Abstract
Breastfeeding is known as the most efficient way to prevent infectious disease in early life. Maternal anti-microbial immunoglobulins transfer through milk confers passive immunity to the breastfed child while his immune system is maturing. Maternal milk also contains bioactive factors that will stimulate this maturation. From the literature on breastfeeding prevention of immune-mediated disease and more specifically from our experiments conducted in the field of allergic disease prevention, we propose that breastfeeding may also induce antigen-specific immune responses in the breastfed child. We found that early oral antigen exposure through breast milk leads to tolerance or immune priming depending on the nature of the antigen transferred and accompanying maternal milk cofactors. Here, we will discuss our data in the light of prevention of infectious disease and will propose that possible milk transfer of microbial antigen could affect actively the immune response in breastfed children and thereby their long-term susceptibility to infectious disease. Further research in this direction may lead to novel strategies of early life vaccination, taking advantage of the possibility to stimulate antigen-specific immune responses through breast milk.
Keywords: breastfeeding, tolerance, neonate immunity, vaccine
1. Introduction
Neonates and infants are at high risk of morbidity and mortality by infectious diseases, highlighting deficient effector immune responses during this period of life. There is no doubt that breastfeeding plays a major role in infectious disease prevention, with less than half the mortality rate attributed to common infections in developing countries compared with that in the absence of breastfeeding [1,2]. Significant less morbidity from infectious disease is also reported in developed countries [1,2]. Reported mechanisms underlying such a potent effect of breastfeeding include passive immunotherapy by transfer through breast milk of maternal IgAs that are specific for mucosal respiratory and enteric pathogens, transfer of antimicrobial factors such as lactoferrin, transfer of molecules that will promote growth of protective enteric bacteria such as oligosaccharide, or of growth factors that will improve barrier function such as EGF (reviewed in [1,2]). This maternal help demonstrates the importance of mother–child interaction through breast milk for infectious disease prevention during the early life of mammals.
Increased risk of allergic disease during early life also points to a deficient immune regulation in this period of life [3–5]. We have been using a mouse model of antigen transfer through breast milk in order to understand how to stimulate immune regulation in early life by oral tolerance induction to improve allergic disease prevention. We choose to assess the immunological outcome of oral antigen administration through breast milk, but not given directly, to include in our research the analysis of the potential impact of maternal milk cofactors on neonate immune regulation. We have discovered maternal and neonate factors that will lead to tolerance or immune priming upon oral antigen administration through breast milk [6–10]. Our data generated in models of allergic disease prevention suggest that antigen transfer through breast milk may affect infant immune reactivity towards that antigen. Data generated by others in the field of autoimmunity in transplantation reach a similar conclusion (see here below). Accordingly, we propose that milk transfer of microbial antigen could affect susceptibility to infectious disease. Here, we will discuss our data in the context of existing literature of early life immune response, and their implications for strategies to prevent infectious disease will be presented.
2. Evidence that breastfeeding can affect actively immune response in the offspring
Evidence from human and animal studies indicates that antigen in breast milk can impact actively a child's immune reactivity in both the short and long term. Here, we will describe briefly the evidence in the context of autoimmune disease and allograft rejection and focus on the evidence from prevention of allergic disease by breast milk.
(a). HLA antigens in breast milk and their impact on transplantation success
In addition to the HLA antigens received genetically, children are exposed to maternal non-inherited allo-antigens (NIMA) that are present in breast milk in soluble forms or expressed on milk cells [11]. Elegant studies in rodents demonstrated that transfer of NIMA from the mother to the pup in utero through breast milk favours the acceptance of heart, skin or bone marrow semi-allogeneic transplants expressing NIMA [11]. In the case of bone marrow transplant, the transfer of HLA antigen through breast milk was sufficient to prevent allogeneic reactions. There is also evidence in humans that renal graft survival is improved when the donor expresses NIMA [11].
(b). Self-antigens in breast milk and their impact on autoimmunity
Self-antigens originating from organs other than mammary gland have been described in breast milk. These include insulin, a major auto-antigen in type 1 diabetes (T1D) [12,13]. Many studies show breastfeeding-associated protection [14,15] against T1D development, although this is not always the case [16,17]. Some authors suggest that in the absence of breastfeeding, bovine insulin present in cow's milk generates cross-reactive immunity to human insulin [18,19]. Other reports suggest that the benefit from breastfeeding would result from tolerance induction to human insulin present in breast milk [12,13,20]. Indeed, Tiittannen et al. [12] observed that the concentration of insulin in breast milk correlated inversely with the plasma levels of IgG antibodies to bovine insulin at 6 months of age in children receiving cow's milk formula, suggesting that insulin in breast milk is tolerogenic. This latter mechanism may, however, not be sufficient to control disease development since many genetic and environmental factors do condition T1D development, explaining the controversy on protection of T1D by breastfeeding.
(c). Allergens in breast milk and prevention of allergy
In humans, the presence of antigens in breast milk derived from maternal diet is well described, and presence of antigens from peanut, wheat and egg can be found in human milk in the range of nanograms per millilitre [21,22]. In adults, allergy prevention is classically based on allergen avoidance. This approach has been extended to the fetus and infants by promoting the avoidance of allergen exposure during pregnancy, lactation and the first years of life. In addition, the rare (less than 0.5%) but nevertheless well-reported cases of food allergy in exclusively breastfed children supported these recommendations [23]. However, such strategy has not yielded the expected results as food allergy has continued to rise over the last decade and prospective studies assessing allergen avoidance have failed to show a significant long-term reduction in food allergy rates [22,24,25]. However, there is no direct correlation between maternal food antigen intake and their concentrations in breast milk [26]. Therefore, from avoidance recommendation, one cannot predict the levels of food antigens in breast milk and the potential impact on allergy exacerbation or prevention or on immune response in the breastfed child.
Animal studies, where a strict control of antigen administration through breast milk and of confounding factors is possible, have clearly demonstrated that the breast milk-mediated transfer of an antigen could prevent antigen-specific immune responses [7,10,27–29] and allergic disease development in rodents [7,10,30,31]. We analysed the mechanisms of breastfeeding-induced tolerance in a mouse model of egg antigen transfer through breast milk, during the whole lactation period. We found that protection was antigen specific and could be transferred to naive mice by the injection of CD4 T lymphocytes from adult mice that had received ovalbumin (OVA) in early life, demonstrating that active immune suppression had been induced in the long term by antigen transferred through breast milk [10]. We further demonstrated that FoxP3 regulatory T cells (Tregs) were not involved in this process of immune regulation since Tregs depletion in adult mice that had received OVA in early life had no impact on their protection [10]. This result was very surprising in view of the significant body of publications showing the role of Tregs in oral tolerance induction in adult mice [32], and the precise mechanisms of protection induced in early life remained unknown [10]. Recently, we demonstrated that soluble OVA transfer through breast milk induced long-term prevention of allergy by induction of T helper type 1 (Th1) cells [33]. Importantly, we also demonstrated that, in contrast to the adult where the sole administration of an antigen is sufficient for tolerance induction, maternal milk transforming growth factor (TGF)-β was required to induce tolerance in offspring to antigen administered through breast milk [7,10]. More recently, we also found that maternal milk-derived vitamin A was key to neonatal gut permeability and immune system maturation, two necessary events for optimal induction of Th1 immune response upon OVA antigen administration in early life. These observations highlight that maternal–child complementarity is also necessary for induction of immune response in early life.
In order to delineate how maternal immune status may affect neonates' immune response to milk-transferred antigen, we also studied impact of OVA transfer through breast milk of immunized mothers. In this case, and in contrast to soluble antigen, we found that this protocol resulted in tolerance induction mediated by regulatory T lymphocytes expressing Foxp3 [7]. We demonstrated that potent induction of Foxp3 Tregs by this protocol resulted from improved transfer of OVA bound to maternal-specific IgG across the neonate gut barrier by the use of neonatal FcR receptor (FcRn), an IgG binding receptor that was shown to be critical for transfer of IgG from mother to young across placental and gut barriers [34,35]. OVA–IgG immune complexes were also better presented to T lymphocytes by dendritic cells (DCs) than soluble OVA [7]. Inhibitory FcgRIIb was not necessary for tolerance induction and OVA-specific IgA played no role in the observed protection [7].
To summarize, we found that, in a mouse model of OVA transfer through breast milk, breastfeeding could actively shape the immune response of the offspring and affect its long-term susceptibility to allergic disease. Importantly, induction of immune response to antigen transferred through breast milk was strictly dependent on maternal milk cofactors such as TGF-β, vitamin A and IgG. Moreover, these cofactors conditioned the type of immune response induced in offspring: Th1 (OVA and TGF-β or vitamin A) or Foxp3 Tregs (OVA and IgG).
In order to understand how breastfeeding could affect respiratory allergies risk in some cases but not in others [36–38], we then hypothesized that respiratory allergen may be transferred through breast milk like dietary antigen and, in the presence of certain cofactors, induce immune tolerance and prevent development of respiratory allergic disease in breastfed children [6–10]. We found that mice exposure to respiratory antigen resulted indeed in the presence of the antigen in breast milk [6,7,10], and more importantly, we could detect respiratory antigen, house dust mite (HDM) Dermatophagoides Pteronyssinus (Der p) 1, in human milk from various regions of the world [6]. Looking at literature in rodents can explain this observation. A total of 2–4% of antigen is found in the lung and 65–80% in the digestive tract 1–2 h after aerosol antigen exposure [39,40]. Therefore, although some airborne antigens penetrate into the distal alveoli, the bulk of inhaled antigen is found in the gut. Indeed, inhaled antigens are either trapped in the nasal passage and swallowed or deposited in the lung and cleared by the mucociliary escalator to the digestive tract [39,40]. The presence of airborne antigens in milk most probably results from the transfer of antigen from the airways to the mammary gland, mainly through the gut and, for a small proportion, through the alveolar–capillary barrier of the lung. While we showed that OVA administration through breast milk resulted in prevention of allergic disease, either by Th1 differentiation in the case of soluble OVA [10] or by Foxp3 Tregs differentiation in the case of OVA in immune complexes [7], oral early exposure to HDM antigens through breast milk resulted in increased allergic sensitization in the progeny even though TGF-β and maternal antibodies were present in milk [6]. There are some important biological and functional differences between OVA and HDM antigens that could explain these observations. Multiple natural adjuvant properties of HDM are thought to be responsible for their allergenicity and have been well studied in the case of Der p [41]. Adjuvant properties of Der p antigens have been attributed to their specific molecules that are able to activate the innate immune system, including proteases such as cysteine (Der p1) and serine protease (Der p3 and Der p6) that have been shown to disrupt respiratory epithelial tight junctions and activate protease-activated receptors and molecule analogues to MD2 involved in LPS signal transduction (Der p2). In addition to mite allergen found mainly in their faecal pellets, components of the mite exoskeleton such as chitin and from Gram-negative bacteria found in Der p extract such as lipopolysaccharide (LPS) also activate the innate immune system. In conclusion, our data highlight that, although milk is usually considered as anti-inflammatory [1,2], transfer of antigen with adjuvant properties can stimulate effector immune response which will persist in the long term.
3. Could breastfeeding immunize infant to microbes?
Possible maternal–child infectious agent transfer through breast milk has been studied with care and child infection by breast milk-mediated transmission of human immunodeficiency virus, human T-lymphotropic virus type 1, cytomegalovirus, hepatitis B and group B streptococcus is reported (reviewed in [42]). What is striking from these studies is that, although high loads of microbe can be found in breast milk, the risk of disease transmission remains rare [42]. Publications demonstrated that maternal milk factors such as IgA, lactoferrin, lymphocytes, lipase and oligosaccharides, were involved in decreased microbe infectivity (reviewed in [1]). According to our observations in the mouse model of antigen transfer, we think it would be worth also looking at both cell mediated and antibody immune response induced in the breastfed child exposed to microbes through breast milk, and compare rates of disease transmission.
The search for the presence of microbial antigens in breast milk is poorly documented, and more particularly in the case of maternal infection without evidence of disease transmission to the infant. Hepatitis B surface antigen in breast milk is reported as present in breast milk [42], but its possible impact on child-specific immune reactivity has not been investigated to our knowledge. The possible impact of microbial antigen transfer through milk on child-specific immune reactivity towards subsequent microbial infection or vaccine strengthens the necessity to search for the presence of microbial antigens in breast milk even though culture or detection of microbial DNA or RNA do not demonstrate the presence of the microbe itself.
Maternal vaccination during late pregnancy has been encouraged in the past few years in order to increase infant IgG levels to specific pathogens and protect them passively until they can mount an efficient active immune response by vaccination [43]. A possible drawback put forward by this approach is that maternal antibodies impede efficient vaccination because of epitope masking or induction of inhibitory response [43,44]. We propose that maternal IgG could also be considered as a strong adjuvant for induction of effector immune response to microbial antigens present in breast milk. As described here above, we found that antigen–IgG immune complexes in breast milk were potent inducers of regulatory T lymphocytes [7]. Others found that upon antigen or pathogen oral exposure in the presence of serum-specific IgG [45–47], antigen-immune complexes allowed a better transfer of antigen across gut barrier, targeting mesenteric lymph node DCs and resulting in stronger induction of effector immune responses in an FcRn-dependent manner [45,46]. In those studies, specific IgG were present in serum and found to be transported from the baso-lateral side to the apical side of the gut epithelium, and then they retro-transported antigen into the lamina propria for processing by DCs and presentation to CD4 T lymphocytes. These data imply that not only milk IgG but also maternal-derived infant serum IgG found in children from immunized mothers could strongly stimulate immune response to microbes or microbial antigens present in present milk. In mice, FcRn expression in the gut is restricted to the first two weeks of life, but in humans this expression remains lifelong [34,35]. However, the possible transfer of milk IgG or, retro-transfer of maternal-derived infant serum IgG via the FcRn in humans is unknown and would require investigation. An important point to solve is to determine which factors will favour the induction of effector instead of regulatory immune responses towards maternal IgG–antigen immune complexes. Immunoglobulin sialylation has been extensively studied as a factor modulating immune outcome [48]. Reports have associated anti-inflammatory properties of IgG to the presence of galactose and sialic acid and their binding to specific glucan receptors such as DC-SIGN (or SIGN-R1 in mice) [48] or DC immunoreceptors responsible for tolerogenic immune response [49]. It was also showed that IgG sialylation decreased with inflammation, favouring effector immune response. In this context, maternal IgG sialylation during pregnancy is worth being investigated and one report indicated that pregnancy may be associated with increased galactosylation and thus induction of anti-inflammatory responses [50]. However, others have not confirmed a role for sialylation in the anti-inflammatory properties of IgG [51] and other factors, such as ratio of antigen to IgG [44,52], FcγR engaged and DC subpopulation targeted [53,54], and the presence of cofactors activating innate immune responses certainly play a role in immune outcome towards immune complexes formed in early life. Conditions of maternal immunization that may affect antibody sialylation and of child vaccination that may affect DC targeting and activation are to us a key factor to look at to improve vaccination success in early life.
Finally, we may also take advantage of our observation in the model of HDM antigen transfer through breast milk and the understanding of the precise mechanisms by which HDM antigens are able to prime the immune system in early life, which may be important for the design of oral vaccine in early life. From our observations, we would also envisage that nasal vaccination of breastfeeding mothers may result in the presence of microbial antigen in their breast milk and thereby affect their child's immune reactivity.
4. Conclusion and perspectives
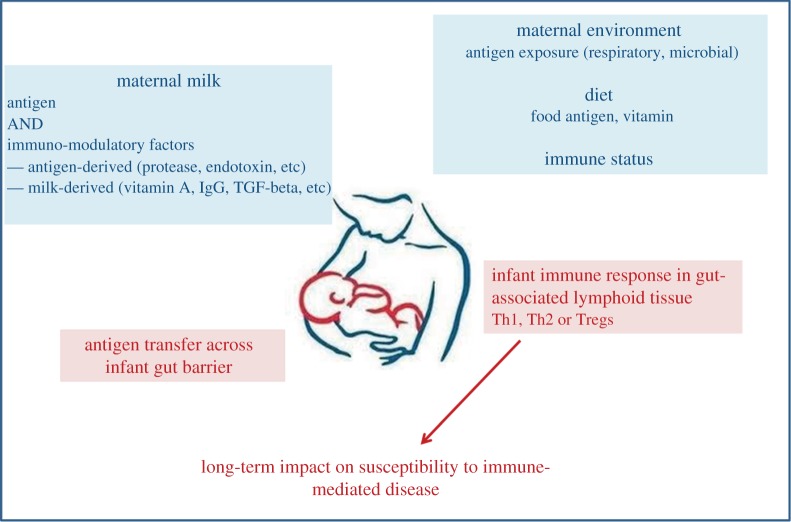
Here, we have reviewed some of the possible immunological outcomes upon early oral exposure to antigen through breast milk. We found that, according to maternal immune status, vitamin A in diet and TGF-β milk content, and according to the nature of the antigen found in breast milk, Th1, Th2 or regulatory immune responses could be induced in the long term in breastfed offspring (figure 1). We believe that these data should strongly encourage the development of research on the possibility to immunize children through maternal milk and confer them long-term protection from infectious disease.
Figure 1.
Evidence from mouse model of allergy prevention on impact of mother–child interaction through breast milk on the education of the immune system. (Online version in colour.)
Acknowledgements
I thank Akila Rekima, Valérie Milcent, Eric Mosconi, Patricia Macchivarni and Mathilde Turfkruyer for their contribution to the experiments that are exposed in this review and discussion we had on interpretation of the data. I also thank Meri Tulic for stimulating discussions and Arnaud Marchant and Beat Kampmann for bringing me into the field of maternal vaccination.
Funding statement
This work was supported by the Institut National de la Santé et Recherche Médicale (INSERM), Université de Nice Sophia-Antipolis (UNS), Fondation Princesse Grace, grant from the Agence Nationale de la Recherche and grant from Fondation de recherche en santé respiratoire.
Conflict of interests
I have no competing interests.
References
- 1.Lawrence RM, Pane CA. 2007. Human breast milk: current concepts of immunology and infectious diseases. Curr. Probl. Pediatric Adolesc. Health Care 37, 7–36. ( 10.1016/j.cppeds.2006.10.002) [DOI] [PubMed] [Google Scholar]
- 2.Labbok MH, Clark D, Goldman AS. 2004. Breastfeeding: maintaining an irreplaceable immunological resource. Nat. Rev. Immunol. 4, 565–572. ( 10.1038/nri1393) [DOI] [PubMed] [Google Scholar]
- 3.Wahn U, von Mutius E. 2001. Childhood risk factors for atopy and the importance of early intervention. J. Allergy Clin. Immunol. 107, 567–574. ( 10.1067/mai.2001.112943) [DOI] [PubMed] [Google Scholar]
- 4.Karlsson MR, Rugtveit J, Brandtzaeg P. 2004. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow's milk allergy. J. Exp. Med. 199, 1679–1688. ( 10.1084/jem.20032121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tulic MK, Hodder M, Forsberg A, McCarthy S, Richman T, D'Vaz N, van den Biggelaar AH, Thornton CA, Prescott SL. 2011. Differences in innate immune function between allergic and nonallergic children: new insights into immune ontogeny. J. Allergy Clin. Immunol. 127, 470–478, e471 ( 10.1016/j.jaci.2010.09.020) [DOI] [PubMed] [Google Scholar]
- 6.Macchiaverni P, et al. 2014. Respiratory allergen from house dust mite is present in human milk and primes for allergic sensitization in a mouse model of asthma. Allergy 69, 395–398. ( 10.1111/all.12332) [DOI] [PubMed] [Google Scholar]
- 7.Mosconi E, et al. 2010. Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol. 3, 461–474. ( 10.1038/mi.2010.23) [DOI] [PubMed] [Google Scholar]
- 8.Verhasselt V. 2010. Neonatal tolerance under breastfeeding influence. Curr. Opinion Immunol. 22, 623–630. ( 10.1016/j.coi.2010.08.008) [DOI] [PubMed] [Google Scholar]
- 9.Verhasselt V. 2010. Oral tolerance in neonates: from basics to potential prevention of allergic disease. Mucosal Immunol. 3, 326–333. ( 10.1038/mi.2010.25) [DOI] [PubMed] [Google Scholar]
- 10.Verhasselt V, Milcent V, Cazareth J, Kanda A, Fleury S, Dombrowicz D, Glaichenhaus N, Julia V. 2008. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat. Med. 14, 170–175. ( 10.1038/nm1718) [DOI] [PubMed] [Google Scholar]
- 11.van Rood JJ, Claas F. 2000. Both self and non-inherited maternal HLA antigens influence the immune response. Immunol. Today 21, 269–273. ( 10.1016/S0167-5699(00)01628-5) [DOI] [PubMed] [Google Scholar]
- 12.Tiittanen M, Paronen J, Savilahti E, Virtanen SM, Ilonen J, Knip M, Akerblom HK, Vaarala O, Finnish TSG. 2006. Dietary insulin as an immunogen and tolerogen. Pediatric Allergy Immunol. 17, 538–543. ( 10.1111/j.1399-3038.2006.00447.x) [DOI] [PubMed] [Google Scholar]
- 13.Shehadeh N, Shamir R, Berant M, Etzioni A. 2001. Insulin in human milk and the prevention of type 1 diabetes. Pediatr. Diabetes 2, 175–177. ( 10.1034/j.1399-5448.2001.20406.x) [DOI] [PubMed] [Google Scholar]
- 14.Malcova H, Sumnik Z, Drevinek P, Venhacova J, Lebl J, Cinek O. 2006. Absence of breast-feeding is associated with the risk of type 1 diabetes: a case-control study in a population with rapidly increasing incidence. Eur. J. Pediatr. 165, 114–119. ( 10.1007/s00431-005-0008-9) [DOI] [PubMed] [Google Scholar]
- 15.Holmberg H, Wahlberg J, Vaarala O, Ludvigsson J. 2007. Short duration of breast-feeding as a risk-factor for beta-cell autoantibodies in 5-year-old children from the general population. Br. J. Nutr. 97, 111–116. ( 10.1017/S0007114507210189) [DOI] [PubMed] [Google Scholar]
- 16.Hummel M, Fuchtenbusch M, Schenker M, Ziegler AG. 2000. No major association of breast-feeding, vaccinations, and childhood viral diseases with early islet autoimmunity in the German BABYDIAB Study. Diabetes Care 23, 969–974. ( 10.2337/diacare.23.7.969) [DOI] [PubMed] [Google Scholar]
- 17.Couper JJ, et al. 1999. Lack of association between duration of breast-feeding or introduction of cow's milk and development of islet autoimmunity. Diabetes 48, 2145–2149. ( 10.2337/diabetes.48.11.2145) [DOI] [PubMed] [Google Scholar]
- 18.Virtanen SM, Laara E, Hypponen E, Reijonen H, Rasanen L, Aro A, Knip M, Ilonen J, Akerblom HK. 2000. Cow's milk consumption, HLA-DQB1 genotype, and type 1 diabetes: a nested case-control study of siblings of children with diabetes. Childhood diabetes in Finland study group. Diabetes 49, 912–917. ( 10.2337/diabetes.49.6.912) [DOI] [PubMed] [Google Scholar]
- 19.Knip M, Veijola R, Virtanen SM, Hyoty H, Vaarala O, Akerblom HK. 2005. Environmental triggers and determinants of type 1 diabetes. Diabetes 54(Suppl. 2), S125–S136. ( 10.2337/diabetes.54.suppl_2.S125) [DOI] [PubMed] [Google Scholar]
- 20.Harrison LC, Honeyman MC. 1999. Cow's milk and type 1 diabetes: the real debate is about mucosal immune function. Diabetes 48, 1501–1507. ( 10.2337/diabetes.48.8.1501) [DOI] [PubMed] [Google Scholar]
- 21.Macchiaverni P, Tulicz MK, Verhasselt V. 2012. Antigens in breast milk: possible impact on immune system education. In Handbook of dietary and nutritonal aspects of human breast milk (eds Zibadi RWR, Preedy S.), pp. 447–459. The Netherlands: Wageningen Academics. [Google Scholar]
- 22.Palmer DJ, Makrides M. 2006. Diet of lactating women and allergic reactions in their infants. Curr. Opin. Clin. Nutr. Metab. Care 9, 284–288. ( 10.1097/01.mco.0000222113.46042.50) [DOI] [PubMed] [Google Scholar]
- 23.Sorva R, Makinen-Kiljunen S, Juntunen-Backman K. 1994. Beta-lactoglobulin secretion in human milk varies widely after cow's milk ingestion in mothers of infants with cow's milk allergy. J. Allergy Clin. Immunol. 93, 787–792. ( 10.1016/0091-6749(94)90259-3) [DOI] [PubMed] [Google Scholar]
- 24.Greer FR, Sicherer SH, Burks AW, N. American Academy of Pediatrics Committee on Nutrition, Section on Allergy and Immunology. 2008. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics 121, 183–191. ( 10.1542/peds.2007-3022) [DOI] [PubMed] [Google Scholar]
- 25.Sicherer SH. 2002. The impact of maternal diets during breastfeeding on the prevention of food allergy. Curr. Opin. Allergy Clin. Immunol. 2, 207–210. ( 10.1097/00130832-200206000-00009) [DOI] [PubMed] [Google Scholar]
- 26.Palmer DJ, Makrides M. 2006. Diet of lactating women and allergic reactions in their infants. Curr. Opin. Clin. Nutr. Metab. Care 9, 284–288. ( 10.1097/01.mco.0000222113.46042.50) [DOI] [PubMed] [Google Scholar]
- 27.Komatsu T, Okao M, Miyamoto H, Chen T, Shinka S. 1988. Effects of early antigen exposure through lactation on later specific antibody responses in mice. J. Immunol. 141, 2895–2906. [PubMed] [Google Scholar]
- 28.Korotkova M, Telemo E, Yamashiro Y, Hanson LA, Strandvik B. 2004. The ratio of n-6 to n-3 fatty acids in maternal diet influences the induction of neonatal immunological tolerance to ovalbumin. Clin. Exp. Immunol. 137, 237–244. ( 10.1111/j.1365-2249.2004.02527.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strobel S, Ferguson A. 1984. Immune responses to fed protein antigens in mice. 3. Systemic tolerance or priming is related to age at which antigen is first encountered. Pediatr. Res. 18, 588–594. ( 10.1203/00006450-198407000-00004) [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto T, Tsubota Y, Kodama T, Kageyama-Yahara N, Kadowaki M. 2012. Oral tolerance induced by transfer of food antigens via breast milk of allergic mothers prevents offspring from developing allergic symptoms in a mouse food allergy model. Clin. Dev. Immunol. 2012, 721085 ( 10.1155/2012/721085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez-Exposito I, Song Y, Jarvinen KM, Srivastava K, Li XM. 2009. Maternal peanut exposure during pregnancy and lactation reduces peanut allergy risk in offspring. J. Allergy Clin. Immunol. 124, 1039–1046. ( 10.1016/j.jaci.2009.08.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pabst O, Mowat AM. 2012. Oral tolerance to food protein. Mucosal Immunol. 5, 232–239. ( 10.1038/mi.2012.4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turfkruyer M, Rekima A, Macchiaverni P, Le Bourhis L, Muncan V, van den Brink GR, Tulic MK, Verhasselt V. Submitted Oral tolerance is inefficient in neonatal mice due to a physiological vitamin A deficiency. [DOI] [PubMed] [Google Scholar]
- 34.Roopenian DC, Akilesh S. 2007. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 7, 715–725. ( 10.1038/nri2155) [DOI] [PubMed] [Google Scholar]
- 35.Qiao SW, Kobayashi K, Johansen FE, Sollid LM, Andersen JT, Milford E, Roopenian DC, Lencer WI, Blumberg RS. 2008. Dependence of antibody-mediated presentation of antigen on FcRn. Proc. Natl Acad. Sci. USA 105, 9337–9342. ( 10.1073/pnas.0801717105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dogaru CM, Nyffenegger D, Pescatore AM, Spycher BD, Kuehni CE. 2014. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am. J. Epidemiol. 179, 1153–1167. ( 10.1093/aje/kwu072) [DOI] [PubMed] [Google Scholar]
- 37.Gdalevich M, Mimouni D, Mimouni M. 2001. Breast-feeding and the risk of bronchial asthma in childhood: a systematic review with meta-analysis of prospective studies. J. Pediatr. 139, 261–266. ( 10.1067/mpd.2001.117006) [DOI] [PubMed] [Google Scholar]
- 38.Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, Trikalinos T, Lau J. 2007. Breastfeeding and maternal and infant health outcomes in developed countries. Evid. Report/Technol. Assess. 153, 1–186. [PMC free article] [PubMed] [Google Scholar]
- 39.Willoughby JB, Willoughby WF. 1977. In vivo responses to inhaled proteins. I. Quantitative analysis of antigen uptake, fate, and immunogenicity in a rabbit model system. J. Immunol. 119, 2137–2146. [PubMed] [Google Scholar]
- 40.Holt PG, Batty JE, Turner KJ. 1981. Inhibition of specific IgE responses in mice by pre-exposure to inhaled antigen. Immunology 42, 409–417. [PMC free article] [PubMed] [Google Scholar]
- 41.Gregory LG, Lloyd CM. 2011. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 32, 402–411. ( 10.1016/j.it.2011.06.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawrence RM, Lawrence RA. 2004. Breast milk and infection. Clin. Perinatol. 31, 501–528. ( 10.1016/j.clp.2004.03.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindsey B, Kampmann B, Jones C. 2013. Maternal immunization as a strategy to decrease susceptibility to infection in newborn infants. Curr. Opin. Infect. Dis. 26, 248–253. ( 10.1097/QCO.0b013e3283607a58) [DOI] [PubMed] [Google Scholar]
- 44.Siegrist CA. 2003. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine 21, 3406–3412. ( 10.1016/S0264-410X(03)00342-6) [DOI] [PubMed] [Google Scholar]
- 45.Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, Lencer WI, Blumberg RS. 2004. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity 20, 769–783. ( 10.1016/j.immuni.2004.05.007) [DOI] [PubMed] [Google Scholar]
- 46.Yoshida M, et al. 2006. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J. Clin. Invest. 116, 2142–2151. ( 10.1172/JCI27821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris NL, et al. 2006. Mechanisms of neonatal mucosal antibody protection. J. Immunol. 177, 6256–6262. ( 10.4049/jimmunol.177.9.6256) [DOI] [PubMed] [Google Scholar]
- 48.Anthony RM, Wermeling F, Ravetch JV. 2012. Novel roles for the IgG Fc glycan. Ann. NY Acad. Sci. 1253, 170–180. ( 10.1111/j.1749-6632.2011.06305.x) [DOI] [PubMed] [Google Scholar]
- 49.Massoud AH, Yona M, Xue D, Chouiali F, Alturaihi H, Ablona A, Mourad W, Piccirillo CA, Mazer BD. 2014. Dendritic cell immunoreceptor: a novel receptor for intravenous immunoglobulin mediates induction of regulatory T cells. J. Allergy Clin. Immunol. 133, 853–863.e855 ( 10.1016/j.jaci.2013.09.029) [DOI] [PubMed] [Google Scholar]
- 50.van de Geijn FE, Wuhrer M, Selman MH, Willemsen SP, de Man YA, Deelder AM, Hazes JM, Dolhain RJ. 2009. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res. Therapy 11, R193 ( 10.1186/ar2892) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramakrishna C, Cantin EM. 2014. Fc-sialylated IgGs in intravenous immunoglobulins are not responsible for induction of regulatory T cells. J. Allergy Clin. Immunol. 134, 1469 ( 10.1016/j.jaci.2014.08.049) [DOI] [PubMed] [Google Scholar]
- 52.Caulfield MJ, Shaffer D. 1987. Immunoregulation by antigen/antibody complexes. I. Specific immunosuppression induced in vivo with immune complexes formed in antibody excess. J. Immunol. 138, 3680–3683. [PubMed] [Google Scholar]
- 53.Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. 2014. The function of Fcgamma receptors in dendritic cells and macrophages. Nat. Rev. Immunol. 14, 94–108. ( 10.1038/nri3582) [DOI] [PubMed] [Google Scholar]
- 54.Nimmerjahn F, Ravetch JV. 2008. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 8, 34–47. ( 10.1038/nri2206) [DOI] [PubMed] [Google Scholar]