Abstract
Environmental enteropathy (EE) is a poorly defined state of intestinal inflammation without overt diarrhoea that occurs in individuals exposed over time to poor sanitation and hygiene. It is characterized pathologically by small intestine villous blunting and inflammation. In children from low-income countries, it is implicated as a cause of malnutrition, oral vaccine failure and impaired cognitive development. Here we review the search for non-invasive biomarkers to measure EE non-invasively, and assess the current evidence linking EE to malnutrition, vaccine failure and neurocognitive development.
Keywords: oral polio vaccine, rotavirus vaccine, environmental enteropathy
1. Introduction
Environmental enteropathy (EE) is a gut disorder that is prevalent among inhabitants of low-income countries living in environments with poor sanitation and hygiene [1–7]. Chronic exposure to faecal pathogens is hypothesized to cause inflammation and resultant structural changes in the small bowel, which ultimately result in functional changes. These functional changes include gut barrier disruption, carbohydrate malabsorption and chronic inflammation, and are hypothesized to contribute to impaired gut immune function and oral vaccine failure, and ultimately growth faltering and impaired child development. This disease is thought to occur in otherwise asymptomatic individuals without evidence of overt diarrhoea [1–3,5]. The lack of both non-invasive tests to diagnose and effective interventions to prevent or treat EE has left a large knowledge gap. Further limiting the development of new approaches to treat or prevent small intestine disease is the lack of understanding of the pathologic state of the diseased small intestine.
2. The burden of enteric infections in low-income countries
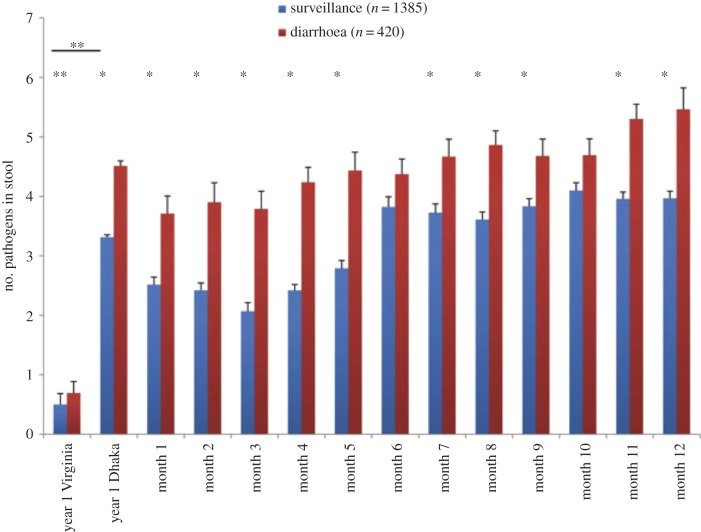
One of the extraordinary aspects of life for infants raised in the urban slums of low-income countries is the impact of diarrhoea and enteric infections on health. It has long been appreciated that diarrhoea and respiratory infections are the leading causes of death of children under age 5 in the developing world. However, only recently with the advent of molecular diagnostics has the magnitude of enteric infections been understood. In pioneering studies, Taniuchi et al. [8] applied modern molecular diagnostics for enteropathogens in a longitudinal birth cohort in the Mirpur slum of Dhaka, Bangladesh (figure 1). Diarrhoea surveillance was conducted through twice weekly household visits, and asymptomatic infections were determined through monthly surveillance stool samples. Nearly 2000 stool samples from 147 children were tested for infection from birth to age 12 months using quantitative PCR-based tests for 32 different enteric pathogens. The results were astounding, showing that the average child had two to four enteropathogens at any one time, even as early as the first month of life when exclusive breastfeeding was universal. In comparison, infants in the USA had far less than one enteropathogen detected at any given time. This work changed the landscape of enteric infections, as suddenly the importance of asymptomatic enteric infections came into question owing to their near universal presence in these infants growing up in poverty. Currently under study is whether the enteropathogen burden, quantified by molecular diagnostics, can be used as a measure of EE.
Figure 1.
Frequency of enteropathogen detection in Dhaka versus Virginia. Diarrhoeal and non-diarrhoeal stool samples were collected at the time points indicated and assayed for 29 enteropathogens by molecular methods. The total number of enteropathogens was summed for each sample; results are shown as mean ± s.e. *Bonferroni adjusted p < 0.05 (determined with linear mixed-effect regression model used to identify differences in the number of pathogens detected between diarrhoeal and surveillance samples for each month during the study period). **Non-parametric Wilcoxon two-sample tests were used to compare numbers of pathogens between Virginia and Dhaka samples and between diarrhoeal and surveillance samples for Virginia alone. Reproduced with permission from [8]. (Online version in colour.)
3. Biomarkers for environmental enteropathy
Biopsy of the small bowel of children in The Gambia revealed a range of pathology, from normal architecture to flattened villi, with crypt hypertrophy and elevated concentrations of intraepithelial CD8+ lymphocytes observed in most [2,5]. Perhaps analogous to coeliac disease, the mucosal changes seen in these children were histologically and immunologically suggestive of a pro-inflammatory state. There has been little done recently with the study of small intestine pathology in children with suspected EE, in part due to ethical considerations of conducting small bowel biopsies in seemingly asymptomatic, yet malnourished children [4]. We are thus left in the awkward position of trying to measure the impact of a disease that may occur in a substantial fraction of children in the developing world without the direct and specific diagnostic tests.
Serum and faecal biomarkers of intestinal inflammation have been developed for evaluation in patients with inflammatory bowel disease. In the absence of the ability to longitudinally follow infants at risk for EE with serial small intestinal biopsies, inflammatory bowel disease markers have been applied to populations at risk. Three recent studies are of note. We [1] measured gut barrier dysfunction in the same longitudinal cohort as Taniuchi et al. [8]. There was an association in infancy of serum endocab antibodies (a measure of the immune response to systemic endotoxin leakage from the gut) with stunting at 1 year of age. Kosek et al. [6], by contrast, studied neopterin, alpha-1-antitrypsin, and myeloperoxidase in stool from 500 infants under longitudinal surveillance for diarrhoeal illness in eight low-income countries. Neopterin is a metabolic product of dendritic cells and macrophages and is a marker of intestinal inflammation. Alpha-1-antitrypsin in stool is a marker of gut barrier dysfunction, as it is a serum protein not normally found in stool. Myeloperoxidase is produced by neutrophils, and provided another faecal marker of gut inflammation. Children were studied over the first 2 years of life.
The absolute values of these tests were in the abnormal range for almost all of the children studied, indicating the commonality of intestinal inflammation and gut barrier disruption. Importantly, the tests did not correlate with each other, supporting that they gave independent and complementary measurements of gut function. When combined to form a disease activity score (calculated by placing the biomarker scores as quartiles and adding them together) children with the highest score grew 1.08 cm less than children with the lowest score over the six-month period following the tests. Prendergast & Kelly [9] conducted a case–control study of 200 infants from Zimbabwe at 18 months. Biomarkers of intestinal damage (intestinal fatty acid-binding protein), inflammation (alpha-1 acid glycoprotein, C-reactive protein, IL-6) and growth hormone-IGF axis (insulin-like growth factor 1 and insulin-like growth factor-binding protein 3) were measured systemically. Infant plasma was tested at 6 weeks and 3, 6, 12 and 18 months, and in paired maternal–infant plasma at birth. Children who ended up stunted at 18 months of age had significantly elevated C-reactive protein and suppressed insulin-like growth factor 1. A picture emerges from these three studies of gut barrier leakage and its resulting chronic inflammation (both gut and systemic) as underlying growth suppression.
A measure of intestinal epithelial health would have the potential to add to the existing portfolio of biomarkers. We have developed a faecal ELISA for Reg1 to test it as a potential biomarker of small intestinal health. Reg1 is known to promote intestinal epithelial cell proliferation, regeneration and repair, and is upregulated in a variety of enteric infections and inflammatory conditions [10]. Reg1A and 1B are 166 amino acid proteins with 88% identity. Human REG1A is the best studied of the two genes. It is known to be expressed in a number of tissues including the pancreas, colon and rectal tumour tissues, the stomach, and importantly the small intestine where it is localized to Paneth cells and non-mature columnar cells of small intestinal crypts. Other data has also shown that Reg1A may be important for tissue repair and regeneration. We hypothesized that children with small intestine damage from enteric infection would have elevated faecal Reg1, and because of damage to their gut would be at increased risk of developing malnutrition. In fact, studies in birth cohorts from Bangladesh and Peru demonstrated that faecal Reg1, measured in 12-week-old children, predicted future stunting through the first 2 years of life (i.e. higher levels of Reg1 are associated with higher risk of future malnutrition; table 1) [10]. Reg1 is currently being tested for its ability to independently predict gut damage.
Table 1.
Linear regression analysis modelling future LAZs at 6–24 months to LAZs, Reg1B and family monthly income for a Bangladesh birth cohort. Reproduced from ref. [10] with permission. LAZ, length for age Z score; REGIB, regenerating gene 1β.
| Bangladesh cohort |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| LAZ at three months (every unit) |
REGIB (every 20 µg ml−1) |
family income (every $100) |
|||||||
| visit | estimate (s.e.)a | contributionb (%) | p-value | estimate (s.e.)a | contributionb (%) | p-value | estimate (s.e.)a | contributionb (%) | p-value |
| month 6 (n = 220) | 0.62 (0.04) | 54.3 | <0.0001 | −0.07 (0.04) | 0.5 | 0.067 | 0.39 (0.13) | 7.1 | 0.004 |
| month 9 (n = 218) | 0.57 (0.05) | 43.8 | <0.0001 | −0.13 (0.04) | 1.8 | 0.001 | 0.24 (0.15) | 4.2 | 0.114 |
| month 12 (n = 220) | 0.57 (0.05) | 42.5 | <0.0001 | −0.11 (0.04) | 1.4 | 0.011 | 0.47 (0.16) | 7.5 | 0.003 |
| month 15 (n = 216) | 0.57 (0.05) | 37.6 | <0.0001 | −0.09 (0.05) | 0.9 | 0.064 | 0.60 (0.18) | 8.2 | 0.001 |
| month 18 (n =214) | 0.55 (0.05) | 38.6 | <0.0001 | −0.10 (0.05) | 1.2 | 0.023 | 0.51 (0.17) | 7.9 | 0.002 |
| month 21 (n = 214) | 0.54 (0.05) | 38.8 | <0.0001 | −0.12 (0.04) | 1.9 | 0.007 | 0.66 (0.16) | 10.9 | <0.0001 |
| month 24 (n = 215) | 0.55 (0.05) | 35.2 | <0.0001 | −0.16 (0.05) | 2.6 | 0.001 | 0.68 (0.17) | 10.4 | <0.0001 |
aEstimated effect of LAZ at three months, REGIB and family income on future LAZ expressed as the magnitude change in future LAZ for every unit increment in LAZ at three months, 20 µg ml−1 increment in REGIB and $100 increment in family income. The s.e. values are for the corresponding covariate effect.
bIndependent contribution of a covariate expressed as the percentage of the explained variation in future LAZ.
While the ability to non-invasively identify infants with EE remains an aspirational goal, a picture is emerging of a heavy and constant burden of enteric infection, leading to gut barrier damage and intestinal and systemic inflammation. Unanswered are what are cause and effect, and whether early interventions based on biomarkers could alter the assumed consequences of oral vaccine failure, growth faltering and impaired neurocognitive development. The PROVIDE study (performance of rotavirus and oral polio vaccines in developing countries) has the goal to more comprehensively apply a portfolio of biomarkers to identify children with EE, in order to discern associations with the problems of vaccine failure, malnutrition and cognitive impairments so common in these populations. PROVIDE is a two-site observational clinical trial to measure and assess the impact of gut damage on infant health and development. Expanded Programme on Immunization (EPI) vaccines are administered by the study with outcomes measured over the first 5 years of life that include oral polio and rotavirus vaccine response, nutrition and development (table 2). The first 2 years of observation of the study are nearing completion, with the anticipation of the first analyses in the coming year. If early in life a common thread of gut damage is found to be predictive of these adverse outcomes, then the hope is that the non-invasive testing will serve as a basis for risk-stratification and early prevention or treatment [11].
Table 2.
PROVIDE biomarkers and outcomes.
| biomarker | outcome measures of small intestine health |
|---|---|
| epithelial health and repair — Reg1 intestinal barrier dysfunction and bacterial translocation — lactulose absorption — α1-antitrypsin — serum endocab antibody — serum endotoxin intestinal absorption — mannitol absorption intestinal inflammation — faecal myeloperoxidase — neopterin — calprotectin systemic inflammation — sCD14 — IL5, IL7 — G-CSF — C-reactive protein hormonal regulators — leptin — adiponectin — grehlin — insulin-like growth factor urine metabolome antibiotic use in first year enteric infections diarrhoea |
nutritional status — stunting (HAZ < −2) — wasting (WHZ < −2) — underweight (WAZ < −2) nutrient malabsorption — ferritin — vitamin A — zinc — vitamin D cognitive dysfunction — Bailey's test of cognitive, language and motor development oral vaccine failure — rotavirus serum IgA and rotavirus diarrhea after vaccination — oral polio serum neutralizing antibody and faecal excretion of Sabin vaccine virus |
4. Small intestine bacterial overgrowth as a form of environmental enteropathy
The gut microbiome is a dynamic complex of both prokaryotic and eukaryotic microorganisms that plays an important role in infant growth and development [12]. While bacteria are found throughout the gastrointestinal tract, the small intestine is relatively pristine with only 102–103 bacteria ml−1 in the upper small intestine compared with 108 bacteria ml−1 in the ileum, and as many as 1011 bacteria ml−1 in the colon [13,14]. However, children and adults with abnormalities in gut structure and function that lead to stasis of gut contents can develop small intestine bacterial overgrowth (SIBO). SIBO is defined as greater than 105 cfu ml−1 of bacterial growth in upper small intestine luminal fluid [14].
Fundamental changes to the gut structure and function found to be associated with EE have led to the hypothesis that SIBO may play a role in EE [2]. Periera et al. [15] investigated SIBO in a cohort of 340 children in rural Burma (Myanmar) and found that the prevalence of SIBO, as measured by lactulose breath testing, reached 27.2% by the second year of life. Two studies in Brazil by Dos Ries et al. [16] and Mello et al. [17] identified roughly similar rates of SIBO (37.5% and 30.9%, respectively) in slum-dwelling children. Notably, the rates of SIBO were much higher in the slum-dwelling children than in children who were able to seek private healthcare, implicating a role for socioeconomic status and likely sanitation in the development of SIBO. Khing-Maung et al. [18] found an association between SIBO and both nutrient malabsorption and stunting in Burmese children [2,14]. Lagos et al. [19] found an association between SIBO and decreased immunogenicity of the oral cholera vaccine CVD 103-HgR in a cohort of Chilean children, implicating SIBO in oral vaccine failure [14].
5. Role of environmental enteropathy in malnutrition
Malnutrition affects nearly a quarter of the world's children [11]. In these children, undernutrition can lead to stunting (length/height for age Z score of less than −2), wasting (weight for length/height Z score of less than −2), severe wasting (weight for height Z score less than −3) or severe acute malnutrition (weight for length/height Z score of −3 or lower or oedema) [20,21]. Several interventions have been shown to decrease maternal and child undernutrition including promotion of breastfeeding, strategies to promote complementary feeding, micronutrient interventions and reduction of disease burden. However, modelling by Bhutta et al. [20] suggests that even if all existing interventions were applied to 99% of the children in the 36 countries that are home to 90% of children with stunted linear growth they would only reduce stunting by 36% and mortality by 25%. This study demonstrated that there is a large gap in our understanding of how to prevent and treat malnutrition. It has been hypothesized that EE plays an important role in malnutrition and may be responsible for the predicted low impact of existing interventions [11].
The EE is characterized pathologically by villous blunting, which is likely to result in a reduction of the surface area of the mature absorptive intestinal epithelial cells [4]. It is plausible that these changes to the gut structure could hinder nutrient uptake; indeed expatriates with enteropathy were found to have reduced intestinal function including decreased absorption of carbohydrates, fat and vitamin B12 [4]. A study of asymptomatic rural Bangladeshi children aged 2–64 months found that the children were impaired in their ability to absorb d-xylose compared with children in the USA. The impairment was likely environmentally acquired as Bangladeshi infants under six months showed similar absorption to American infants of the same age [22]. A study of healthy rural Thai children found that half had abnormal absorption of d-xylose [4]. The children with absorption abnormalities in both of these studies have been described as having EE [4].
Dual sugar absorption tests, such as the lactulose–mannitol test, have also been used to assess gut function. Lactulose is too large to cross normal mucosa unless the epithelium has become permeable, while mannitol is taken up in proportion to small intestinal absorptive capacity. The ratio of lactulose to mannitol (L : M) is used to assess intestinal permeability and absorptive capacity. Studies in Africa, Asia and South America have shown increased L : M ratios in asymptomatic children and a study of asymptomatic Gambian children 2–5 years old found an inverse relationship between L : M ratios and height for age [4]. A second study of Gambian infants also found an association between growth faltering and a chronic inflammatory enteropathy of the small intestine [23]. It should be noted that other studies have failed to find similar associations between intestinal permeability and growth [4,24]. However, on the whole, the available data support a role for EE in malnutrition.
6. Infant development and environmental enteropathy
The EE may also have a significant impact on infant development. It is estimated that a full one-third of children below age 5 years in low- and middle-income countries fail to meet their full developmental potential [7,25,26]. These children are at increased risk of becoming trapped in a vicious cycle of poor health and poverty [25,26].
Poverty is associated with insufficient food and poor sanitation leading to high rates of infections and stunting in children, as well as low maternal education and inadequate stimulation in the home, all of which have a negative impact on child development [26]. Thus, it is not surprising that studies in many countries have shown an association between socioeconomic status and school enrolment, achievement and later cognitive attainment. A number of studies have also found an association between stunting and lower school enrolment, poor progress in school and poor cognitive ability [26]. More recently, it has also been appreciated that inflammation and infection during infancy and early childhood can have a profound impact on future cognitive development. Jiang et al. [25] assessed the impact of inflammation on the neurodevelopment of children living in a urban slum in Dhaka, Bangladesh, using febrile illness and elevated inflammation-related cytokines (IL-1β, IL-6, TNF-α, IL-4, IL-10) as markers of inflammation. Every additional 10 days of febrile illness was associated with a significant decrease in both language and motor scores. Elevated IL-1β and IL-6 were both associated with significantly lower motor scores, while IL-4 was associated with increased cognitive scores (figure 2). This study demonstrates that both clinical and biological markers of inflammation are implicated in poor neurodevelopmental outcomes in these low-income children. Both chronic inflammation and stunting have also been associated with EE, and thus EE may play a significant role in the impairment of infant development in impoverished environments.
Figure 2.
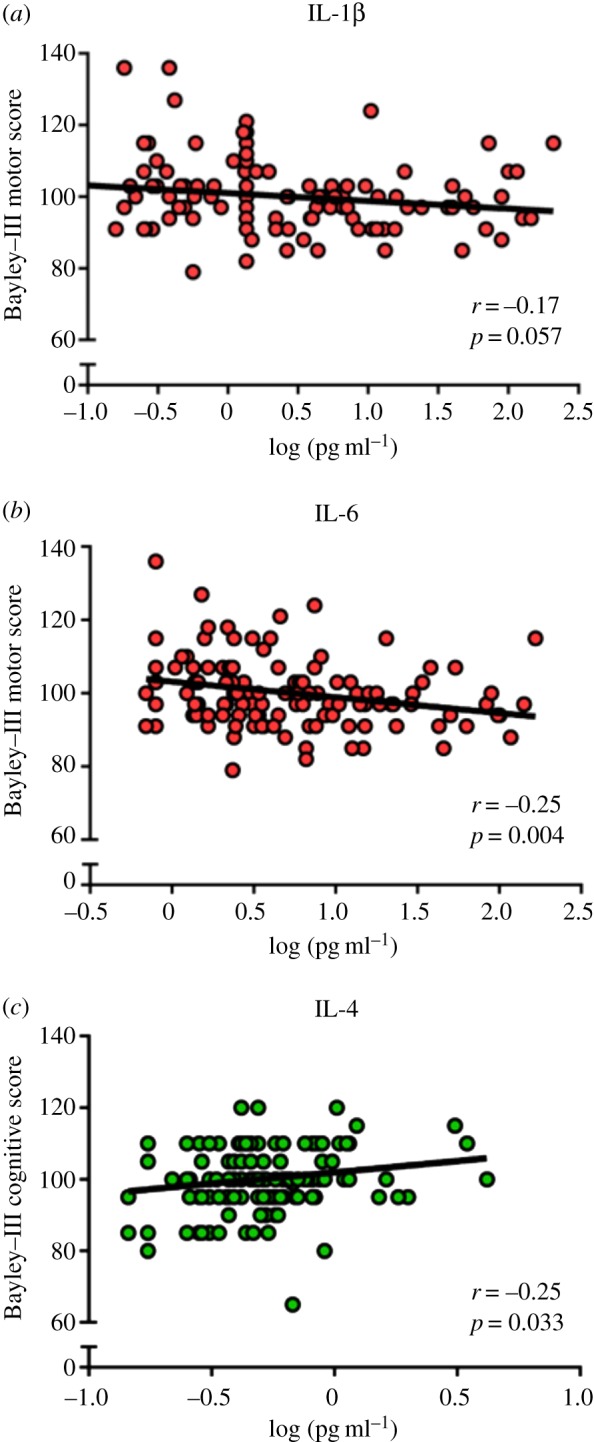
Cytokine levels in six-month sera are associated with developmental outcomes at 12 months. Plots show the associations of log-transformed individual cytokine concentrations with developmental outcomes at 12 months of age in a subset of 127 infants. Reproduced with permission from [25]. (Online version in colour.)
7. Could environmental enteropathy impair oral vaccination?
Oral vaccines are particularly attractive for use in low-income countries [13,27] owing to their relative ease of administration and because both children and adults often prefer oral vaccines to parenteral vaccines [13,28]. Oral vaccines are also chosen because of their superior ability to induce mucosal immunity [27]. However, it has been well established that many oral vaccines show decreased immunogenicity in low-income countries [13,29]. Grassly et al. [27] reported that trivalent oral polio vaccine had an efficacy of just 9% in the Indian state of Uttar Pradesh and only 21% throughout the rest of India, despite the fact that children in India are vaccinated with more doses of oral polio vaccine than anywhere else in the world, on average 15 doses in some parts of the country. Similarly, the two currently available oral rotavirus vaccines prevent, on average, only 50–60% of severe rotavirus cases in low-income countries with high rotavirus mortality, compared with 80–90% of severe rotavirus cases in higher income countries with low mortality [30–33]. Oral vaccines against cholera and shigella have also shown diminished immunogenicity in low-income countries.
There are several possible explanations for the decreased immunogenicity and efficacy of oral vaccines observed in low-income countries. It is possible that maternal antibodies, passed either via the placenta or via breast milk, could inhibit the replication of live oral vaccines and impair the ability of the vaccinated child to develop their own adaptive immune response [13,32]. It is also possible that enteropathogen infections at the time of vaccination could impair vaccine efficacy. As we have noted, children in low-income countries face a high burden of enteropathogens [8]. In support of this hypothesis, a recent meta-analysis of 25 oral polio vaccine trials by Grassly and colleagues [34] found that concurrent infections with non-polio enteroviruses significantly reduced the odds of per-dose seroconversion for type 1 poliovirus.
Environmental enteropathy may also play a role in oral vaccine failure in children from low-income countries [2,4,13]. Profound changes in the architecture and function of the small intestine are associated with EE, as demonstrated by biopsies and abnormal sugar absorption tests. In children with EE, constant exposure to enteropathogens leads to chronic activation of both the innate and adaptive immune responses [2]. It is entirely possible that this chronic immune activation could result in impaired mucosal immunity.
8. Concluding remarks
Children living in communities with poor sanitation are at increased risk of developing EE, a condition marked by significant changes in gut structure and function, as well as intestinal inflammation and a lack of overt diarrhoea. EE may play an important role in malnutrition, impaired infant development and oral vaccine failure. To date, efforts to study EE have been hampered by a lack of diagnostic tests. Potential tests based on serum and faecal biomarkers of intestinal health and inflammation may provide a solution. A recent study by Smith et al. [35] found that supplementation with multiple micronutrients could transiently ameliorate EE in Malawian children aged 12–35 months, providing a potential avenue for treatment. However, new studies are urgently needed to better understand EE and its consequences.
Funding statement
The work of the authors was supported by the Bill & Melinda Gates Foundation and the National Institutes of Health grant no. 5 R01 AI043596-14.
Authors' contributions
Each author contributed equally to the drafting and revising of the article and gave final approval for it to be published.
Conflict of interests
We have no competing interests.
References
- 1.Mondal D, Minak J, Alam M, Liu Y, Dai J, Korpe P, Liu L, Haque R, Petri WA. 2012. Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin. Infect. Dis. 54, 185–192. ( 10.1093/cid/cir807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korpe PS, Petri J, William A. 2012. Environmental enteropathy: critical implication of a poorly understood condition. Trends Mol. Med. 18, 328–336. ( 10.1016/j.molmed.2012.04.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prendergast A, Kelly P. 2012. Enteropathies in the developing world: neglected effects on global health. Am. J. Trop. Med. Hyg. 86, 756–763. ( 10.4269/ajtmh.2012.11-0743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keusch GT, et al. 2014. Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Clin. Infect. Dis. 59, S207–S212. ( 10.1093/cid/ciu485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan PB, Lunn PG, Northrop-Clewes CA, Crowe PT, Marsh MN, Neale G. 1992. Persistent diarrhea and malnutrition—the impact of treatment on small bowel structure and permeability. J. Pediatr. Gastroenterol. Nutr. 14, 208–215. ( 10.1097/00005176-199202000-00016) [DOI] [PubMed] [Google Scholar]
- 6.Kosek M, et al. 2013. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am. J. Trop. Med. Hyg. 88, 390–396. ( 10.4269/ajtmh.2012.12-0549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker SP, Wachs TD, Meeks Gardner J, Lozoff B, Wasserman GA, Pollitt E, Carter JA. 2007. Child development: risk factors for adverse outcomes in developing countries. Lancet 369, 145–157. ( 10.1016/S0140-6736(07)60076-2) [DOI] [PubMed] [Google Scholar]
- 8.Taniuchi M, et al. 2013. Etiology of diarrhea in Bangladeshi infants in the first year of life analyzed using molecular methods. J. Infect. Dis. 208, 1794–1802. ( 10.1093/infdis/jit507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prendergast AJ, et al. 2014. Stunting is characterized by chronic inflammation in Zimbabwean infants. PLoS ONE 9, e86928 ( 10.1371/journal.pone.0086928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson KM, et al. 2013. REG1B as a predictor of childhood stunting in Bangladesh and Peru. Am. J. Clin. Nutr. 97, 1129–1133. ( 10.3945/ajcn.112.048306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petri WA, Naylor C, Haque R. 2014. Environmental enteropathy and malnutrition: do we know enough to intervene? BMC Med. 12, 187 ( 10.1186/s12916-014-0187-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arrieta M-C, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. 2014. The intestinal microbiome in early life: health and disease. Front. Immunol. 5, 427 ( 10.3389/fimmu.2014.00427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine MM. 2010. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol. 8, 129 ( 10.1186/1741-7007-8-129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donowitz JR, Petri WA., Jr 2014. Pediatric small intestinal bacterial overgrowth in low-income countries. Trends Mol. Med. 21, 6–15. ( 10.1016/j.molmed.2014.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira SP, Khin Maung U, Bolin TD, Duncombe VM, Nyunt Nyunt W, Myo K, Linklater JM. 1991. A pattern of breath hydrogen excretion suggesting small bowel bacterial overgrowth in Burmese village children. J. Pediatr. Gastroenterol. Nutr. 13, 32–38. ( 10.1097/00005176-199107000-00006) [DOI] [PubMed] [Google Scholar]
- 16.Dos Reis JC, de Morais MB, Oliva CAG, Fagundes-Neto U. 2007. Breath hydrogen test in the diagnosis of environmental enteropathy in children living in an urban slum. Dig. Dis. Sci. 52, 1253–1258. ( 10.1007/s10620-006-9288-9) [DOI] [PubMed] [Google Scholar]
- 17.Mello CS, Tahan S, Melli LC, Rodrigues MS, de Mello RM, Scaletsky IC, de Morais MB. 2012. Methane production and small intestinal bacterial overgrowth in children living in a slum. World J. Gastroenterol. 18, 5932–5939. ( 10.3748/wjg.v18.i41.5932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khin Maung U, Bolin TD, Duncombe VM, Myo K, Nyunt Nyunt W, Pereira SP, Linklater JM. 1992. Epidemiology of small bowel bacterial overgrowth and rice carbohydrate malabsorption in Burmese (Myanmar) village children. Am. J. Trop. Med. Hyg. 47, 298–304. [DOI] [PubMed] [Google Scholar]
- 19.Lagos R, Fasano A, Wasserman SS, Prado V, Martin OS, Abrego P, Losonsky GA, Alegria S, Levine MM. 1999. Effect of small bowel bacterial overgrowth on the immunogenicity of single-dose live oral cholera vaccine CVD 103-HgR. J. Infect. Dis. 180, 1709–1712. ( 10.1086/315051) [DOI] [PubMed] [Google Scholar]
- 20.Bhutta ZA, et al. 2008. What works? Interventions for maternal and child undernutrition and survival. Lancet 371, 417–440. ( 10.1016/S0140-6736(07)61693-6) [DOI] [PubMed] [Google Scholar]
- 21.Victora CG, de Onis M, Hallal PC, Blössner M, Shrimpton R. 2010. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics 125, e473–e480. ( 10.1542/peds.2009-1519) [DOI] [PubMed] [Google Scholar]
- 22.Einstein LP, Mackay DM, Rosenberg IH. 1972. Pediatric xylose malabsorption in East Pakistan: correlation, with age, growth retardation, and weanling diarrhea. Am. J. Clin. Nutr. 25, 1230–1233. [DOI] [PubMed] [Google Scholar]
- 23.Campbell DI, Elia M, Lunn PG. 2003. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J. Nutr. 133, 1332–1338. [DOI] [PubMed] [Google Scholar]
- 24.Goto R, Panter-Brick C, Northrop-Clewes CA, Manahdhar R, Tuladhar NR. 2002. Poor intestinal permeability in mildly stunted Nepali children: associations with weaning practices and Giardia lamblia infection. Br. J. Nutr. 88, 141–149. ( 10.1079/BJN2002599) [DOI] [PubMed] [Google Scholar]
- 25.Jiang NM, et al. 2014. Febrile illness and pro-inflammatory cytokines are associated with lower neurodevelopmental scores in Bangladeshi infants living in poverty. BMC Pediatr. 14, 50 ( 10.1186/1471-2431-14-50) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B, the International Child Development Steering Group 2007. Developmental potential in the first 5 years for children in developing countries. Lancet 369, 60–70. ( 10.1016/S0140-6736(07)60032-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grassly NC, Jafari H, Bahl S, Sethi R, Deshpande JM, Wolff C, Sutter RW, Aylward RB. 2012. Waning intestinal immunity after vaccination with oral poliovirus vaccines in India. J. Infect. Dis. 205, 1554–1561. ( 10.1093/infdis/jis241) [DOI] [PubMed] [Google Scholar]
- 28.Sow SO, et al. 2012. Efficacy of the oral pentavalent rotavirus vaccine in Mali. Vaccine 30, A71–A78. ( 10.1016/j.vaccine.2011.11.094) [DOI] [PubMed] [Google Scholar]
- 29.John TJ. 1976. Antibody response of infants in tropics to five doses of oral polio vaccine. Br. Med. J. 1, 812 ( 10.1136/bmj.1.6013.812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soares-Weiser K, MacLehose H, Bergman H, Ben-Aharon I, Nagpal S, Goldberg E, Pitan F, Cunliffe N. 2012. Vaccines for preventing rotavirus diarrhoea: vaccines in use. In Cochrane database of systematic reviews. New York: John Wiley & Sons. [Google Scholar]
- 31.Armah GE, et al. 2010. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 376, 606–614. ( 10.1016/S0140-6736(10)60889-6) [DOI] [PubMed] [Google Scholar]
- 32.Glass RI, Parashar U, Patel M, Gentsch J, Jiang B. 2014. Rotavirus vaccines: successes and challenges. J. Infect. 68(Suppl. 1), S9–S18. ( 10.1016/j.jinf.2013.09.010) [DOI] [PubMed] [Google Scholar]
- 33.Zaman K, et al. 2010. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 376, 615–623. ( 10.1016/S0140-6736(10)60755-6) [DOI] [PubMed] [Google Scholar]
- 34.Parker EPK, Kampmann B, Kang G, Grassly NC. 2014. Influence of enteric infections on response to oral poliovirus vaccine: a systematic review and meta-analysis. J. Infect. Dis. 210, 853–864. ( 10.1093/infdis/jiu182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith HE, et al. 2014. Multiple micronutrient supplementation transiently ameliorates environmental enteropathy in Malawian children aged 12–35 months in a randomized controlled clinical trial. J. Nutr. 144, 2059–2065. ( 10.3945/jn.114.201673) [DOI] [PubMed] [Google Scholar]