Abstract
Despite significant progress in reducing the burden of mortality in children under the age of five, reducing mortality in newborns remains a major challenge. Infection plays a significant role in infant deaths and interventions such as early vaccination or antenatal immunization could make a significant contribution to prevention of such deaths. In the last few years, we have gained new insights into immune ontogeny and are now beginning to understand the impact of vaccines, nutrition and environmental factors on ‘training′ of the immune response in early life. This review article sets out to explain why vaccine responses can be heterogeneous between populations and individuals by providing examples chosen to illustrate the impact of host, pathogen and environmental factors on shaping the immune ‘interactome′ in young children.
Keywords: immune ontogeny, vaccine responses, maternal immunization, BCG vaccine
1. Introduction
There can be no doubt that the inclusion of additional life-saving vaccines in the infant vaccination schedule has had a major beneficial impact on global health [1]. While fully acknowledging the considerable progress that has been achieved internationally in lowering mortality in children under the age of 5, neonatal mortality continues to demand our urgent attention, as 40% of deaths are attributable to infection in this early period of life [2,3]. The causes of neonatal mortality are multifactorial and ultimately require a multi-disciplinary approach to be addressed, but infection is a key contributor: of the over 4 million neonatal deaths annually, three-quarters occur in the first week of life and one-third are estimated to be related to infection [4].
Over 90% of these early deaths are found in resource-constrained settings where exposure to pathogens and colonization with commensals and pathogens alike is also known to occur at very early stages in life [5,6].
Apart from bacille Calmette–Guérin (BCG), polio and hepatitis B, no other vaccines are given to infants younger than 6–8 weeks of age, after the majority of deaths have already occurred. The potential impact of vaccines in this younger age group remains insufficiently explored.
In the last few years, an increasing number of investigators have contributed data describing the ontogeny of the neonatal immune system. In light of these results, the perception of the neonatal immune system as somewhat non-functional should now be re-examined [7]. The notion that vaccines will not work well in neonates, or that the presence of maternal antibody significantly interferes with their protective efficacy is currently being re-examined in the laboratory and in field trials.
This review article will focus on recent insights into immune regulation in neonates, describes environmental, maternal and infant factors which might influence the immune ontogeny and illustrate the opportunities of maternal immunization to add protection during the most delicate phase of life, the neonatal period.
2. Transition from the in utero environment to a world full of pathogens and maturation of immune pathways shortly after birth
Following the relatively limited exposure to antigens in utero, the neonate will rapidly transition to a state of colonization of internal and external surfaces with bacteria, some of which are potentially pathogenic. The innate immune response, which initially is largely weighted towards maintaining tolerance between mother and fetus, has to rapidly evolve and generate adaptive responses able to distinguish between self and non-self antigens in order to mount suitable generic and antigen-specific defences [8].
The availability of novel laboratory tools and applicability to optimized small-volume whole blood assays has provided us with an opportunity to study maturation of innate immunity in greater detail than was possible in the past. This has resulted in renewed interest in immune ontogeny and several publications have now reported that the expression of innate pattern-recognition receptors (PRRs) changes in the first year of life, with the major changes occurring in the early neonatal period.
In a study conducted in The Gambia, Burl et al. [9] demonstrated that pro-inflammatory responses induced through stimulation of Toll-like receptor (TLR) 4, 5 and 8 changed significantly in the first month of life. Similar data were reported from Papua New Guinea and across a number of countries using comparable methodology [10–12].
In summary, it is now well established that the ability of the innate immune system to recognize specific pathogens undergoes maturation. By adding our increasing knowledge about the changing microbiome straight after birth, the field can now start to dissect the interactions between host and pathogen in more detail [13].
In addition to host-specific maturation of innate immunity, other factors are likely to influence the magnitude and quality of immune responses in infants, as summarized in figure 1.
Figure 1.
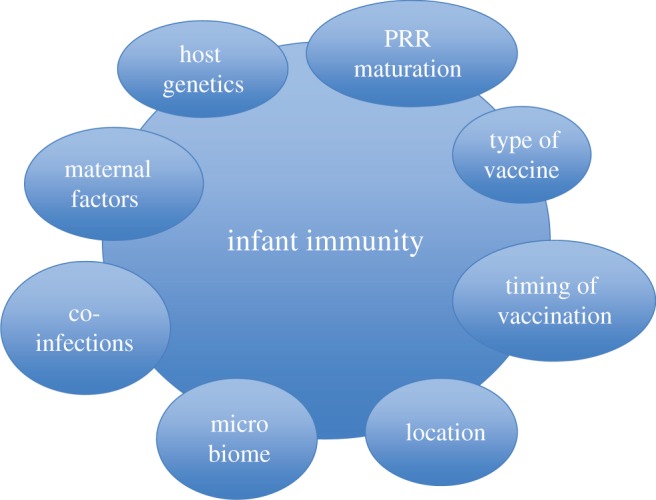
Key factors known to influence early infant immunity and responses to vaccines. (Online version in colour.)
3. Vaccines as experimental tools to probe immune pathways
While colonization of the neonate with different organisms depends on the immediate environment and appears somewhat circumstantial, the use of vaccines as experimental tools to probe immune pathways provides us with an opportunity to control for specific types of antigen exposure and examine the downstream effects: we can vary time and dose and dissect in more detail the innate and acquired responses triggered by a well-defined immune stimulus. This model has already been substantially investigated in the context of BCG vaccination, which is of particular interest to the immunology and vaccine community; the vaccine elicits inconsistent levels of protection and has been shown to have heterologous effects, making the availability of a better vaccine against tuberculosis urgent [14].
Data from studies of alterations of BCG strains and timing of vaccination show that the acquired immune response can be influenced by either factor [15]. By comparing acquired vaccine responses to BCG given at birth or delayed until 4.5 months, our group in The Gambia showed that BCG vaccination at birth induced a strong mixed Th1 and Th2 response, which was significantly different to those responses obtained at 4.5 months in unvaccinated infants [16]. However, these changes were only transient, as by 9 months both early and late vaccinated infants showed the same level of responses. This study was limited to examining acquired responses.
Shey et al. [17] have since examined the innate components of the immune response to BCG in more detail. They measured whole blood or peripheral blood mononuclear cells (PBMCs) from newborns, 10- and 36-week-old infants, incubated with BCG or TLR ligands and a generic positive control. Innate cytokines and maturation markers, and activation of pro-inflammatory NF-κB and mitogen-activated protein kinase (MAPK) signalling pathways were investigated. The results showed that levels of secreted cytokines in whole blood supernatants increase with age, as did the monocyte-specific expression of pro-inflammatory cytokines in newborns and infants. The authors concluded that innate immune responses to BCG and signal-transduction pathways downstream to TLR mature between birth and 9 months to a more pro-inflammatory capacity in monocytes but not in myeloid dendritic cells.
4. Location, location, location
It is also well established that not all infants respond to the same vaccine in the same way in all locations: data comparing BCG responses in infants in different parts of Africa and Europe have shown significant differences in immune parameters measured, somewhat reflecting the heterogeneity of protection in these geographical areas, well known to affect this vaccine in particular [18].
Hur et al. [19] reported that location affected the acquired responses in blood from BCG-immunized infants from Malawi, the Gambia and the UK. The study showed significant differences in the production of key cytokines in a 6-day whole blood stimulation assay. This type of assay cannot predict cell-type specific responses, hence we cannot be sure which cells were primarily involved in mediating such differences. The same authors also compared results between infants born in the dry season as opposed to the wet season and concluded that season of birth also impacted on the responses; however, mechanisms were not elucidated.
A comparative study by Smolen et al. [20] examined the cellular composition of blood samples taken from 2-year old children in Belgium, Canada, South Africa and Ecuador. The work also aimed to identify the cellular sources of secreted cytokines in response to PRR stimulation, found to be different in previous studies between children from different settings. Whole blood from 2-year old children at the four distinct sites was incubated with TLR ligands for 6 h, cell pellets were harvested and frozen, and an 11-colour flow panel identified key cell populations and single-cell specific intracellular cytokine production following TLR stimulation. The results varied between sites, with single-cell specific cytokine responses to TLR/Nod-like receptor (NLR) ligands being significantly lower in South African children.
There is no current explanation for this phenomenon, as all assays were conducted using the same protocols and technician for all settings and experiments.
However, given this data, it is not surprising that innate and acquired immunity to vaccines might show differences in infants from different regions of the world.
5. The impact of maternal or infant co-infections on vaccine responses
Significant insights into the impact of co-morbidity on immune responses in general and vaccine responses in particular have been gained through studies of children exposed to malaria, helminths or HIV. For example, Hartgers et al. [21] examined the impact of malaria as a co-infection on TLR expression/responsiveness in schoolchildren in Ghana. The authors reported higher expression of TLR2 and MAPK in malaria co-infection. In vitro incubation of PBMCs with Plasmodium falciparum-infected red blood cells led to enhanced responsiveness to TLR ligands. Co-infection with malaria therefore showed altered innate responses, which might alter vaccine responses; however, these were not measured. Elliott et al. [22] studied the impact of maternal helminth infections on infant vaccine responses and found some impact on a number of cytokine responses. The authors concluded, though, that maternal helminth infection may have little, if any, adverse effect on the outcome of infant immunization.
We have recently conducted observational studies to understand the impact of maternal infection on immune priming and vaccine responses in the neonate. In a South African mother–infant cohort, we showed that maternal HIV infection is associated with significantly lower concentrations of specific antibody to vaccine antigens in HIV-exposed, uninfected infants at birth compared with infants not exposed to HIV; however, HIV exposure did not affect the infant's ability to mount a robust response to infant immunization [23]. We also examined the consequences of maternal HIV infection and/or sensitization to mycobacteria and found that HIV-exposed, uninfected infants have normal responses to BCG vaccination administered at birth [24]. Even though the infants of HIV-infected mothers remained uninfected, in utero HIV exposure did affect specific T-cell subsets in these uninfected infants at birth. Our key findings were that TNF-α concentration was significantly increased in HIV-exposed infants born to Mycobacterium tuberculosis (Mtb) sensitized mothers, suggesting that in utero exposure to infection can prime the developing immune response, even in the absence of infant infection. Furthermore, we observed that in the presence of maternal Mtb sensitization, frequencies of maternal and newborn infant BCG-specific proliferating CD4+ T cells were positively correlated, again suggesting an effect of maternal cellular responses on infant responses to BCG antigen. These effects were all transient; following infant BCG vaccination administered at 6 weeks of age, there was no effect of in utero HIV exposure or maternal Mtb infection on infant BCG-specific T-cell proliferative responses or concentrations of secreted cytokines and chemokines at 16 weeks of age.
It can therefore be concluded that despite the effect of maternal HIV and Mtb infection on infant immune profiles at birth, HIV-exposed, non-infected infants have the same potential to respond to, and be protected by, BCG vaccination as HIV-unexposed infants and that any bias in innate responses at birth appears to be transient in nature.
The deficiency in antibody-mediated protection among HIV-exposed infants prior to vaccination, however, is of concern. Since maternal antibody concentration is inversely correlated with maternal immunosuppression, the best strategy for enhancing protection of the young infant is to optimize maternal health by administering highly active antiretroviral treatment (HAART) to HIV-infected women. Therefore, the better the management of maternal HIV, the better antibody protection afforded to the neonate. We could demonstrate this concept in our maternal–infant cohort study in the UK, where—unlike in South Africa—we did not find significantly lower vaccine-specific antibody in HIV-infected women. These women had been managed consistently with ART and were less immunocompromised in both B- and T-cell compartments, indirectly affording protection to their infants [25].
6. Immunization of pregnant women and influence on infant immune responses
The regulation of infant immune responses to a variety of stimuli is likely to start well before birth. In the early perinatal period, infants are largely protected by the presence of maternal antibody, which is transmitted transplacentally in the third trimester of pregnancy [26]. This principle of passive immunization has been exploited by immunizing women during pregnancy, where a vaccine given to the mother generates sufficient antibody to protect the newborn, as already established practice in resource poor settings to protect against neonatal tetanus. Providing passive protection through tetanus vaccination of women in pregnancy is now standard practice in developing countries and has globally decreased the rates of neonatal tetanus from 200 000 in the year 2000 to 49 000 in 2013 [27]. Potentially, this mechanism can be harnessed to protect against other infections in the neonatal period and beyond, and the same concepts are currently being explored for group B streptococcus, respiratory syncytial virus pneumococcus, meningococcus and potentially other candidates, with pertussis and influenza vaccination in pregnancy already in place in some settings, but not routinely in developing countries [7].
Antenatal pertussis immunization programmes have been implemented in countries that have experienced a recent resurgence of pertussis, which particularly affected infants too young to be protected by existing infant immunization programmes. In the UK alone, 14 infants died as a result of confirmed pertussis in 2012 [28]. The UK antenatal pertussis immunization programme has been very successful, with coverage of over 60% and vaccine effectiveness of 91% against pertussis in infants less than 3 months [28,29]. However, high concentrations of maternally derived antibody will alter the baseline for responses to vaccines as part of the EPI schedule and therefore have the potential to blunt the infant's own response to vaccination. When comparing vaccine responses in infants born to mothers who had or had not received immunization against pertussis in pregnancy, the rises in antibody titres in infants born to vaccinated mothers have not been as substantial, but our own data and data from other groups indicate that the overall infant antibody responses to the routine childhood vaccines are well within the range of putative protection [30,31]. Administration of the influenza vaccine to pregnant women has also shown significant positive impact on both maternal and infant health: pregnant women who received H1N1 influenza vaccine were less likely to give birth preterm, and their babies were heavier at birth [32]. Immune responses to subsequent vaccines were not specifically measured in these trials, but we know that gestational age and birth weight impact on immunity in general.
Presence of maternal antibody can potentially interfere with the subsequent immunogenicity of vaccines given to newborns and infants, as has been shown in the context of maternally derived measles antibodies inhibiting immunogenicity of the measles vaccine given to infants [33]. However, antibody levels presumed to be indicative of protection are ultimately achieved for all vaccines used in current schedules in EPI programmes where such studies have been carried out. Whether quality and longevity of this antibody response could be affected in the longer term by the presence of maternal antibody is the subject of ongoing cohort studies.
7. The concept of ‘innate immune memory’
In the context of the well-known heterologous effects of BCG vaccine [29], a novel and somewhat still controversial concept that is now sometimes referred to as ‘trained immunity’ or ‘innate immune memory’ has arisen [34–36]. Trained immunity is defined as enhanced non-specific innate immune protection that is suggested to be mediated by epigenetic mechanisms. It basically describes the ability of an innate immune cell population to generate immunological memory and to influence the response to a second stimulus, as proposed by Netea et al. [37]. The experimental work shows that primary exposure to microbial ligands alters the fate of monocytes, dependent on the nature and concentration of the ligand, with impact on subsequent immune responses and mediated by epigenetic mechanisms.
Similar principles can obviously apply to all the factors mentioned above and the observation of heterologous effects of vaccines—or possibly other infectious diseases, nutrition and also the microbiome—is therefore not all that surprising.
8. Host genetics
It has to be assumed of course that some heterogeneity of observed vaccine responses can be ascribed to host genetic factors [38]. Marchant et al. [39] measured antibody responses to tetanus toxoid, measles and total IgG in 210 Gambian twin pairs in The Gambia. The authors compared the intra-pair correlations for monozygous and heterozygous pairs to estimate environment versus genetic components of variation in response. The authors concluded that genetic determinants control the early phase of the vaccine antibody response, while the environment predominantly influences antibody persistence and avidity maturation.
Several large cohort studies are currently being undertaken to identify the contribution of specific genotypical markers to immune responses, mostly vaccines. This area of work is discussed extensively by Mentzer et al. [40] in this issue.
9. Where next for immune ontogeny and protection of the most vulnerable?
In order to understand the underlying mechanisms for the phenomena described above, deeper insights into the ‘immune interactome’ are still required. Future studies should aim to further delineate the impact of microbial exposure on innate immunity and dissect molecular and cellular mechanisms and novel epigenetic phenomena.
Detailed longitudinal studies of prospectively followed groups of infants in several locations are likely to be required to relate innate responses to acquired, antigen-specific immune responses, and to gain an in-depth understanding of immune priming and the interplay of factors determining immune trajectories.
Given our current data on maturation of PRRs, the potential role of TLR adjuvants in optimizing vaccine responses in neonates and older children remains to be established, and the impact of maternal immunization on enhancing protection and ‘innate immune memory’ in infants could potentially be increased by additional vaccines given in pregnancy.
Given the rapid changes occurring straight after birth, future studies ought to be conducted in narrowly defined age groups, using distinct vaccines or naturally occurring organisms as probes. Many lessons can also be learnt from ‘extreme phenotypes’, i.e. immunocompromised hosts and vaccine failures. Comprehensive age-related studies, tools and populations are required, and epidemiological confounders need to be carefully considered to best exploit the immune interactome for combating neonatal morbidity and mortality.
Acknowledgements
We acknowledge the Thrasher Foundation and the European Society for Paediatric Infectious Diseases for additional support to our vaccine-related research.
Funding statement
B.K. is supported by a Program grant from the MRC (MR/K007602/1, MR/K011944/1, MC_UP_A900/1122) and the Biomedical Research Centre at Imperial College, London.
Conflict of interest
We have no competing interests.
References
- 1.Koff WC, Burton DR, Johnson PR, Walker BD, King CR, Nabel GJ, Ahmed R, Bhan MK, Plotkin SA. 2013. Accelerating next-generation vaccine development for global disease prevention. Science 340, 1232910 ( 10.1126/science.1232910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill K, You D, Inoue M, Oestergaard MZ. 2012. Child mortality estimation: accelerated progress in reducing global child mortality, 1990–2010. PLoS Med. 9, e1001303 ( 10.1371/journal.pmed.1001303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, et al. 2012. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379, 2151–2161. ( 10.1016/S0140-6736(12)60560-1) [DOI] [PubMed] [Google Scholar]
- 4.Lawn JE, Cousens S, Zupan J, Lancet Neonatal Survival Steering Team. 2005. 4 million neonatal deaths: when? where? why? Lancet 365, 891–900. ( 10.1016/S0140-6736(05)71048-5) [DOI] [PubMed] [Google Scholar]
- 5.Waters D, et al. 2011. Aetiology of community-acquired neonatal sepsis in low and middle income countries. J. Glob. Health 1, 154–170. [PMC free article] [PubMed] [Google Scholar]
- 6.Kwambana BA, Barer MR, Bottomley C, Adegbola RA, Antonio M. 2011. Early acquisition and high nasopharyngeal co-colonisation by Streptococcus pneumoniae and three respiratory pathogens amongst Gambian new-borns and infants. BMC Infect. Dis. 11, 175 ( 10.1186/1471-2334-11-175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindsey B, Kampmann B, Jones C. 2013. Maternal immunization as a strategy to decrease susceptibility to infection in newborn infants. Curr. Opin. Infect. Dis. 26, 248–253. ( 10.1097/QCO.0b013e3283607a58) [DOI] [PubMed] [Google Scholar]
- 8.Levy O. 2007. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat. Rev. Immunol. 7, 379–390. ( 10.1038/nri2075) [DOI] [PubMed] [Google Scholar]
- 9.Burl S, et al. 2011. Age-dependent maturation of Toll-like receptor-mediated cytokine responses in Gambian infants. PLoS ONE 6, e18185 ( 10.1371/journal.pone.0018185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lisciandro JG, et al. 2012. Ontogeny of Toll-like and NOD-like receptor-mediated innate immune responses in Papua New Guinean infants. PLoS ONE 7, e36793 ( 10.1371/journal.pone.0036793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kollmann TR, et al. 2009. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 183, 7150–7160. ( 10.4049/jimmunol.0901481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kollmann TR. 2013. Variation between populations in the innate immune response to vaccine adjuvants. Front. Immunol. 4, 81 ( 10.3389/fimmu.2013.00081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houghteling PD, Walker WA. 2015. Why is initial bacterial colonization of the intestine important to infants’ and children's health? J. Pediatr. Gastroenterol. Nutr. 60, 294–307. ( 10.1097/MPG.0000000000000597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kampmann B, Tena GN, Mzazi S, Eley B, Young DB, Levin M. 2004. Novel human in vitro system for evaluating antimycobacterial vaccines. Infect. Immun. 72, 6401–6407. ( 10.1128/IAI.72.11.6401-6407.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lutwama F, et al. 2014. Distinct T-cell responses when BCG vaccination is delayed from birth to 6 weeks of age in Ugandan infants. J. Infect. Dis. 209, 887–897. ( 10.1093/infdis/jit570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burl S, et al. 2010. Delaying bacillus Calmette–Guérin vaccination from birth to 4 1/2 months of age reduces postvaccination Th1 and IL-17 responses but leads to comparable mycobacterial responses at 9 months of age. J. Immunol. 185, 2620–2628. [DOI] [PubMed] [Google Scholar]
- 17.Shey MS, et al. 2014. Maturation of innate responses to mycobacteria over the first nine months of life. J. Immunol. 192, 4833–4843. ( 10.4049/jimmunol.1400062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trunz BB, Fine P, Dye C. 2006. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 367, 1173–1180. ( 10.1016/S0140-6736(06)68507-3) [DOI] [PubMed] [Google Scholar]
- 19.Hur Y-G, et al. 2014. Factors affecting immunogenicity of BCG in infants, a study in Malawi, The Gambia and the UK. BMC Infect. Dis. 14, 184 ( 10.1186/1471-2334-14-184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smolen KK, et al. 2014. Single-cell analysis of innate cytokine responses to pattern recognition receptor stimulation in children across four continents. J. Immunol. 193, 3003–3012. ( 10.4049/jimmunol.1400895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartgers FC, Obeng BB, Voskamp A, Larbi IA, Amoah AS, Luty AJF, Boakye D, Yazdanbakhsh M. 2008. Enhanced Toll-like receptor responsiveness associated with mitogen-activated protein kinase activation in Plasmodium falciparum-infected children. Infect. Immun. 76, 5149–5157. ( 10.1128/IAI.01579-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott AM, et al. 2010. Effects of maternal and infant co-infections, and of maternal immunisation, on the infant response to BCG and tetanus immunisation. Vaccine 29, 247–255. ( 10.1016/j.vaccine.2010.10.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. 2011. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. J. Am. Med. Assoc. 305, 576–584. ( 10.1001/jama.2011.100) [DOI] [PubMed] [Google Scholar]
- 24.Jones CE, Hesseling AC, Tena-Coki NG, Scriba TJ, Chegou NN, Kidd M, Wilkinson RJ, Kampmann B. 2015. The impact of HIV exposure and maternal Mycobacterium tuberculosis infection on infant immune responses to bacille Calmette–Guérin vaccination. AIDS 29, 155–165. ( 10.1097/QAD.0000000000000536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones C, Pollock L, Barnett SM, Battersby A, Kampmann B. 2013. Specific antibodies against vaccine-preventable infections: a mother–infant cohort study. BMJ Open 3, e002473 ( 10.1136/bmjopen-2012-002473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindsey B, Jones C, Kampmann B. 2012. Bridging the gap: maternal immunisation as a means to reduce neonatal deaths from infectious diseases. Pathog. Glob. Health 106, 137–138. ( 10.1179/204777312X13462106637684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan R, Vandelaer J, Yakubu A, Raza AA, Zulu F. 2015. Maternal and neonatal tetanus elimination: from protecting women and newborns to protecting all. Int. J. Women's Health 7, 171–180. ( 10.2147/IJWH.S50539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Donegan K, Fry NK, Miller E, Ramsay M. 2014. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet 384, 1521–1528. ( 10.1016/S0140-6736(14)60686-3) [DOI] [PubMed] [Google Scholar]
- 29.Dabrera G, Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Fry NK, Ramsay M. 2015. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012–2013. Clin. Infect. Dis. 60, 333–337. ( 10.1093/cid/ciu821) [DOI] [PubMed] [Google Scholar]
- 30.Jones C, Pollock L, Barnett SM, Battersby A, Kampmann B. 2014. The relationship between concentration of specific antibody at birth and subsequent response to primary immunization. Vaccine 32, 996–1002. ( 10.1016/j.vaccine.2013.11.104) [DOI] [PubMed] [Google Scholar]
- 31.Munoz FM, et al. 2014. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants. J. Am. Med. Assoc. 311, 1760–1769. ( 10.1001/jama.2014.3633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richards JL, et al. 2013. Neonatal outcomes after antenatal influenza immunization during the 2009 H1N1 influenza pandemic: impact on preterm birth, birth weight, and small for gestational age birth. Clin. Infect. Dis. 56, 1216–1222. ( 10.1093/cid/cit045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cáceres VM, Strebel PM, Sutter RW. 2000. Factors determining prevalence of maternal antibody to measles virus throughout infancy: a review. Clin. Infect. Dis. 31, 110–119. ( 10.1086/313926) [DOI] [PubMed] [Google Scholar]
- 34.Ota MOC, et al. 2002. Influence of Mycobacterium bovis bacillus Calmette-Guérin on antibody and cytokine responses to human neonatal vaccination. J. Immunol. 168, 919–925. ( 10.4049/jimmunol.168.2.919) [DOI] [PubMed] [Google Scholar]
- 35.Freyne B, Marchant A, Curtis N. 2015. BCG-associated heterologous immunity, a historical perspective: intervention studies in animal models of infectious diseases. Trans. R. Soc. Trop. Med. Hyg. 109, 52–61. ( 10.1093/trstmh/tru197) [DOI] [PubMed] [Google Scholar]
- 36.Netea MG, van Crevel R. 2014. BCG-induced protection: effects on innate immune memory. Semin. Immunol. 26, 512–517. ( 10.1016/j.smim.2014.09.006) [DOI] [PubMed] [Google Scholar]
- 37.Ifrim DC, et al. 2014. Trained immunity or tolerance: opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clin. Vacc. Immunol. 21, 534–545. ( 10.1128/CVI.00688-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newport MJ, Goetghebuer T, Weiss HA, Whittle H, Siegrist CA, Marchant A. 2004. Genetic regulation of immune responses to vaccines in early life. Genes and immunity. Nature 5, 122–129. ( 10.1038/sj.gene.6364051) [DOI] [PubMed] [Google Scholar]
- 39.Marchant A, et al. 2006. Predominant influence of environmental determinants on the persistence and avidity maturation of antibody responses to vaccines in infants. J. Infect. Dis. 193, 1598–1605. ( 10.1086/503775) [DOI] [PubMed] [Google Scholar]
- 40.Mentzer AJ, O'Connor D, Pollard AJ, Hill AVS. 2015. Searching for the human genetic factors standing in the way of universally effective vaccines. Phil. Trans. R. Soc. B 370, 20140341 (doi:10.1098.rstb.2014.0341) [DOI] [PMC free article] [PubMed] [Google Scholar]


