-specific accumulation of the imaging probes, mice were anesthetized by inhalation of isoflurane. Two consequent intravenous injections of Affibody-DyLight 750 conjugate were performed with a time interval of 24 h. Mice were imaged at several predetermined time points after each injection. The dose of the probe was ~10 μg in 100 ml of PBS. The mean and standard deviations of the fluorescence signal were calculated by averaging the maximum pixel
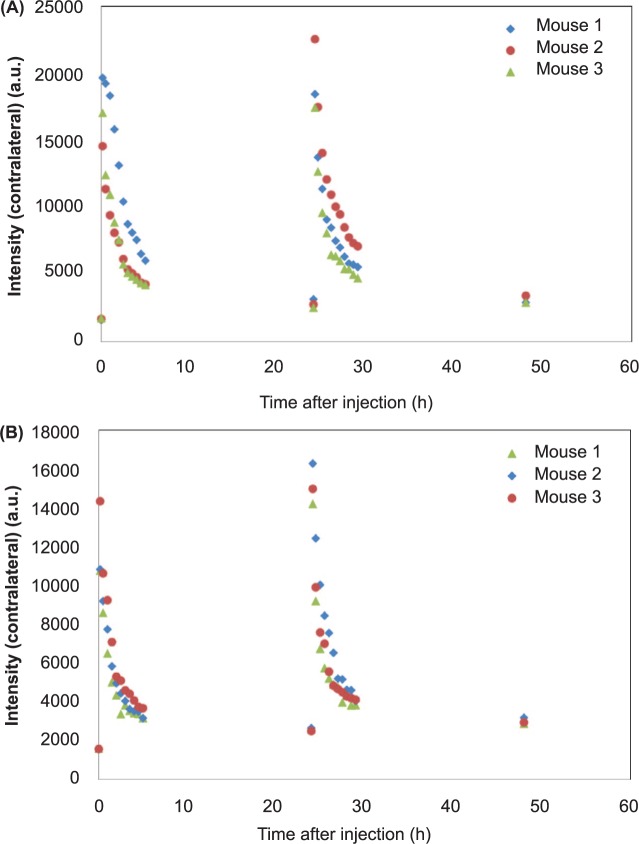
Characteristic dependencies of fluorescence intensities, measured for mice with BT-474 and MD-MBA-361, are presented in Figures 1 and 2. The data were obtained by averaging over 6 pixels at the maximum intensity area of the tumor. In all measurements, the photons (intensity) were counted over two seconds integration time. Much higher fluorescence intensities from the tumor in the first case indicate higher expression of HER 2 in BT-474 than MD-MBA-361, as expected. However, fluorescence intensities, increased considerably further after the second injection, show that in both cases the binding saturation (all HER2 receptors are bound with the probe) is not achieved after the first injection. Comparison of the data before and after the second probe injection within kinetic model framework can be used to assess the HER2 expression level.
Figure 1:
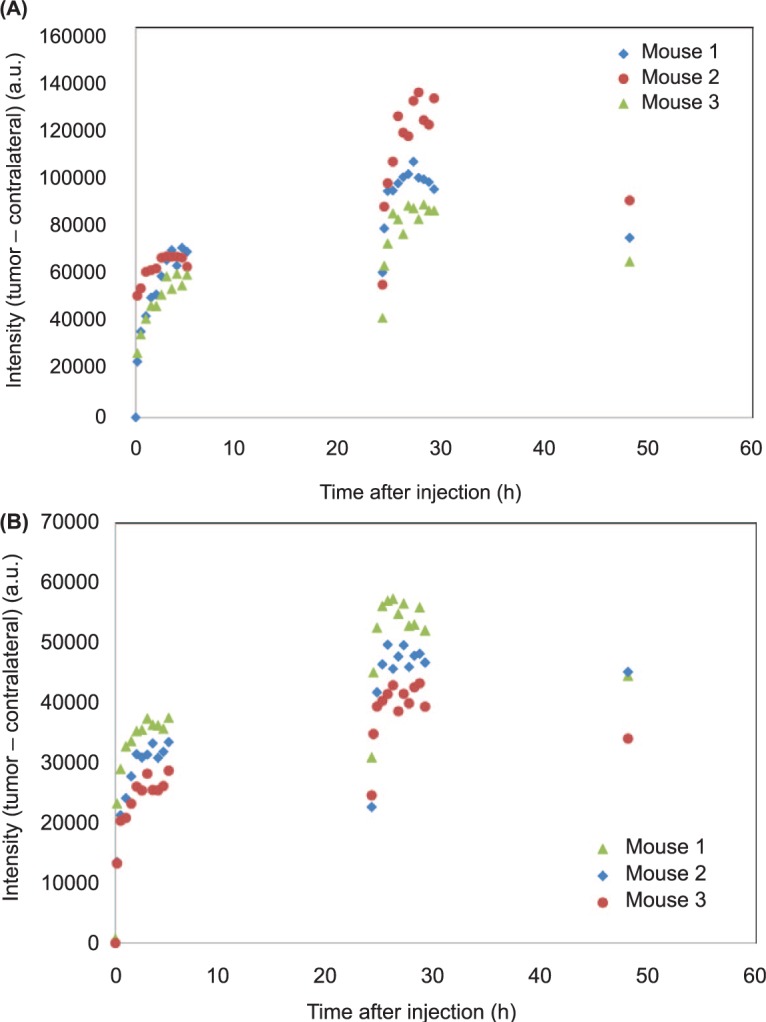
Fluorescence intensities (tumor – contralateral side) over the observation period of 48 h, including two injection points at times 0 and 24 h: (A) BT-474 and (B) MD-MBA-361.
Figure 2:
Fluorescence intensities (contralateral side) over the observation period of 48 h, including two injection points at times 0 and 24 h: (A) BT-474 and (B) MD-MBA-361.
Kinetic Model
The compartmental ligand-receptor model has been used initially for quantification of the specific receptor density in the PET studies (19). In our previous papers (9, 12, 15) we accommodated this model to assess HER2 expression, using initial NRA of the specific fluorescent probe in the tumor area. The non-equilibrium, non-linear model includes 3 compartments (free ligand in blood plasma, free ligand in tissue, and specifically HER2-bound ligand with concentrations , F*, and B*, respectively). Binding of fluorescent ligands (Affibodies) with HER2 is determined by kinetic rate kon. The total concentration of available HER2 in the tumor, potentially limits the binding process. It should be noted that without knowledge of kon or F*, only relative values of for different tumor types can be found from such kinetic model.
As has been shown in (12) for ABD-Affibody based probe, asymptotic values of fluorescence intensities after normalization on initial concentration of free ligands in the circulation are proportional to average HER2 expression for different types of breast carcinomas. However, for probes with much faster kinetics, as those based on monomer Affibody, the relationship between concentration of available HER 2 receptors (i.e. HER 2 expression) and asymptotic fluorescence intensities is not that straightforward: a large fraction of receptors can be left unbound for the lack of free fluorescent ligands at longer times t >> τ, where τ is washout time.
If the dissociation rate constant is low enough to disregard the ligands-receptor dissociation during the observation period of 5-7 h after injection, maximum fluorescence intensities from the tumor depend on the probe doze and washout time. At the first approximation, accumulation of the HER2-bound fluorescent-labeled Affibody molecules in the tumor is described by an equation:
| 1 |
similar to Equation 6A from the paper by Delforge et al. (19), where t1 is the time of injection, B1 is the concentration of the bound ligands just before the injection. B1 is equal to 0 for the first of two injections.
Measurements of the fluorescence intensities from the contralateral side show that concentration of free ligands in blood is decreasing exponentially after the probe injection. For Affibody-DyLight 750 conjugate corresponding washout time is rather short τ ~1 h (9). Assuming equilibrium between free ligands in blood and tissue, we obtain:
| 2 |
Thus maximum intensities at the tumor site, reached after the first injection, can be given by the formula:
| 3 |
Similarly, the asymptotic value of B* after the second injection is:
| 4 |
where γ is the ratio of doses of both injections.
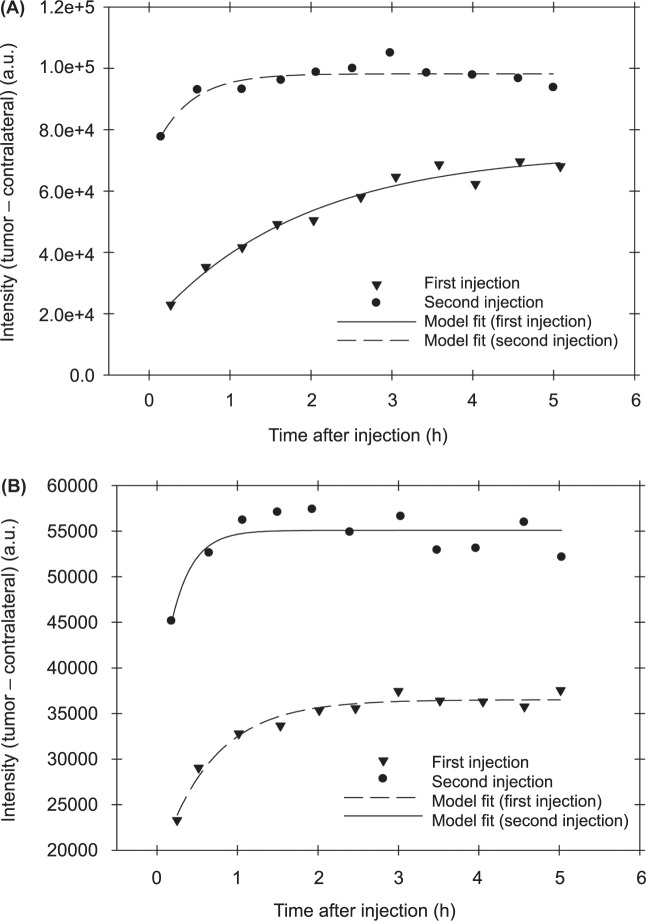
Combining Equations [3] and [4], one can obtain the final relationship between the HER 2 expression level and parameters that can be directly evaluated from experimental data, i.e. asymptotic intensities after each of the probe injections ( and ), measured intensity from the tumor just before the second injection B1 and the ratio of doses γ ≈ 1. The latter parameter can be determined by the corresponding intensities from the contralateral side, i.e., by calculating the average ratio of these intensities from the contralateral side after injections under assumption that circulation system does not change during the observation period. Asymptotic values and are determined by fitting the experimental time dependencies of the fluorescence after each injection to an exponential rise to maximum model y(t) = a[l − exp(−bt)] (we have subtracted initial level B1 from intensities after the second injection before fitting and added B1 to the found asymptote ab to find ). Figure 3A and 3B, illustrate this fitting procedure for two mice with BT-474 and MDMBA-361 xenografts, respectively. In Table II, we present obtained values of γ, , , B1 for all six mice.
Figure 3:
Two examples of the model fitting procedure to evaluate parameters, required for assessment of HER2 expression in the case of carcinoma xenografts of (A) BT-474 and (B) MD-MBA-361.
Table II:
Derived values of parameters for all six mice, required to solve numerically Equation [5].
| Carcinoma | Mouse | γ | B1 | ||
|---|---|---|---|---|---|
| #1a | 0.76 | 67500 | 97739 | 59540 | |
| BT-474 | #2a | 1.60 | 62750 | 125277 | 54544 |
| #3a | 1.05 | 54910 | 83698 | 40850 | |
| #1b | 1.20 | 35380 | 55086 | 31000 | |
| MD-MBA-361 | #2b | 1.38 | 31434 | 47639 | 22700 |
| #3b | 1.01 | 25735 | 41112 | 24646 |
To find an unknown one should solve numerically the following equation that can be derived from the system of Equations [3] and [4]:
| 5 |
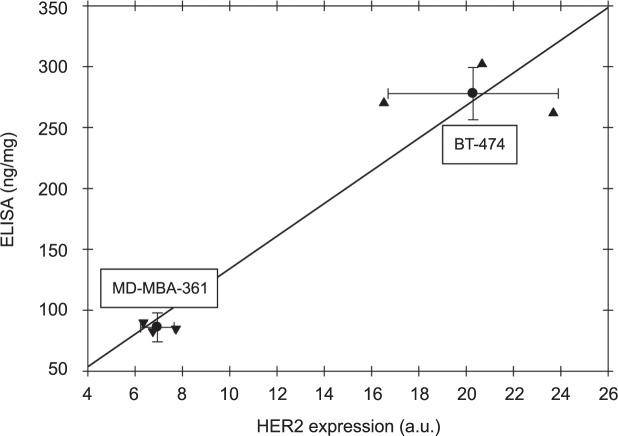
Since no analytical solution is available for Equation [5], we have developed a simple program, using Matlab routine fzero to realize this solving procedure. Our estimates of were compared with ex vivo ELISA readings of the HER 2 expression (Table I), obtained for the same tumors after completion of the tumor fluorescence imaging series.
Discussion
In a recent work (12) we have used a compartmental ligandreceptor kinetic model to show that for HER2 specific ligands, based on Albumin-binding domain-fused-(ZHER2:342) 2-Cys Affibody (so-called ABD-Affibody), both initial rate accumulation and asymptotic values of fluorescence intensities in the tumor, are proportional to HER2 expression level in different types of breast carcinomas (mouse model), if one normalizes these parameters to take into account initial concentrations of the free probe ligands in blood.
Moreover, according to Hassan et al. (15), this NRA of HER2 specific contrast agent can be used as a quantitative indicator of the total concentration of HER2 receptors in the tumor. It was shown, in particular, that good linear correlation is observed between the NRAs for individual tumors (BT-474) and ELISA assay reading, obtained ex vivo for the same tumor/mouse.
Our paper (15) was the first to demonstrate the potential of fluorescence imaging to monitor the treatment of the tumors with Hsp90 therapy (17-DMAG). Using this in vivo method we have found ~30% decrease in the average level of HER2 expression after the treatment. Corresponding ELISA values confirmed this finding. In addition, our measurements with Affibody-Alexa Fluor 750 probe at early times after its injection imply significant anti-angiogenic effect of 17-GMAG. We directly confirmed this effect by fluorescence imaging with fluorescent blood pool imaging agent AngioSense 750.
Our initial studies involved ABD Affibody based probes that have relatively slow kinetics in the circulation (washout time in the mouse model t ~ 27 h). Lately we moved to probes with much faster kinetics, i.e., Affibody monomer (Affibody-DyLight 750) based conjugates (t ~ 1 h). Results of our latest paper (9) confirm that our approach to characterize HER2 expression from initial rate of accumulation of this new probe holds.
However, in the case of relatively fast washout of the dye from the blood stream comparing to the binding rate to HER2 receptors, the relationship between asymptotic fluorescence intensities and HER2 expression becomes more complicated than in the case of slow kinetics, considered in (12), where at longer times practically all HER2 receptors are bound to the probe molecules.
In this study, we have shown that fluorescence imaging, based on repeated injections of HER2-specific Affibody-DyLight conjugates, can provide quantitative assessment on HER2 expression level in HER2-positive carcinoma xenografts. This information is extracted from time series of fluorescent images, analyzed in the framework of developed kinetic model that describes probe binding after each injection. In our model we can expect linear relationship between these parameters with corresponding linear regression line, passing through the origin. Figure 4 illustrates such comparison both for individual mice and data, averaged over carcinoma type subsamples. While noticeable deviations from the linear dependence (≤21%) are observed for individual mice, obtained average ratio of HER2 expression levels for BT-474 and MD-MBA-361 carcinomas 3.2 ± 0.6, is close to theratio of ex vivo ELISA readings of HER2 receptors for these tumors (2.9 ± 0.4). The latter observation substantiates presented approach to characterize HER2 expression level of the tumors (in other words, concentration of HER2 receptors on the cell surface) by two injections analysis, presented in this paper.
Figure 4:
Comparison between HER2 expression (a.u.), estimated in vivo for individual mice from two injections approach. Average values for carcinoma type subsamples are shown as error crosses.
It should be noted that in recent papers (13, 14) an alternative method to quantify the cell receptors concentration has been successfully applied. Unlike our approach, it uses more complicated experimental setup with simultaneous injection of two reporters: receptor-specific and non-specific with different emission/excitation wavelengths.
It is obvious that the information on HER2 expression can become instrumental to optimize the regimen of cancer therapy, especially if receptor specific monoclonal antibodies are involved. We believe that optical imaging using HER2 molecules labeled with an NIR fluorescent target may provide robust information about HER2 status in pre-clinical models in a reliable and cost-effective manner, avoiding exposure to ionizing radiation. In the future, it might become a convenient, complementary tool for selection of breast cancer patients for HER2-targeted therapies if validated in clinical trials. Due to its minimally-invasive character, this method can potentially be also used for monitoring the response of individual tumors to therapies. Currently, clinical applications of the fluorescence imaging are limited by penetration depth of NIR photons into tissue and require proper selection of targets not deeper than several centimeters. Nevertheless, the method could provide a unique tool for preclinical studies of targeted therapies and their effect or dependence on receptor expression.
Acknowledgement
This research is supported by the Intramural Research Program of National Cancer Institute, NIH, and Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH.
Abbreviations
- IHC:
Immunohistochemistry
- FISH:
Fluorescence in situ Hybridization
- HER2:
Human Epidermal Growth Factor Receptor 2
- ELISA:
Enzyme-linked Immuno Sorbent Assay
- mAb:
Monoclonal Antibody
- ROI:
Region of Interest
- NRA:
Normalized Rate of Accumulation
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol 23, 1147-1157 (2005). DOI: 10.1035/Nbt1137. [DOI] [PubMed] [Google Scholar]
- 2.Nahta R, Esteva FJ. Herceptin: mechanisms of action and resistance. Cancer Letters 232, 123-138 (2006). DOI: 10.1016/j.canlet.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 3.Ardeshirpour Y, Chernomordik V, Zielinski R, Capala J, Griffiths G, Vasalatiy O, Smirnov AV, Knutson JR, Lyakhov I, Achilefu S, Gandjbakhche A, Hassan M. In vivo fluorescence lifetime imaging monitors binding of specific probes to cancer biomarkers. PloS One 7, e31881 (2012). DOI: 10.1371/journal.pone.0031881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol 200, 290-297 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235, 177-182 (1987). [DOI] [PubMed] [Google Scholar]
- 6.Allison M. The HER2 testing conundrum. Nat Biotechnol 28, 117-119 (2010). DOI: 10.1038/nbt0210-117. [DOI] [PubMed] [Google Scholar]
- 7.Kramer-Marek G, Capala J. Can PET imaging facilitate optimization of cancer therapies? Curr Pharm Design 18, 2657-2669 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Nutt R, Vento LJ, Ridinger MHT. In vivo molecular imaging biomarkers: clinical pharmacology’s new “PET” Clin Pharmacol Ther 81, 792-795 (2007). DOI: 10.1038/sj.clpt.2007.6100213. [DOI] [PubMed] [Google Scholar]
- 9.Zielinski R, Hassan M, Lyakhov I, Needle D, Chernomordik V, Garcia-Glaessner A, Ardeshirpour Y, Capala J, Gandjbakhche A. Affibody-DyLight conjugates for in vivo assessment of HER2 expression by near-infrared optical imaging. PloS One 7, e41016 (2012). DOI: 10.1371/journal.pone.0041016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SB, Hassan M, Fisher R, Chertov O, Chernomordik V, Kramer-Marek G, Gandjbakhche A, Capala J. Affibody molecules for in vivo characterization of HER2-positive tumors by near-infrared imaging. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research 14, 3840-3849 (2008). DOI: 10.1158/1078-0432.CCR-07-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ardeshirpour Y, Chernomordik V, Capala J, Hassan M, Zielinsky R, Griffiths G, Achilefu S, Smith P, Gandjbakhche A. Using in-vivo fluorescence imaging in personalized cancer diagnostics and therapy, an image and treat paradigm. Technology in Cancer Research & Treatment 10, 549-560 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chernomordik V, Hassan M, Lee SB, Zielinski R, Gandjbakhche A, Capala J. Quantitative analysis of Her2 receptor expression in vivo by near-infrared optical imaging. Mol Imaging 9, 192-200 (2010). [PMC free article] [PubMed] [Google Scholar]
- 13.Tichauer KM, Samkoe KS, Sexton KJ, Hextrum SK, Yang HH, Klubben WS, Gunn JR, Hasan T, Pogue BW. In vivo quantification of tumor receptor binding potential with dual-reporter molecular imaging. Molecular Imaging and Biology: the Official Publication of the Academy of Molecular Imaging 14, 584-592 (2012). DOI: 10.1007/s11307-011-0534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pogue BW, Samkoe KS, Hextrum S, O’Hara JA, Jermyn M, Srinivasan S, Hasan T. Imaging targeted-agent binding in vivo with two probes. Journal of Biomedical Optics 15, 030513 (2010). DOI: 10.1117/1.3449109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassan M, Chernomordik V, Zielinski R, Ardeshirpour Y, Capala J, Gandjbakhche A. In vivo method to monitor changes in HER2 expression using near-infrared fluorescence imaging. Mol Imaging 11, 177-186 (2012). DOI: 10.2310/7290.2011.00038. [PMC free article] [PubMed] [Google Scholar]
- 16.Ikoma Y, Watabe H, Hayashi T, Miyake Y, Teramoto N, Minato K, Iida H. Quantitative evaluation of changes in binding potential with a simplified reference tissue model and multiple injections of [11C] raclopride. NeuroImage 47, 1639-1648 (2009). DOI: 10.1016/j.neuroimage.2009.05.099. [DOI] [PubMed] [Google Scholar]
- 17.Kramer-Marek G, Kiesewetter DO, Martiniova L, Jagoda E, Lee SB, Capala J. [18F]FBEM-Z(HER2:342)-Affibody molecule-a new molecular tracer for in vivo monitoring of HER2 expression by positron emission tomography. European Journal of Nuclear Medicine and Molecular Imaging 35, 1008-1018 (2008). DOI: 10.1007/s00259-007-0658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassan M, Riley J, Chernomordik V, Smith P, Pursley R, Lee SB, Capala J, Gandjbakhche AH. Fluorescence lifetime imaging system for in vivo studies. Mol Imaging 6, 229-236 (2007). [PMC free article] [PubMed] [Google Scholar]
- 19.Delforge J, Mesangeau D, Dolle F, Merlet P, Loc’h C, Bottlaender M, Trebossen R, Syrota A. In vivo quantification and parametric images of the cardiac beta-adrenergic receptor density. J Nucl Med 43, 215-226 (2002). [PubMed] [Google Scholar]