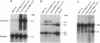Abstract
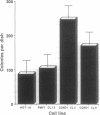
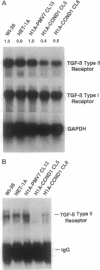
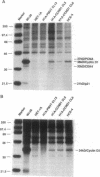
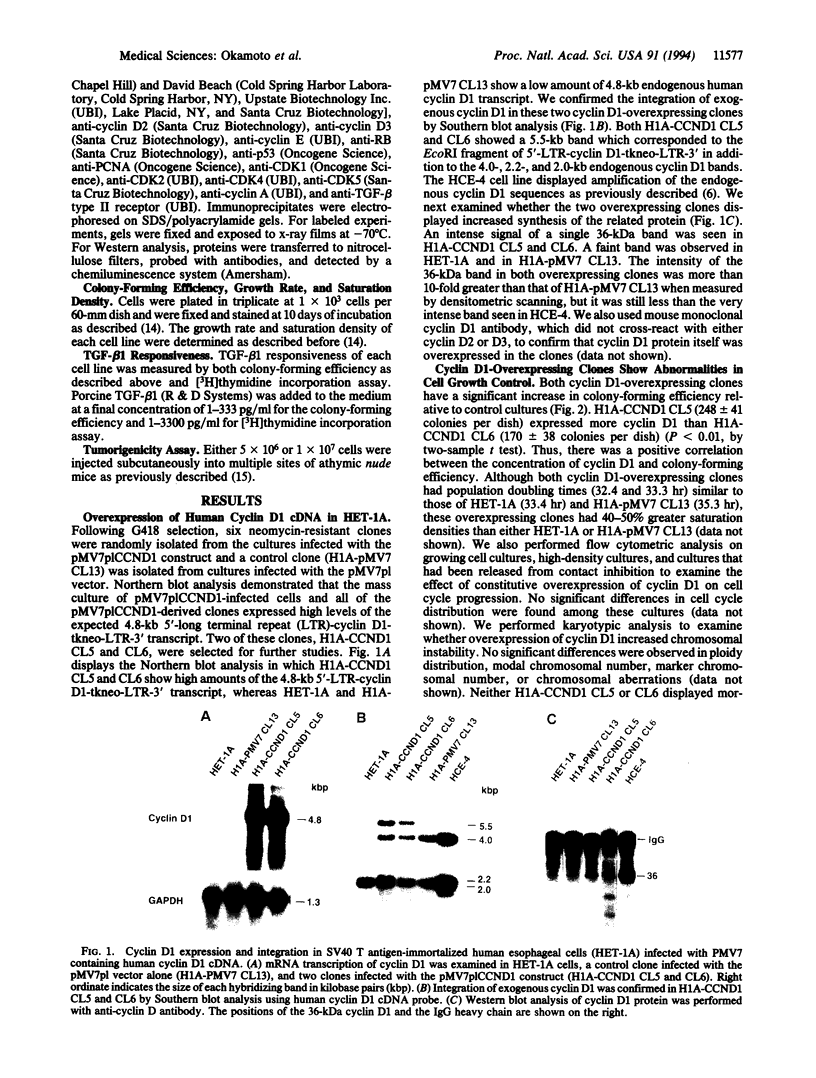
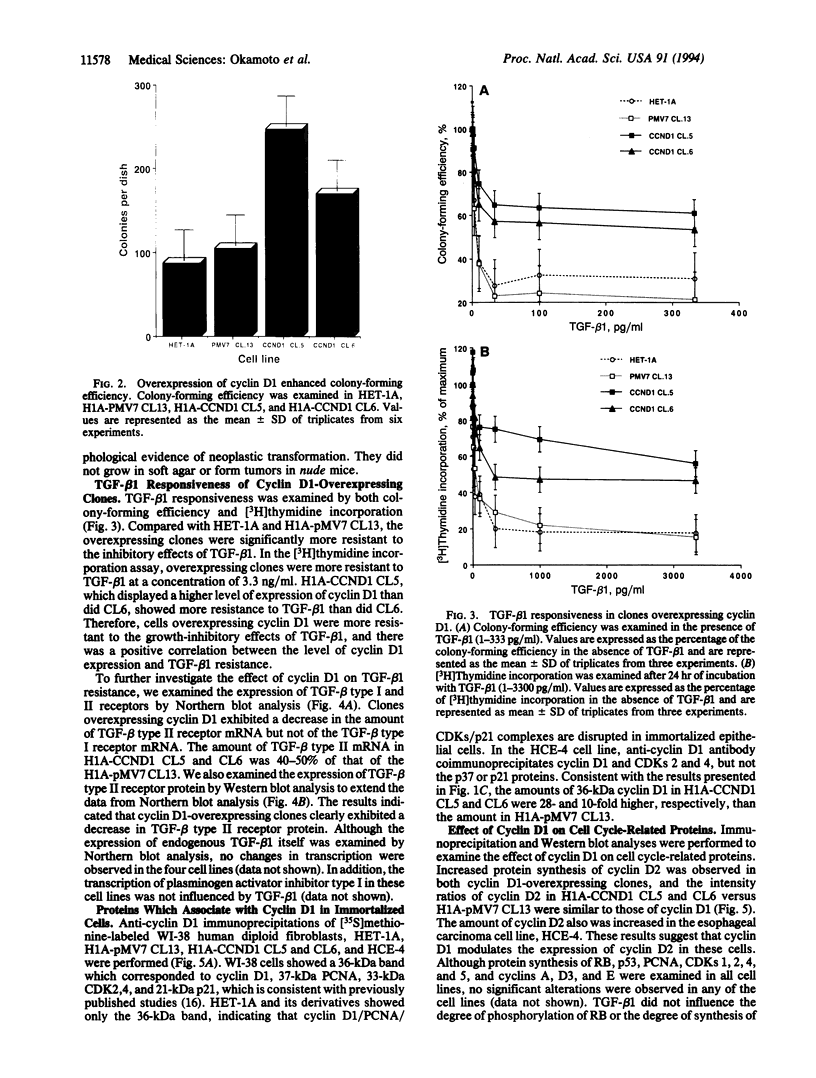
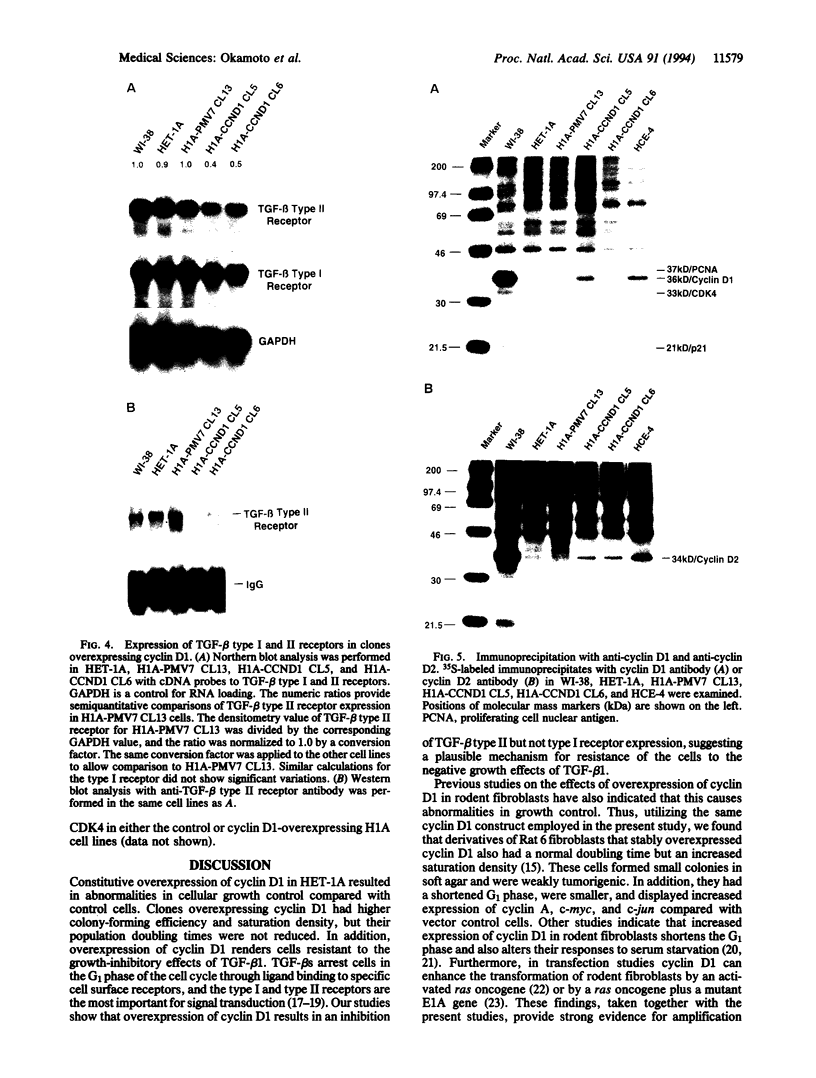
Cyclin D1 has been implicated in G1 cell cycle progression and is frequently amplified, overtranscribed, and oversynthesized in human tumors, including esophageal carcinomas. To further address the role of cyclin D1 in cell cycle control and tumorigenesis, we have stably transfected the human cyclin D1 in the nontumorigenic esophageal epithelial cell line HET-1A. These transfected cells, which express increased amounts of cyclin D1, have enhanced colony-forming efficiency and saturation density and are resistant to growth inhibition by TGF-beta 1 compared with the parental cell line or a control vector cell clone. The clones which express increased amounts of cyclin D1 exhibited a decrease in the amount of TGF-beta type II receptor, indicating a plausible mechanism for their diminished response to TGF-beta 1. Therefore, deregulated expression of the cyclin D1 gene can modulate the negative growth factor pathway of TGF-beta 1 and may disturb the control of epithelial cell proliferation in esophageal carcinogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldin V., Lukas J., Marcote M. J., Pagano M., Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993 May;7(5):812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- Bassing C. H., Yingling J. M., Howe D. J., Wang T., He W. W., Gustafson M. L., Shah P., Donahoe P. K., Wang X. F. A transforming growth factor beta type I receptor that signals to activate gene expression. Science. 1994 Jan 7;263(5143):87–89. doi: 10.1126/science.8272871. [DOI] [PubMed] [Google Scholar]
- Boyd F. T., Massagué J. Transforming growth factor-beta inhibition of epithelial cell proliferation linked to the expression of a 53-kDa membrane receptor. J Biol Chem. 1989 Feb 5;264(4):2272–2278. [PubMed] [Google Scholar]
- Ewen M. E., Sluss H. K., Whitehouse L. L., Livingston D. M. TGF beta inhibition of Cdk4 synthesis is linked to cell cycle arrest. Cell. 1993 Sep 24;74(6):1009–1020. doi: 10.1016/0092-8674(93)90723-4. [DOI] [PubMed] [Google Scholar]
- Geng Y., Weinberg R. A. Transforming growth factor beta effects on expression of G1 cyclins and cyclin-dependent protein kinases. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10315–10319. doi: 10.1073/pnas.90.21.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hinds P. W., Dowdy S. F., Eaton E. N., Arnold A., Weinberg R. A. Function of a human cyclin gene as an oncogene. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):709–713. doi: 10.1073/pnas.91.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein M. C., Metcalf R. A., Welsh J. A., Montesano R., Harris C. C. Frequent mutation of the p53 gene in human esophageal cancer. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9958–9961. doi: 10.1073/pnas.87.24.9958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M., Moustakas A., Lin H. Y., Lodish H. F., Carr B. I. Growth inhibition by transforming growth factor beta (TGF-beta) type I is restored in TGF-beta-resistant hepatoma cells after expression of TGF-beta receptor type II cDNA. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5359–5363. doi: 10.1073/pnas.90.11.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Kahn S. M., Zhou P., Zhang Y. J., Cacace A. M., Infante A. S., Doi S., Santella R. M., Weinstein I. B. Overexpression of cyclin D1 in rat fibroblasts causes abnormalities in growth control, cell cycle progression and gene expression. Oncogene. 1993 Dec;8(12):3447–3457. [PubMed] [Google Scholar]
- Jiang W., Zhang Y. J., Kahn S. M., Hollstein M. C., Santella R. M., Lu S. H., Harris C. C., Montesano R., Weinstein I. B. Altered expression of the cyclin D1 and retinoblastoma genes in human esophageal cancer. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9026–9030. doi: 10.1073/pnas.90.19.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Koff A., Ohtsuki M., Polyak K., Roberts J. M., Massagué J. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-beta. Science. 1993 Apr 23;260(5107):536–539. doi: 10.1126/science.8475385. [DOI] [PubMed] [Google Scholar]
- Laiho M., DeCaprio J. A., Ludlow J. W., Livingston D. M., Massagué J. Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell. 1990 Jul 13;62(1):175–185. doi: 10.1016/0092-8674(90)90251-9. [DOI] [PubMed] [Google Scholar]
- Laiho M., Weis M. B., Massagué J. Concomitant loss of transforming growth factor (TGF)-beta receptor types I and II in TGF-beta-resistant cell mutants implicates both receptor types in signal transduction. J Biol Chem. 1990 Oct 25;265(30):18518–18524. [PubMed] [Google Scholar]
- Lovec H., Sewing A., Lucibello F. C., Müller R., Möröy T. Oncogenic activity of cyclin D1 revealed through cooperation with Ha-ras: link between cell cycle control and malignant transformation. Oncogene. 1994 Jan;9(1):323–326. [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Laiho M., Ralph D. A., Weis F. M., Zentella A. Transforming growth factor-beta. Cancer Surv. 1992;12:81–103. [PubMed] [Google Scholar]
- Matsushime H., Roussel M. F., Ashmun R. A., Sherr C. J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991 May 17;65(4):701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- Motokura T., Bloom T., Kim H. G., Jüppner H., Ruderman J. V., Kronenberg H. M., Arnold A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991 Apr 11;350(6318):512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- Mulder K. M., Zhong Q., Choi H. G., Humphrey L. E., Brattain M. G. Inhibitory effects of transforming growth factor beta 1 on mitogenic response, transforming growth factor alpha, and c-myc in quiescent, well-differentiated colon carcinoma cells. Cancer Res. 1990 Dec 1;50(23):7581–7586. [PubMed] [Google Scholar]
- Nobori T., Miura K., Wu D. J., Lois A., Takabayashi K., Carson D. A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994 Apr 21;368(6473):753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- Okamoto A., Demetrick D. J., Spillare E. A., Hagiwara K., Hussain S. P., Bennett W. P., Forrester K., Gerwin B., Serrano M., Beach D. H. Mutations and altered expression of p16INK4 in human cancer. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11045–11049. doi: 10.1073/pnas.91.23.11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Pfeifer A. M., Lechner J. F., Masui T., Reddel R. R., Mark G. E., Harris C. C. Control of growth and squamous differentiation in normal human bronchial epithelial cells by chemical and biological modifiers and transferred genes. Environ Health Perspect. 1989 Mar;80:209–220. doi: 10.1289/ehp.8980209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K., Kato J. Y., Solomon M. J., Sherr C. J., Massague J., Roberts J. M., Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994 Jan;8(1):9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Polyak K., Lee M. H., Erdjument-Bromage H., Koff A., Roberts J. M., Tempst P., Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994 Jul 15;78(1):59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Quelle D. E., Ashmun R. A., Shurtleff S. A., Kato J. Y., Bar-Sagi D., Roussel M. F., Sherr C. J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993 Aug;7(8):1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- Resnitzky D., Gossen M., Bujard H., Reed S. I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994 Mar;14(3):1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B. Physiological actions and clinical applications of transforming growth factor-beta (TGF-beta). Growth Factors. 1993;8(1):1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- Schuuring E., Verhoeven E., Mooi W. J., Michalides R. J. Identification and cloning of two overexpressed genes, U21B31/PRAD1 and EMS1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene. 1992 Feb;7(2):355–361. [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Stoner G. D., Kaighn M. E., Reddel R. R., Resau J. H., Bowman D., Naito Z., Matsukura N., You M., Galati A. J., Harris C. C. Establishment and characterization of SV40 T-antigen immortalized human esophageal epithelial cells. Cancer Res. 1991 Jan 1;51(1):365–371. [PubMed] [Google Scholar]
- Toyoshima H., Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994 Jul 15;78(1):67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Cárcamo J., Zentella A., Doody J., Laiho M., Wang X. F., Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992 Dec 11;71(6):1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Connolly T., Futcher B., Beach D. Human D-type cyclin. Cell. 1991 May 17;65(4):691–699. doi: 10.1016/0092-8674(91)90100-d. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993 Dec 16;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Zhang H., Beach D. Subunit rearrangement of the cyclin-dependent kinases is associated with cellular transformation. Genes Dev. 1993 Aug;7(8):1572–1583. doi: 10.1101/gad.7.8.1572. [DOI] [PubMed] [Google Scholar]
- Zhang Y. J., Jiang W., Chen C. J., Lee C. S., Kahn S. M., Santella R. M., Weinstein I. B. Amplification and overexpression of cyclin D1 in human hepatocellular carcinoma. Biochem Biophys Res Commun. 1993 Oct 29;196(2):1010–1016. doi: 10.1006/bbrc.1993.2350. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]