Abstract
In April 2010, a Medicines and Healthcare Products Regulatory Agency safety alert concerning all metal-on-metal (MOM) hip replacements recommended measuring chromium and cobalt concentrations when managing patients with painful prostheses. The need for this review is illustrated by the recent surge in requests for these blood tests from orthopaedic surgeons following this alert. The aim is to provide guidance to laboratories in assessing these requests and advising clinicians on interpretation. First, we summarize the basic terminology regarding the types of hip replacements, with emphasis on the MOM type. Second, we describe the clinical concerns over implant-derived wear debris in the local tissues and distant sites. Analytical aspects of the measurement of the relevant metal ions and what factors affect the levels measured are discussed. The application of inductively coupled plasma mass spectrometry techniques to the measurement of these metals is considered in detail. The biological effects of metal wear products are summarized with local toxicity and systemic biological effects considered, including carcinogenicity, genotoxicity and systemic toxicity. Clinical cases are used to illustrate pertinent points.
Introduction
Clinical chemistry laboratories in the UK are receiving increased numbers of requests for blood cobalt (Co) and chromium (Cr) concentrations because orthopaedic surgeons have shown a relationship of concentrations of these metals to clinical events affecting hip replacements. This trend has been accelerated by the recent issue by the Medicines and Healthcare Products Regulatory Agency (MHRA) in the UK of a Medical Device Alert advising that all patients with a metal-on-metal (MOM) implant be followed annually for at least five years, with blood Cr and Co measurements when indicated.1
Hip replacements were first used in the 1930s by Dr Phillip Wiles from the Middlesex Hospital in the UK, but it was not until Sir John Charnley's cemented metal-on-plastic (MOP) hip replacements in the 1960s that a design suitable for widespread use was widely available. The first implants used a stainless-steel ball with a Teflon cup. The Teflon was later replaced with high-density polyethylene due to the high incidence of osteolysis due to wear debris from the Teflon. Variants of this design have proliferated, and a variety of materials have been used, including titanium stems and titanium cup shells, different plastics and ceramics.
Every year one million hip replacements are implanted worldwide and 70,000 in the UK (UK data from the UK National Joint Registry, www.njrcentre.org.uk). Broadly, there are three types of implants, classified according to their bearing surfaces: MOP, ceramic-on-ceramic and MOM. Each type has different advantages and disadvantages such as: low wearing; suitability for hip re-surfacing (involving capping of the femur); and long clinical history. The most successful type has not yet been determined; however, there has been a dramatic increase in MOM hips over the last 10 y.
MOM replacement
The use of MOM hip implants was led by Europe (initially Germany with small 28-mm diameter femoral head components and latterly the UK with large diameter [>36 mm] femoral head components). The latest data from the USA show that 35% of all hip implants are now MOM. We have estimated that the total number of MOM implants that have been implanted is approximately one million, with the majority over the last 10 y. Many studies have shown that these have good early (<5 y) and possibly medium-term (5–10 y) results.
MOM re-surfacings have the advantage that the metal components can be made thin enough, yet maintain strength and the ability to bond to bone (often with a coating or roughened surface) so that large diameter femoral head components are feasible. Large diameter femoral head components are desirable because they offer increased range of motion (if used with a narrow femoral neck component), reduced risk of dislocation and the ability to re-surface or cap the femoral head.
All materials used for hip implants will wear in use. Particles released from MOP implants, primarily polythene particles, are typically tens of micrometres in diameter and can clearly be seen in the surrounding tissue by light microscopy. They are responsible for osteolysis and implant loosening in approximately 90% of patients at 10 y postoperatively. MOM implants generate nanometre-sized metal particles and metal ions at approximately one trillion per year (one million particles generated per step times one million steps walked per year).2,3 These 40-nm particles are not visible using light microscopy, although it is possible that aggregates may be visible.
The MOM implants have two bearing surfaces of a Co–Cr–molybdenum (Mo) alloy (most commonly ASTM F754 in the ratio of Co:Cr:Mo of 60:30:7, although other alloys such as F90, F799, F562 and F1537 may also be used) that are separated by a thin film of synovial fluid when tested in a hip simulator. However, when used in patients it is likely that this lubricant film is not constant and thick enough to prevent the surfaces from contacting and generating wear debris, as evidenced by the reports of a wide range in blood metal ion concentrations. Typical blood levels of Co and Cr in patients with unilateral, well functioning hip implants are 30 and 45 nmol/L (1.7 and 2.3 μg/L), respectively. However, blood Co concentrations can rise as high as 6550 nmol/L (387 μg/L) and Cr 3400 nmol/L (179 μg/L).5 We have recently recorded concentrations of these metals in fluid surrounding these hips of 400 mg/L of Cr and 22 mg/L of Co.
There is an important distinction between MOM hip replacement and hip re-surfacing. In the former, a stemmed (femoral) component is used, whereas in the latter the femur is capped or re-surfaced. Hip re-surfacing therefore conserves femoral bone by capping the femoral head rather than removing it. If a later redo/revision operation is required, then a conventional ‘stemmed’ hip replacement is straightforward on the femoral side and so hip re-surfacing is favoured for younger patients who are likely to need more than one hip replacement during their lives. For the purposes of this review, we have used the term MOM hip replacement rather than refer to the subset of hip re-surfacing because it is likely that the most important and pertinent clinical problems relate to the bearing surface.
Since 1996, approximately one million patients have had MOM hips, with revision due to poor biocompatibility ranging from 1% to 20% at five years postoperatively, depending on the type of prosthesis. The full economic cost of a revision has been estimated at up to £30,000.
There are many designs of these implants, but all use the same Co–Cr–Mo alloy, although there are manufacturer differences in the treatment of the alloy in the manufacturing process. The implants are based on alloys predominantly manufactured from Co, Cr and Mo, but other metals such as tungsten, nickel and titanium may also be present. It is unclear whether these have any effect on the wear of the implant or on metal release. The lubrication of the space between the head and the cup by a thin film of synovial fluid6 will remove any released metal from the joint.
The mechanism of metal release is primarily by wear of the bearing surfaces, but there may also be an element of corrosion especially in the shaft. The linear wear rate of these implants is approximately 5 μm/y,7 but in some failed implants we have measured wear rates up to 257 μm/y.8 Metals may also be released from implants by corrosion. This may be caused by the action of body fluids on the surfaces or by an electrochemical couple being formed between different metal components.
Local effects of MOM hips
Soft tissue inflammatory reactions surrounding MOM implants have only recently been reported, partly because imaging techniques such as magnetic resonance imaging (MRI) previously suffered from artefact due to the prosthesis. These reactions have been described as pseudotumours.9,10 Their true prevalence will be unknown until large-scale MRI screening studies are carried out. However, studies of small numbers of failures have found that females are more affected than males, with one study reporting 14% of young females with MOM implants affected. Even if the prevalence for the whole population is much lower, the problem is important because it can cause destruction of bone and muscle, which may be either unreconstructable or leave the patient with long-term poor function. One type of MOM implant (the Ultima; Depuy, Warsaw, IN, USA) was withdrawn by the manufacturers because of this problem.11,12 The Depuy ASR implant has recently been withdrawn due to a higher than expected failure rate as shown in statistics from the National Joint Registry (NJR, a register of all orthopaedic joint replacements performed in England and Wales).
The British Hip Society in collaboration with the NJR and the MHRA has recently conducted an audit of this problem and we await their findings. Meanwhile, surgeons are requesting blood Co and Cr concentrations on patients with MOM implants as a means of understanding how well the hip is performing in vivo. Research in this area has increased dramatically. This review summarizes the evidence that has stimulated surgeons to request these tests and highlights the gaps in understanding that may be useful to clinical biochemists when understanding the clinical interpretation of blood concentrations of Co and Cr in patients with MOM hip replacements.
Systemic effects of MOM hip replacements
Cr(VI) is a well-known environmental and occupational carcinogen and mutagen, so many reports have focused on the carcinogenic and mutagenic potential of these implants. Some reports have shown increased occurrence of some cancers, but the same reports also showed decreased incidence of other cancers in patients with MOM hip replacements.13–15 A recent report has suggested that there may be an increased incidence of some cancers in rheumatoid and osteoarthritis patients who have had knee replacements,16 although this increased cancer risk in such patients may possibly be related to the inflammatory disease.17
Concern over the effects of metal ions has prompted various authorities to examine the potential carcinogenic effects of MOM hips. Firstly, the UK Government's Committee on Mutagenicity of Chemicals in Food, Consumer Products and the Environment published a statement saying that there was evidence of genetic damage in patients with all types of MOM and some MOP implants, but not from stainless steel on polythene implants.18 The committee concluded that ‘However, it was not possible to make any definite conclusions as to which metal ions, or interactions between metal ions or particulate metals might be responsible for the observed genotoxicity…There was limited evidence available to suggest a possible interaction between chromium and cobalt ions and possible mutagenicity/DNA damage in vitro but not in vivo. There was no convincing evidence for metal-specific effects of wear debris with regard to potential for clastogenicity or aneugenicity’. A series of papers from Learmonth and Case in Bristol19–21 was considered by the committee. They showed that DNA damage, including aneuploidy and chromosomal translocations, could be linked to Cr–Co containing implants, but not to stainless steel-containing implants. However, the report also concludes that evidence for an association of these effects with implants is not in itself evidence of a causal relationship.
Secondly, a review by the National Institute for Health and Clinical Excellence (NICE) recommended further research into many of the unknown questions surrounding metal ions from MOM implants.22 Thirdly, in 2006, the British Orthopaedic Association organized a national two-day meeting entitled, ‘Biological Implications of Metal on Metal Hip Replacements’ which concluded with the need for more research into this area and has recently conducted a UK-wide audit of all failed MOM implants with adverse soft tissue changes. Lastly, the US Food and Drug Administration (FDA) have recommended that the first 350 patients of the initial cohort receiving MOM hip re-surfacing devices should have blood concentrations of metal ions measured for 10 y postoperatively.23,24 More recent advice from the FDA25 is more cautious than the MHRA, ‘At the current time, there is no evidence to support the need for checking metal ion levels in the blood or special imaging if patients with MOM hip implants have none of the signs or symptoms described above and the orthopaedic surgeon feels the hip is functioning’. No advice on action limits for metal concentrations is given, and the advice is to interpret concentrations in the context of the overall specific clinical scenario including symptoms, physical findings and other diagnostic results.
The British Hip Society has issued revised guidance,26 specifically aimed at larger diameter implants. The advice is that the MHRA advice still applies, but that patients may need to be followed up for the life of the implant.
NICE guidance on the use of MOM hip re-surfacings22 has highlighted the need for further studies on the effects of metal wear particles from these products, but emphasized the safety of these products and general lack of adverse effects. However, there is a relatively high rate of revision of these operations, and unexplained pain in many patients has been related to elevated blood metal concentrations and increased blood metal concentrations have been correlated to the position of the implant.5,27,28 There have been little published data on appropriate reference ranges for these metals or the clinical utility of the data.
Measurement of Cr, Co and other metals
Cr and Co are nowadays usually measured in biological samples by inductively coupled plasma mass spectrometry (ICPMS), although electrothermal atomization atomic absorption spectrometry (ETAAS) has also been used, and was commonly used before the widespread introduction of ICPMS. The sensitivity of ETAAS for these elements is limited; for example, in a method for measuring Co in serum, multiple injections into the graphite tube were needed to attain the desired sensitivity.29 The large amount of sample required to obtain the needed sensitivity will cause a large background absorbance which may have stretched the capabilities of older instruments. Nevertheless, the concentrations found in these subjects are well within the range of ETAAS, and this technique has been used in several studies.30–32
ICPMS has lower detection limits than ETAAS and is also capable of simultaneous measurement of Cr and Co, and of many other elements33,34 and is used in the majority of studies considered here. Accurate measurements can be made using either collision/reaction cell quadrupole ICPMS (Q-ICPMS) or high-resolution ICPMS (HR-ICPMS). The use of either of these is essential to cope with the interference on the measurement of 52Cr from 40Ar12C. All of the available collision/reaction cell instruments are capable of reducing this interference to acceptable levels while retaining good sensitivity. Although there is in principle interference in the measurement of 59Co from ions such as 44Ca14N, there does not seem to be any significant effect of these, and measurements can be made in standard mode.
High resolution ICPMS, using a double focusing magnetic sector mass spectrometer, is available in a few laboratories and overcomes the uncertainties of interference reduction with collision/reaction cells.35–37 The interferences can usually be completely resolved, but the expense of the hardware will preclude the use of this technique in all but a few specialist and research laboratories. Krachler et al.38 looked in detail at method validation, especially the use of currently available quality control (QC) samples. The accuracy of values assigned to their product by one manufacturer of QC materials was questioned.
The electrochemical technique anode stripping voltametry has been used occasionally,39 but is not common. This is a much more labour-intensive technique and requires a total acid digestion of the sample before analysis.
Other metals may also be measured. Mo is a component of the alloy commonly used, but measurement will add little diagnostic value. Some other implants may contain other metals such as iron, nickel, vanadium and titanium in the alloy used or in other components such as the shell surrounding the cup and in the shaft. Measurement of Ni or Ti may be of value in some circumstances where metal release may be due to corrosion rather than wear at the bearing surface. Mo and vanadium may also be measured by Q-ICPMS. The sensitivity for Mo measurement is limited by the number of isotopes of the element, but it is readily measured without the need for the use of collision cell technology. Vanadium suffers from interference from 35Cl16O, but this is readily removed by collision cells. Nickel may also be measured using collision cell Q-ICPMS. Titanium measurements by Q-ICPMS are difficult in clinical samples at normal concentrations, although it is possible to use quadrupole instruments to measure higher concentrations of titanium. Some workers (Andrew Taylor, personal communication) have claimed to be able to measure titanium using optical emission spectroscopy. Measurement of the major isotope, 48Ti, is interfered with by 48Ca, which although only 0.19% of total calcium, would still be in considerable excess over titanium. Other titanium isotopes, 47Ti and 49Ti, although the abundance of each is less than 10%, can be measured by HR-ICPMS using medium resolution settings. Ti measurements are not practicable using Q-ICPMS.
There are no Certified Reference Material quality standard reference materials available, and there is a limited number of commercial products including both elements at suitable concentrations. Until now, most quality assessment materials and External Quality Assurance programmes have focused on the industrial exposure requirements for samples. Cr and Co, in blood, serum and urine, are now included in the monthly Trace Element Quality Assessment Scheme (TEQAS) quality assessment scheme in the UK (part of NEQAS, National External Quality Assessment Service) organized from the University of Surrey in Guildford and are also included in the Quebec Multielement Eternal Quality Assessment Scheme organized by the Institut National de Santé Publique in Quebec, Canada.
Cr and Co can be measured in either blood or serum. For either it is essential to use trace element-free blood collection tubes, and suitable tubes with no additives, heparin or EDTA are available from all blood collection device suppliers. Contamination of samples from standard blood collection tubes and from needles and syringes has been tested in the author's (BS) laboratory and found to be negligible for most trace elements measured, with the possible exception of titanium (Table 1). In our laboratory, we have standardized the protocol using K2EDTA-containing trace element-free tubes.5 This provides a more stable sample when tubes are supplied to surgeons in other hospitals for return to the Implant Retrieval Centre at Imperial College. For these elements, it is usually recommended that samples are collected via a Teflon catheter, with the first draw through the catheter discarded or used for other routine testing. In practice, we find that the expected concentrations in these patients are so much higher than the reference range in the unexposed population that using a standard needle will have little impact, although it is still recommended to take a first draw for other testing before the sample for trace element analysis. Walter et al.40 suggested that most of the Cr and Co is in the serum, with little associated with erythrocytes.
Table 1.
Metal contamination from blood collection system
| Cr | Co | Mo | V | Ni | Ti | |
|---|---|---|---|---|---|---|
| Blood | 11.6 ± 2.9 (0.60 ± 0.03) | 15.8 ± 0.3 (0.93 ± 0.02) | 36.0 ± 1.1 (3.45 ± 0.11) | 2.0 ± 0.1 (0.1 ± 0.005) | 22.7 ± 2.5 (1.05 ± 0.12) | |
| Blood + tube | 12.9 ± 1.4 (0.67 ± 0.07) | 15.4 ± 0.5 (0.91 ± 0.03) | 28.4 ± 5.9 (2.72 ± 0.57) | 2.1 ± 0.4 (0.11 ± 0.02) | 16.3 ± 1.1 (0.96 ± 0.06) | 36.8 ± 11.6 (1.76 ± 0.56) |
| Blood + tube + needle | 15.0 ± 4.4 (0.78 ± 0.23) | 14.8 ± 1.1 (0.87 ± 0.06) | 28.0 ± 3.6 (2.68 ± 0.35) | 2.1 ± 0.3 (0.11 ± 0.02) | 19.6 ± 4.6 (1.15 ± 0.27) | 49.1 ± 22.8 (2.35 ± 1.07) |
Equine blood was analysed by high-resolution inductively coupled plasma mass spectrometry before and after standing in trace element-free blood collection tubes (BD K2EDTA tubes, BD, Oxford, UK) and after being aspirated into the tube via the Vacutainer needle. All results expressed as nmol/L (μg/L), mean and standard deviation of six measurements. The Ni assay in the ‘blood’ samples showed high contamination
There have been some reports of urine metal concentrations in these patients,36 but urinary metal measurements are rarely used. Although urine is a non-invasive specimen, there is potentially a higher variation in urinary excretion attributable to factors including renal function and urinary concentration. A random collection is suggested, with the metal concentration normalized to creatinine concentration.
Synovial fluid metal concentrations can also be measured,41 and may be of value in some situations. The fluid surrounding the implant can have a very high concentration of metal. Samples of fluid may be taken by needle biopsy prior to operation for microbiological testing and to reduce the fluid pressure in the cavity. A high metal content in this fluid will be an obvious indicator of wear in the implant, and has been shown to correlate with blood concentrations, but does not always add any diagnostic value. Metal analysis of the tissue removed during the revision operation is also possible, and can complement the histological findings, but is largely of research interest.
Blood, serum and urine can be analysed by direct analysis after simple dilution but synovial fluid and tissue samples need prior digestion with acid. This is often performed with a microwave digestion system, but digestion can also be effectively performed with a heated digestion block. The need for digestion of synovial fluids is in part due to the variable nature of the matrix: many of the samples presented to the author's (BS) laboratory have a large amount of solid material, presumably protein aggregates, which may contain bound metal and thus should not be excluded from analysis. Fluid samples may also contain intact wear particles which may not be totally vaporised in the analytical system.
Reference concentrations
Unexposed population
Brune et al.42 published a systematic review of 87 papers on reference concentrations of Cr, in relation to occupational exposure. The reference range for serum concentrations was 1–3 nmol/L (0.05–0.15 μg/L) and for urine was 2–10 nmol/L (0.1 − 0.5 μg/L), 0.2–1.0 nmol/mmol creatinine. There have been many studies reporting reference intervals for Cr and Co in the unexposed population and in occupational exposure (Table 2). There is a good consensus on the normal concentration of these metals in blood, serum and urine. Blood and serum Cr concentrations are less than 5 nmol/L in many of the studies summarized. This is in line with the current reference range suggested by the UK Supra Regional Assay Service (SAS) laboratories of less than 10 nmol/L (0.5 μg/L), which will allow for a degree of sample contamination. It has generally been accepted that there is no lower limit, and it is not possible to use serum or blood concentrations to identify rare cases of Cr deficiency. Similarly, for Co it is generally accepted that reference concentrations are less than 10 nmol/L (0.51 μg/L) in both serum and blood.
Table 2.
Reference intervals for metals from non-surgical studies
| Subjects | Methods | Results | Comments | Reference | |||
|---|---|---|---|---|---|---|---|
| Systematic review | Serum chromium 1–3 nmol/L (0.05–0.15 μg/L) | Urine chromium 2–10 nmol/L (0.1–0.5 μg/L), 0.2–1.0 nmol/mmol creatinine | 42 | ||||
| Normal subjects, age 19–71, N = 234 | ETAAS | Serum chromium <6 nmol/L (<0.3 μg/L) | 43 | ||||
| Non-exposed subjects, N = 120 | ETAAS | Blood chromium 2.9–10 nmol/L (0.15–0.52 μg/L) | 44 | ||||
| Controls, N = 44 | HR-ICPMS | Blood chromium 4.2 ± 5.0 nmol/L (0.22 ± 0.26 μg/L) | Blood cobalt 2.9 ± 2.9 nmol/L (0.17 ± 0.17 μg/L) | Blood nickel 17.1 ± 19.8 nmol/L (0.99 ± 1.15 μg/L) | Blood molybdenum 6.5 ± 3.1 nmol/L (0.62 ± 0.29 μg/L) | 42% of samples below detection limit for Ni, 8.5 % for Cr, 4.2% for Co | 35 |
| Control subjects, N = 35 Haemodialysis patients, N = 29 | ETAAS | Normal serum cobalt 5.9 ± 2.5 nmol/L (0.35 ± 0.15 μg/L) | Haemodialysis serum cobalt 24.7 ± 9.7 nmol/L(1.46 ± 0.57 μg/L) | 29 | |||
HR-ICPMS, high-resolution inductively coupled plasma mass spectrometry; ETAAS, electrothermal atomization atomic absorption spectrometry
There are less data available on some of the other metals mentioned, although there is monitoring of all in occupationally exposed subjects, usually in urine.
Exposed population
One of the earliest case reports on metal release and accumulation, from 1980, reported accumulation of metal in the tissue around the MOM joint, but not around the contralateral hip, which had an MOP prosthesis.45 High metalconcentrations were also recorded in several other tissues and in hair. There is now much interest in quantifying the metal release from implants as a means of identifying the causes of joint failure and possible adverse reactions to the metal. There is a consensus that increased Cr and Co concentrations are found in serum, blood and urine. In most studies there has been no significant increase in Mo concentrations, but in some cases where the metal is present, increased nickel and titanium concentrations can be found. Some of the more recent reports are summarized in Table 3. The studies suggest that the average concentrations in patients with pain-free well-functioning implants is 20–40 nmol/L (1–2 μg/L) for both Cr and Co. Most studies suggest that there is a ‘running-in’ period of up to a year, with concentrations gradually increasing to a stable level. In some cases, the running-in period may be followed by a slight decline. The majority of reported studies have been on patients with hip replacements, but there have been some studies on metal release from other orthopaedic implants and from dental implants. A selection of such reports on blood or serum and tissue metal concentrations is listed in Table 4. Increased metal concentrations can be seen in all of these implants, but do not reach the high concentrations found with hip implants.
Table 3.
Studies of metals in blood, serum and urine in patients with hip replacements and other surgical implants
| Duration of follow-up | Results | ||||||
|---|---|---|---|---|---|---|---|
| Analysis method | Type of implant | Sample | Chromium | Cobalt | Comments | Reference | |
| AAS (Cr), ASV (Co) | Sikomet (Zimmer) | 5 y | Serum | Control: 0.30 ± 0.05 pg/L, median 0.26 (5.8 ± 0.96 nmol/L, median 5.0) | 0.11 ± 0.04 μg/L, median 0.10 (1.86 ± 0.68 nmol/L, median 1.69) | Follow-up; changes after removal also reported (see text) | 39 |
| Patients: 1.31 ± 1.37 μg/L, median 0.71, range 0.29–6.53 (25.2 ± 26.3 nmol/L, median 12.0, range 6–126) | 0.33 ± 0.18 μg/L, median 0.26 μg/L, range 0.13–0.92 (5.6 ± 3.05 nmol/L, median 4.4, range 2–52) | ||||||
| HR-ICPMS | MOM Conserve plus (Wright Medical, Arlington, TN, USA) | 15 months | Serum | Control: 1.47 ± 0.37 μg/L, range 0.31–1.97 μg/L (28.3 ± 7.1 nmol/L, range 6–38) | 3.46 ± 1.34 μg/L, range 1.42–6.30 μg/L (58.6 ± 22.7 nmol/L, range 24–107) | Urine Cr and Co and serum and urine Mn, Mo and Ni also reported | 35 |
| Patients: 2.88 ± 2.22 μg/L, range 0.45–4.98 μg/L (55 4 ± 42.7 nmol/L, range 9–82) | 14.0 ± 10.9 μg/L, range 3.70–43.2 μg/L (237 ± 184.4 nmol/L, range 63–3125) | ||||||
| AAS | BHR, MTHR | 2 y | Serum | Control: <0.25 μg/L, <4 nmol/L | 0.25 μg/L, 4 nmol/L, Mo 2.11 μg/L, 22 nmol/L | Median concentrations given, no significant rise in Mo in patients; MTHR levels fall from year 1, BHR levels continue to rise | 30 |
| Unilateral MTHR 2 y 1.22 μg/L | 1.70 μg/L | ||||||
| Bilateral MTHR 2 y 2.5 μg/L | 3.18 μg/L | ||||||
| BHR 2 y 5.12 μg/L | 4.28 μg/L | ||||||
| AAS | THR | 10 y | Serum | Median 0.95 μg/L, range 0.3–58.6 μg/L (median 18.3 nmol/L, range 6–113) | Median 0.75 μg/L, range 0.3–50.1 μg/L (median 12.7 nmol/L, range 5–85) | 31 | |
| HR ICPMS | Durom (Zimmer) | 2 y | Serum, blood, erythrocytes | Pre-op 0.92 ± 0.55 μg/L, 17.7 ± 10.6 nmol/L | 0.15 ± 0.15 μg/L, 2.5 ± 2.5 nmol/L | Initial rise followed by fall, concentrations inversely related to head size | 37 |
| 3 month 2.01 ± 0.12 μg/L | 0.90 ± 0.42 μg/L | ||||||
| 2 y 1.37 ± 0.65 μg/L | 0.59 ± 0.26 μg/L | ||||||
| Q-ICPMS | MOM re-surfacing | Whole-blood | 3.0 μg/L, range 0.8–179.0 (57.7 nmol/L, range 15–3442) | 4.5 μg/L, range 0.5–386.5 (76.3 nmol/L, range 8–6550) | Painful joints prerevision surgery | 5 | |
| AAS | MTHR | 7 y | Serum | 6 m; 0.75 ± 0.80 μg/L, range 0.1–3.1 (14.4 ± 15.4 nmol/L, range 2–60) | 7 y follow-up; data given annually. No significant outliers | 46 | |
| 110 subjects | 7 y; 1.68 ± 1.28 μg/L, range 0.3–5.3 (32.3 ± 24.6 nmol/L, range 38–102) | ||||||
AAS, atomic absorption spectroscopy, ASV, anode stripping voltametry, Q-ICPMS, quadrupole inductively coupled plasma mass spectrometry, HR-ICPMS, high resolution ICPMS, THR, total hip replacement (type not specified), BHR, Birmingham hip replacement (Smith & Nephew, London, UK); MTHR, Metasul bearing hip replacement (Zimmer, Warsaw, IN, USA); MOM, metal-on-metal
Data have been re-calculated to show molar concentrations in addition to mass units where appropriate. Papers have been listed in ascending order of publication
Table 4.
Reported studies on other joint replacements
| Reference | Joint | Metals | Conclusions |
|---|---|---|---|
| 47 | Knee | Cr, Co, Mo | Serum Cr and Co increased, no significant increase in Mo |
| 48 | Elbow | Not measured | Tissue deposition of particles and study of recovered implants |
| 49 | Spine | Serum Ni, blood, urine Cr | No increase in blood Cr or Ni but increased urine Cr. Urine Cr reduced after removal of implant |
| 50 | Vascular stents | Ni, Cr, Mn, Mo | In vitro study. Platelet activation by metals may contribute to thrombosis. Diamond coating reduces metal release |
| 51 | Orthodontic implants | Ni | Ni concentration in saliva increased up to 10 weeks after implantation, then decreased |
| 52 | Metal plates and screws | Al, Ti | Al but not Ti accumulates in soft tissue around implant |
| 53 | Intramedullary nails | Cr, Mo, Ti, Al, V | Increased serum concentrations of Cr and Ti |
There have been few studies of metals in tissues, largely because such studies, looking at tissues other than local to the implant, have to be at postmortem. Case et al.53 looked at patients primarily with stainless steel implants, but also some Cr–Co implants. Metal was found in lymph nodes, liver and spleen.54 Similar results from patients with hip or knee replacements were reported by Urban et al.55 More widespread dissemination of metals was seen in patients with a failed implant. In at least one case, chromium phosphate particles were detected. Polyethylene particles from MOP implants were also detected. In both of these studies, the cause of death was unrelated to the implant. More recently, Hart et al.56 have studied tissue immediately surrounding the implant using X-ray synchrotron spectroscopy. Widespread deposition of Cr was found, with few Co particles remaining in the tissues. The Cr was found to be almost entirely chromium phosphate. Co and Mo were found as isolated metallic particles.
Causes of increased metal concentrations
Implant-derived metal debris is a consequence of wear, corrosion or both. Modular components have potential for increased metal release – when compared with one-piece (monobloc) components such as a hip re-surfacing – because of the additional wearing surfaces. Clinical studies relating the mechanism of wear in these implants to blood metal concentrations and in vitro studies using hip simulators have been important in defining some of the factors affecting metal release. The factors shown to be important include: orientation of the components (particularly the cup); implant design; head size and patient activity. Khan et al.57,58 measured the rise in serum metal concentrations after exercise. Significant increases in serum Cr and Co concentrations were found after just one hour of exercise, with differences between implant types. The BHR (Birmingham Hip Replacement; Smith & Nephew, Memphis, TN, USA) and Cormet (Corin, Cirencester, UK) implants, with a 46.8- or 48-mm diameter head, gave 8.5 and 6.5 times larger increases than Metasul (Zimmer, Warsaw, IL, USA) implants, with a 28-mm diameter head. A much lower effect of activity on metal concentrations was reported by Heisel et al.59 Marginal increases in blood metal concentrations were found even after treadmill exercise, and no change in normal exercise. In a more recent study, no correlation was found between activity and metal concentration.60
There are reports of a strong positive correlation between cup inclination angle and either wear rate of removed MOM hips61 or blood metal ion levels.5,62,63 However, there are confounding variables that may also influence wear rate; these can be divided into the following groups: patient factors (small [<50 mm] femoral head size,61,62 gender, activity); surgical factors (horizontal femoral offset,64 cup version angle,62 incorrect sizing); and manufacturing factors (metallurgy and design). Interestingly, among the five most used types of MOM hip (BHR, Cormet, ASR, Metasul, Adept [Finsbury Orthopaedics, Surrey, UK]), there are no proven statistically significant differences in metal ion release and wear rates despite differences in metallurgy (e.g. heat-treated versus non-heat-treated) and design (e.g. amount of hemisphere, clearance between two bearing surfaces). The angles of inclination and version or abduction, which define the orientation of the cup within the pelvis, are both important. Blood metal concentrations in patients with an angle of inclination of greater than 55° have been shown to be significantly raised. This seems to be a more important parameter than the duration of the implant or the manufacturer.
Biological effects of wear products and metal toxicity: systemic effects
Cr and Co are both essential trace elements. The role of Co in vitamin B12 is well known. Cr is thought to be essential for glucose metabolism, and probably helps the binding of insulin to cell receptors.65 Mo is also essential, but only as Mo co-factor for sulphite oxidase and xanthine oxidase.66 There is some evidence that nickel67 and vanadium68 may have some biological function, but this is controversial. There is no evidence that titanium has any biological role. However, the concern in this context is the possible toxic hazard from the metal ions and particles. Toxicity has most been studied in the metal working industries, but other toxic effects are also well known.
The effects of the metal wear debris on the local tissues have dominated the recent debate regarding the use of MOM hips, but for many years concerns about the potential biological effects of metal particles have been raised. The areas of concern are primarily the chemical form of the metal released from the implant and the metabolic fate of the metal. The toxicity of Cr and Co has been recognized as a concern in industrial uses of these metals for many years.69,70 Fears of carcinogenicity of particles were raised as early as 1971,71 and a report by the International Agency for Research on Cancer in 200072 concluded that alloys containing nickel and Co are possibly carcinogenic and that there are insufficient data for other more complex alloys. The current state of knowledge of hazards from wear metals has recently been reviewed.73
The main concern has been the possibility that Cr is present as Cr(VI), which is a well-known carcinogen. There are reports that the Cr released from implants may be as hexavalent Cr,74 but this is not supported by other reports. Clinical studies have linked MOM hip replacements to white blood cell DNA and chromosomal damage and immunological function disturbances,19–21,75–81 although as noted previously, epidemiological studies have not found any evidence for an overall increase in cancer risk.
Co poisoning is known to be associated with cardiomyopathy82,83 and in vitro studies have shown neurotoxic84 effects. Although not so well known, there have also been reports of neurotoxicity following treatment of anaemia with cobalt chloride85 and occupational exposure to Co.86 There have been a few case reports87–89 linking high Co concentrations in these patients to neurotoxicity. It seems likely that this may be a more significant risk in orthopaedic patients.
One other aspect of concern, especially now that these operations are being offered to younger patients, is the potential for reproductive effects. There are little data published on the effects of metals on semen, but there are suggestions that Mo may have adverse effects on semen quality.90 Cr and Co have not been reported as having any adverse effects. Brodner et al.91 found that metal concentrations in cord blood were undetectable, in contrast to maternal blood concentrations. However, Novak et al.92 found that metal concentrations in blood from neonates whose mothers had implants were higher than those in controls.
Biological effects of wear products and metal toxicity: local effects
There is also concern that the metal wear debris causes exaggerated periprosthetic tissue inflammation surrounding MOM hips and will cause loosening of the joint,93 bone loss94 or tissue damage.5 This may be the cause of the high rate of unexplained pain in failed MOM hips when compared with MOP hips, 43% and 12% respectively.95 In addition, these severe inflammatory changes have been seen surrounding the Ultima MOM hip96 and were responsible for its withdrawal from the UK health-care market by the MHRA. The large mass that can develop surrounding the tissue, sometimes referred to as a ‘pseudotumour’, can be readily visualized by MRI scans.9,10 There is also a typical inflammatory response in the tissue which has been termed ALVAL: aseptic lymphocytic vasculitis-associated lesion.97 There are no published data suggesting that measurement of circulating concentrations of inflammatory markers, such as serum C-reactive protein, has any diagnostic value.
Hur et al.98 published a short study of five patients with a MOM implant who had renal failure. The patients with renal failure had higher Co concentrations than patients with normal renal function, but the Cr concentrations were similar. It was not stated whether the patients were dialysis-dependant, although one patient underwent renal transplantation, and concentrations in comparative patients with renal failure but without a hip replacement or other surgical implant were not given.
Clinical value of metal measurements
It is possible to use the published data to propose acceptable blood concentrations of Cr and Co in these patients and to define action levels to determine the need for further clinical investigation and action. This is the approach taken in defining the action concentrations in the recent MHRA safety alert. The level of 7 μg/L Cr or Co (135 nmol/L Cr or 119 nmol/L Co) was derived from the data in a recent study75,76 and comes from the statistical extreme outlier definition of third quartile (Q3)+2×interquartile range. The range of concentrations found in this study is shown in Table 5.
Table 5.
Median and interquartile range for chromium and cobalt in different patient groups (data from refs75,76)
| MOP (n 33) | COC (n 25) | Unilateral MOM (n 88) | Bilateral MOM (n 18) | |
|---|---|---|---|---|
| Age in years (range) | 64 (55–67) | 59 (54–60) | 56 (51–61) | 56 (51–61) |
| Months after surgery | 25 (18–20) | 25 (20–33) | 43 (37–52) | 29 (24–34) |
| Cobalt (nmol/L, μg/L) | 7.5 (5.4–12.7) | 2.0 (1.7–4.2) | 29 (21.9–39.5) | 41.5 (26.5–73) |
| 0.44 (0.32–0.75) | 0.21 (0.17–0.25) | 1.71 (1.29–2.33) | 2.45 (1.56–4.30) | |
| Chromium (nmol/L, μg/L) | 12.5 (4.0–21.1) | 6.1 (4.6–8.7) | 44.8 (34.4–56) | 45.2 (32.7–64.6) |
| 0.65 (0.21–1.10) | 0.32 (0.24–0.45) | 2.33 (1.79–2.91) | 2.35 (1.70–3.36) |
MOP, metal-on-plastic, COC, ceramic-on-ceramic, MOM, metal-on-metal
A potential use of blood metal ion concentrations is to identify those patients who may go on to develop further problems. High concentrations in an asymptomatic patient may be an indication of increased wear which may indicate need for an early revision before tissue necrosis becomes a problem. Lower concentrations can be used to reassure non-symptomatic patients and minimize the need for long-term follow-up.
Clinical case examples
Case 1 – Silent osteolysis in a 29-year-old woman
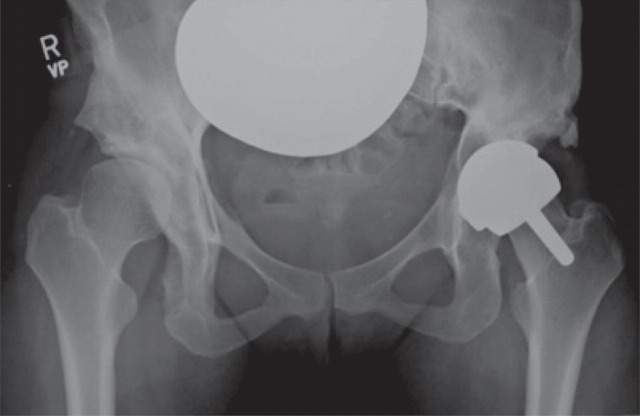
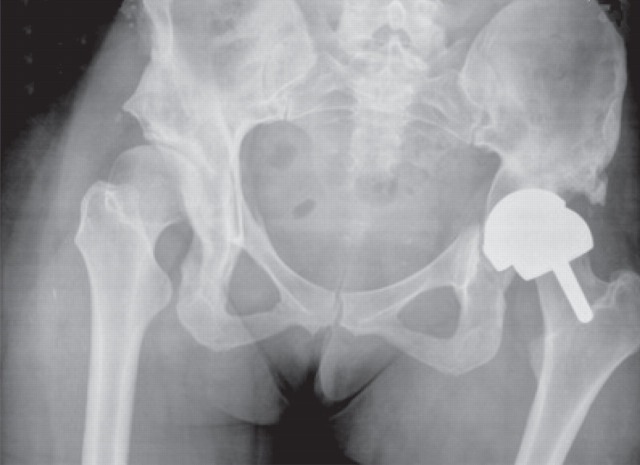
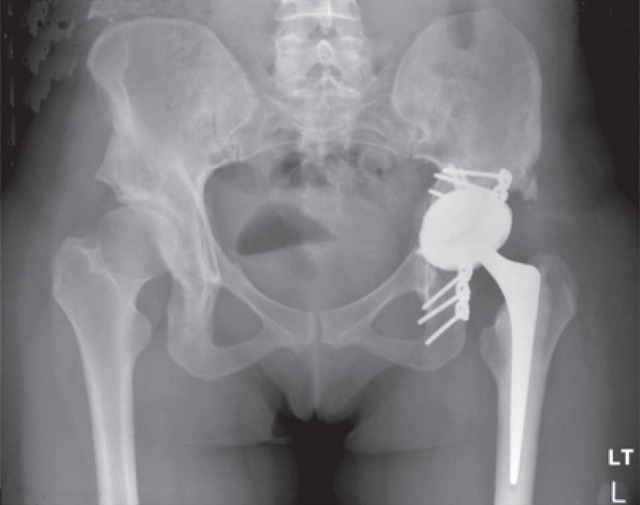
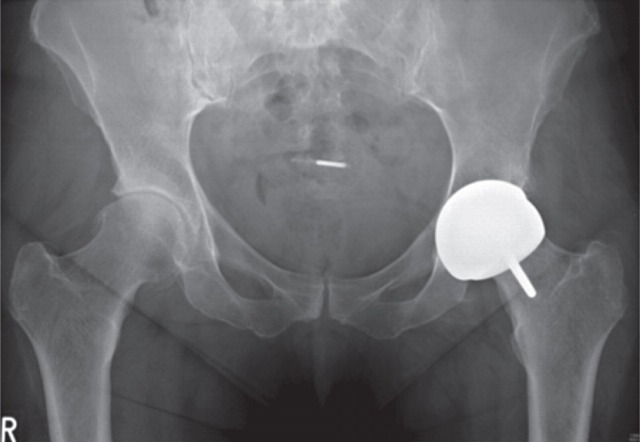
A 29-year-old woman presented with sudden onset of left hip pain and inability to bear weight following a low-energy wakeboarding incident. She had had a pelvic osteotomy in 2000 and subsequently a Birmingham Hip Resurfacing (Smith and Nephew) in 2001. In 2006, a plain antero-posterior (AP) radiograph of the pelvis showed evidence of neck narrowing (Figure 1). There appears to be rarification and reduced bone density with thinning of the iliopectineal cortical outline medially. Blood metal ion levels were Co 621 nmol/L (36.6 μg/L) and Cr 730 nmol/L (38 μg/L). Figure 2 shows an AP radiograph from 2007 immediately after her wakeboarding accident. There is a fracture of her pelvis adjacent to the left hip re-surfacing. Cup inclination measured 55° with uncovering of head and edge loading. Component head size and outer shell diameter were 42 and 48 mm, respectively. Note the right hip dysplasia with evidence of previous periacetabular osteotomy and a gracile femur. Metal concentrations were also measured in synovial fluid: Cr 228,000 nmol/L (11,850 μg/L) and Co 12,600 nmol/L (745 μg/L). Figure 3 shows an AP radiograph postfixation and total hip replacement.
Figure 1.
Case 1. Plain antero-posterior radiograph of the pelvis showed evidence of neck narrowing. There appears to be rarification and reduced bone density with thinning of the iliopectineal cortical outline medially
Figure 2.
Case 1. Antero-posterior radiograph from 2007 immediately after her wakeboarding accident. There is a fracture of her pelvis adjacent to the left hip re-surfacing. There appears to be rarification and reduced bone density with thinning of the iliopectineal cortical outline medially
Figure 3.
Case 1. Antero-posterior radiograph postfixation and total hip replacement
The clinical ‘metal ion’ questions raised by this case are: did blood metal ion levels return to normal following revision? This was very important to the psychological wellbeing of the patient who was of reproductive age, planning a family and had read about carcinogenicity following MOM hips. Subsequent metal concentrations were Cr 199 nmol/L (10.3 μg/L) and Co 28 nmol/L (1.7 μg/L) in October 2008 and Cr 91 nmol/L (4.7 μg/L) and Co 13 nmol/L (0.8 μg/L) in October 2009. As in other patients with metallosis, Cr concentrations can remain elevated after revision, while Co concentrations can fall relatively quickly.
Case 2 – Silent osteolysis and imminent acetabular fracture and high metal ion levels in a 65-year-old man
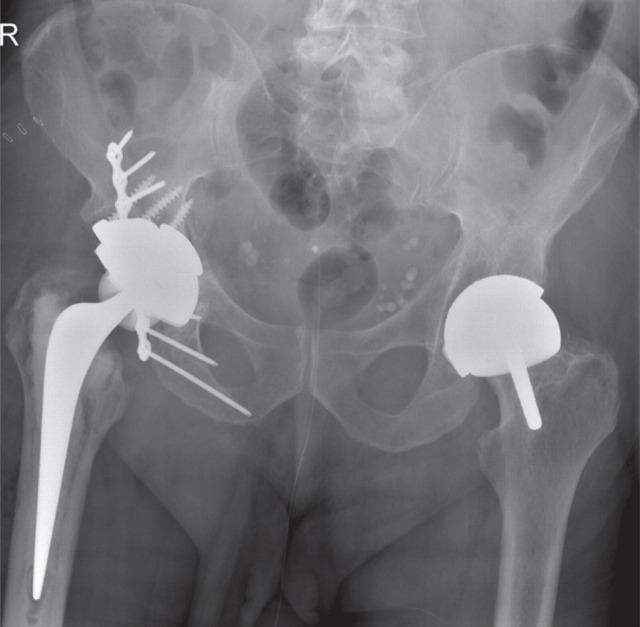
Figure 4 shows an AP radiograph from a 65-year-old man who presented with a painful right MOM hip re-surfacing (Birmingham Hip Resurfacing, Smith and Nephew) five years postoperatively. The femoral component was loose and the acetabular bone was so thin that fracture was imminent. High blood metal ions were recorded (Cr 698 nmol/L [36.3 μg/L] and Co 1212 nmol/L [71.4 μg/L]), and may have predicted such severe osteolysis if they had been measured prior to this. A postrevision AP radiograph (Figure 5) shows the extent of the reconstruction needed to stabilize the bony defects found at operation. By six months postrevision, he was able to return to sailing the Atlantic Ocean. A series of later pre- and postoperative measurements of blood Co and Cr revealed a dramatic rate of decay for Co levels and a slower rate for Cr (Figure 6). The blood metal concentrations had increased in the period following the initial outpatient appointment.
Figure 4.
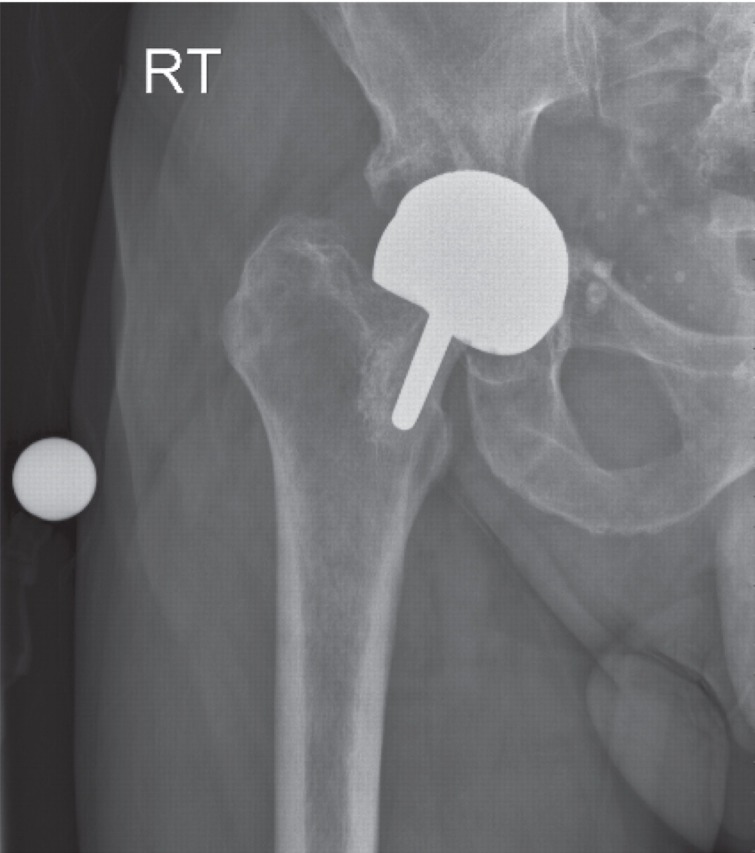
Case 2. Antero-posterior radiograph from a 65-year-old man who presented with a painful right metal-on-metal hip re-surfacing five years postoperatively. The femoral component is loose and the acetabular fracture was so thin that fracture was imminent
Figure 5.
Case 2. Antero-posteriorradiograph showing the extent of the reconstruction needed to stabilize the bony defects found at operation
Figure 6.
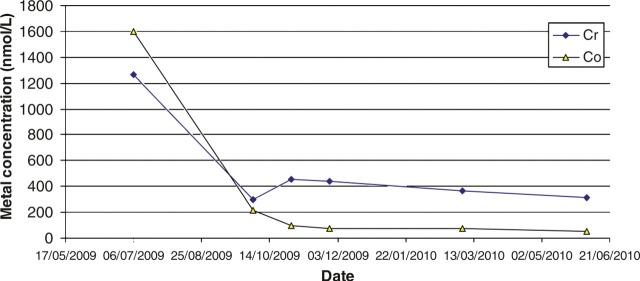
Case 2. Pre- and postoperative changes in blood chromium and cobalt concentrations
The clinical ‘metal ion’ questions posed by this case are: is the other hip at risk? Did the levels of metal ions return to that of a typical unilateral MOM hip? Was the loss of bone caused by local metal ion poisoning? We once again show that the Co concentration falls to normal concentrations much faster than does Cr concentration. There can be no direct proof in an individual case that the loss of bone density is directly caused by metallosis, but we suggest that it is a reasonable assumption that this is the case.
Case 3 – Silent muscle necrosis with very high metal ions and rapid deterioration requiring revision and inability to reconstruct muscle
A 54-year-old lady attended a research clinic with a good hip function score and very high blood metal ion concentrations with an ASR hip re-surfacing (DePuy, Leeds, UK). Blood Cr concentration was 2700 nmol/L (140 μg/L) and Co concentration was 6551 nmol/L (386.5 μg/L). An AP radiograph is shown in Figure 7. Two years later her hip function deteriorated dramatically. This might have been better predicted from the high metal ion concentrations. Three-dimensional computed tomography measurement (Figure 8) revealed cup angles of 70° inclination (the optimum is 45° because angles higher than this cause edge loading and high rates of wear of the hip) and 49° anteversion (the optimum is between 5 and 25°, depending on the femoral version). Metal artefact reduction sequence MRI99 revealed a large trochanteric bursa (Figure 9) with evidence of nodularity within the bursal sac and an irregular wall. A large amount of black fluid surrounded the hip at revision operation. Synovial fluid analysis showed Cr concentration 5063 μmol/L (263.3 mg/L) and Co concentration 207.1 μmol/L (12.2 mg/L). Analysis of the explanted hip revealed a wear rate that was 100 times greater than predicted by hip simulator studies.
Figure 7.
Case 3. Antero-posterior radiograph from a 54-year-old lady who attended a research clinic. She had a good hip function score and very high blood metal ion concentrations
Figure 8.
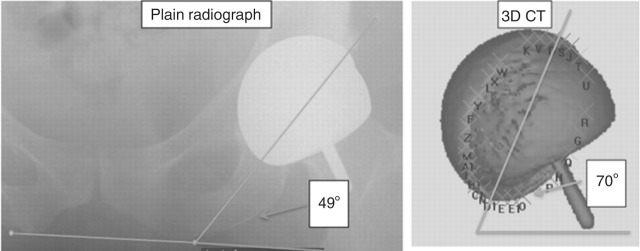
Case 3. Three-dimensional computed tomography (3D CT) measurement showing cup angles of 70° and 49° anteversion
Figure 9.
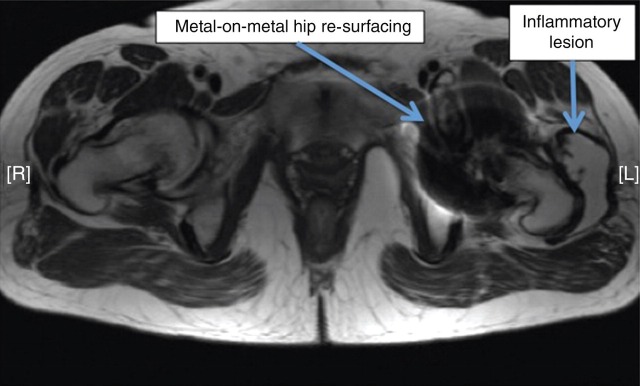
Case 3. Metal artefact reduction sequence magnetic resonance imaging revealed a large trochanteric bursa with evidence of nodularity within the bursal sac and an irregular wall
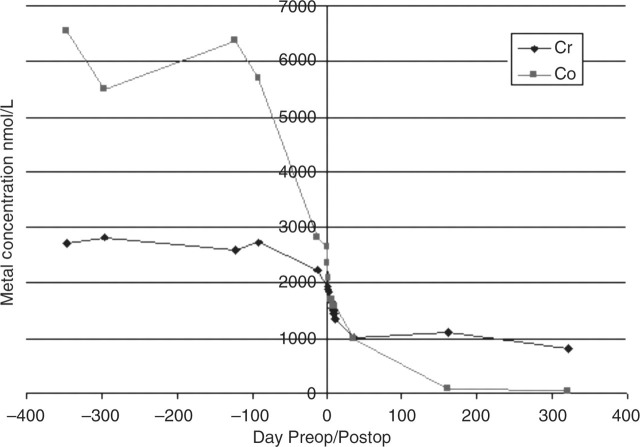
The clinical ‘metal ion’ questions raised by this case are: the patient wanted to know why this had happened? Did the metal ion levels return to normal? Could other patients be spared the muscle destruction if earlier intervention is taken? The change in blood metal concentrations following the operation is shown in Figure 10. The blood Co concentration showed a significant fall in the weeks before the revision surgery, probably due to her reduced activity as a result of the pain from the joint. There is an immediate and ongoing fall in metal concentrations postsurgery, although concentrations remained very high for a considerable time after the operation, and Cr continues to remain high more than 12 months later. We are continuing to monitor metal concentrations to see if there is an eventual fall to ‘acceptable’ levels. The conclusion is that there remains a slow release pool of Cr, possibly with the capsular tissues. There appears to be no toxic effect of this very high metal concentration. The very high metal concentrations seen preoperatively are a clear indication of high wear in the joint. The patient was reviewed for a year before the revision operation. It may be that the final tissue damage may have been less severe had the operation been performed sooner during this period. This highlights the need to take clinical action when raised metal concentrations are seen in these patients, even if there is as yet no pain.
Figure 10.
Case 3. Pre- and postoperative changes in blood chromium and cobalt concentrations. Preop, preoperative; Postop, postoperative
Conclusions
Increased circulating concentrations of Cr and Co can be used to monitor wear in MOM hip replacements. These operations are very successful but a minority of patients suffer wear in the joint, which will be reflected in increased metal concentrations. These may be used in conjunction with radiological and clinical assessments to inform the decision about revision of the original operation. The recent MHRA safety alert will serve to bring this potential problem to the attention of all concerned and expedite the clinical decision before major clinical problems arise.
DECLARATIONS
Competing interests and funding: The case reports and funding of the data was through The London Implant Retrieval Centre (LIRC), which is funded by the British Orthopaedic Association (BOA). The LIRC was set up and is run by two consultant orthopaedic surgeons Alister Hart and John Skinner. It is a joint venture between Imperial College London, the Royal National Orthopaedic Hospital, the British Orthopaedic Association and nine orthopaedic companies (JRI, Corin, Mathys, Zimmer, Depuy, Finsbury, Stryker, Smith & Nephew, and Biomet). It has the support of the Medicines and Healthcare Regulatory Agency (MHRA). Neither AH or BS have consultancies, share options or funding separate to this from industry.
Ethical approval: Cases referred to in this report were approved by the Riverside Research Ethics Committee (REC reference: 07/Q0401/25).
Guarantor: BS takes final responsibility for the review.
Contributorship: BS suggested the need for this review and was responsible for the first draft and structure. AH contributed much of the discussion on clinical aspects of the topic and the clinical cases. BS wrote the final manuscript in collaboration with AH.
REFERENCES
- 1. Medicines and Healthcare Products Regulatory Agency. Medical Device Alert. All metal-on-metal (MoM) hip replacements (MDA/2010/033) London: MHRA, 2010. [Google Scholar]
- 2. Catelas I, Bobyn JD, Medley JB, et al. Size, shape, and composition of wear particles from metal-metal hip simulator testing: effects of alloy and number of loading cycles. J Biomed Mater Res A 2003;67:312–27 [DOI] [PubMed] [Google Scholar]
- 3. Bowsher JG, Hussain A, Williams PA, Shelton JC. Metal-on-metal hip simulator study of increased wear particle surface area due to ‘severe’ patient activity. Proc Inst Mech Eng [H] 2006;220:279–87 [DOI] [PubMed] [Google Scholar]
- 4. ASTM F75-07. Standard Specification for Cobalt-28 Chromium-6 Molybdenum Alloy Castings and Casting Alloy for Surgical Implants (UNS R30075). ASTM International, West Conshohocken, PA, 2007. DOI: 10.1520/F0075-07. See www.astm.org (last checked 21 September 2011)
- 5. Hart AJ, Sabah S, Henckel J, et al. The painful metal-on-metal hip resurfacing. J Bone Joint Surg Br 2009;91-B:738–44 [DOI] [PubMed] [Google Scholar]
- 6. Jin ZM, Dowson D, Fisher J. Analysis of fluid film lubrication in artificial hip joint replacements with surfaces of high elastic modulus. Proc Inst Mech Eng [H] 1997;211:247–56 [DOI] [PubMed] [Google Scholar]
- 7. Rieker C, Kottig P. In vivo tribological performance of 231 metal-on-metal hip articulations. Hip Int 2002;12:73–6 [DOI] [PubMed] [Google Scholar]
- 8. Matthies A, Underwood R, Cann P, et al. Retrieval analysis of 240 metal-on-metal hip components, comparing modular total hip replacement with hip resurfacing. J Bone Joint Surg Br 2011;93:307–14 [DOI] [PubMed] [Google Scholar]
- 9. Pandit H, Glyn-Jones S, McLardy-Smith P, et al. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br 2008;90-B:847–51 [DOI] [PubMed] [Google Scholar]
- 10. Toms AP, Marshall TJ, Cahir J, et al. MRI of early symptomatic metal-on-metal total hip arthroplasty: a retrospective review of radiological findings in 20 hips. Clin Radiolo 2008;63:49–58 [DOI] [PubMed] [Google Scholar]
- 11. Medicines and Healthcare Products Regulatory Agency. Medical Device Alert. Total hip replacement: DePuy Ultima TPS femoral stem used in combination with Ultima metal-on-metal articulation (MDA/2007/054) London: MHRA, 2007. [Google Scholar]
- 12. Medicines and Healthcare Products Regulatory Agency. Medical Device Alert. ASR™ hip replacement implants manufactured by DePuy International Ltd (MDA/2010/069). London: MHRA, 2010.
- 13. Visuri TI, Pukkala E, Pulkkinen P, Paavolainen P. Cancer incidence and causes of death among total hip replacement patients: a review based on Nordic cohorts with a special emphasis on metal-on-metal bearings. Proc Inst Mech Eng [H] 2006;220:399–407 [DOI] [PubMed] [Google Scholar]
- 14. Signorello LB, Ye W, Fryzek JP, et al. Nationwide study of cancer risk among hip replacement patients in Sweden. J Natl Cancer Inst 2001;93:1405–10 [DOI] [PubMed] [Google Scholar]
- 15. Onega T, Baron J, MacKenzie T. Cancer after total joint arthroplasty: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15:1532–7 [DOI] [PubMed] [Google Scholar]
- 16. Wagner P, Olsson H, Lidgren L, et al. Increased cancer risks among arthroplasty patients: 30 year follow-up of the Swedish Knee Arthroplasty Register. Eur J Cancer 2011;47:1061–71 [DOI] [PubMed] [Google Scholar]
- 17. Askling J, Fored CM, Backlund E, et al. Haematopoietic malignancies in rheumatoid arthritis: lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann Rheum Dis 2005;64:1414–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Committee on Mutagenicity of Chemicals in Food, Consumer Products and the Environment (COM). Annual Report 2006. London: Food Standards Agency, 2007 [Google Scholar]
- 19. Doherty AT, Howell RT, Ellis LA, et al. Increased chromosome translocations and aneuploidy in peripheral blood lymphocytes of patients having revision arthroplasty of the hip. J Bone Joint Surg Br 2001;83-B:1075–81 [DOI] [PubMed] [Google Scholar]
- 20. Ladon D, Doherty A, Newson R, et al. Changes in metal levels and chromosome aberrations in the peripheral blood of patients after metal-on-metal hip arthroplasty. J Arthroplasty 2004;19(Suppl 3):78–83 [DOI] [PubMed] [Google Scholar]
- 21. Davies AP, Sood A, Lewis AC, et al. Metal-specific differences in levels of DNA damage caused by synovial fluid recovered at revision arthroplasty. J Bone Joint Surg Br 2005;87-B:1439–44 [DOI] [PubMed] [Google Scholar]
- 22. National Institute for Clinical Excellence. Technology Appraisal No. 44. Guidance on the Use of Metal on Metal Hip Resurfacing Arthroplasty. Issue Date: June 2002 London: NICE; [Google Scholar]
- 23. United States Food & Drug Administration. New Device Approval: Birmingham Hip Resurfacing P040033. Rockville, MD: US FDA, 2006. See http://www.accessdata.fda.gov/cdrh_docs/pdf4/p040033a.pdf (last checked 9 May 2006)
- 24. United States Food and Drug Administration. New Device Approval: Cormet Hip Resurfacing P050016. Rockville, MD: US FDA, 2007. See http://www.accessdata.fda.gov/cdrh_docs/pdf5/p050016a.pdf (last checked 3 July 2007)
- 25. United States Food & Drug Administration. Metal-on-metal hip implants. Rockville, MD: US FDA, 2011. See http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/MetalonMetalHipImplants/default.htm (last checked 2 February 2011)
- 26. British Hip Society. Large Diameter Metal on Metal Bearing Total Hip Replacements 2011. See http://www.britishhipsociety.com/pdfs/BHS_MOM_THR.pdf (last checked March 2011)
- 27. Schmalzried TP, Guttmann D, Grecula M, Amstutz HC. The relationship between the design, position, and articular wear of acetabular components inserted without cement and the development of pelvic osteolysis. J Bone Joint Surg Am 1994;76:677–88 [DOI] [PubMed] [Google Scholar]
- 28. Brodner W, Grübl A, Jankovsky R, Meisinger V, Lehr S, Gottsauner-Wolf F. Cup inclination and serum concentration of cobalt and chromium after metal-on-metal total hip arthroplasty. J Arthroplasty 2004;19:66–70 [DOI] [PubMed] [Google Scholar]
- 29. Sampson B. Determination of cobalt in plasma and urine by electrothermal atomisation – atomic absorption spectrometry using palladium matrix modification. J Anal Atomic Spectrom 1988;3:465–9 [Google Scholar]
- 30. Jacobs JJ, Skipor AK, Doorn PF, et al. Cobalt and chromium concentrations in patients with metal on metal total hip replacements. Clin Orthop Relat Res 1996;329:S256–63 [DOI] [PubMed] [Google Scholar]
- 31. Witzleb W-C, Ziegler J, Krummenauer F, et al. Exposure to chromium, cobalt and molybdenum from metal-on-metal total hip replacement and hip resurfacing arthroplasty. Acta Orthop 2006;77:697–705 [DOI] [PubMed] [Google Scholar]
- 32. Grubl A, Marker M, Brodner W, et al. Long-term follow-up of metal-on-metal total hip replacement. J Orthop Res 2007;25:841–8 [DOI] [PubMed] [Google Scholar]
- 33. Sampson B. Clinical applications of inductively coupled plasma mass spectrometry. CPD Bull Clin Biochem 2004;6:24–31 [Google Scholar]
- 34. Morton J, Quinn ZA, Baranov VI, et al. Clinical applications of ICPMS. In: Nelms S, ed. Inductively Coupled Plasma Mass Spectrometry Handbook. Oxford: Blackwell, 2005:385–96 [Google Scholar]
- 35. Case CP, Ellis L, Turner JC, Fairman B. Development of a routine method for the determination of trace metals in whole blood by magnetic sector inductively coupled plasma mass spectrometry with particular relevance to patients with total hip and knee arthroplasty. Clin Chem 2001;47:275–80 [PubMed] [Google Scholar]
- 36. Iavicoli I, Falcone G, Alessandrelli M, et al. The release of metals from metal-on-metal surface arthroplasty of the hip. J Trace Elem Med Biol 2006;20:25–31 [DOI] [PubMed] [Google Scholar]
- 37. Vendittoli PA, Mottard S, Roy AG, et al. Chromium and cobalt ion release following the Durom high carbon content, forged metal-on-metal surface replacement of the hip. J Bone Joint Surg Br 2007;89:441–8 [DOI] [PubMed] [Google Scholar]
- 38. Krachler M, Heisel C, Kretzer JP. Validation of ultratrace analysis of Co, Cr, Mo and Ni in whole blood, serum and urine using ICP-SMS. J Anal At Spectrom 2009;24:605–10 [Google Scholar]
- 39. Milosev I, Pisot V, Campbell P. Serum levels of cobalt and chromium in patients with Sikomet metal-metal total hip replacements. J Orthopaedic Res 2005;23:526–35 [DOI] [PubMed] [Google Scholar]
- 40. Walter LR, Marel E, Harbury R, Wearne J. Distribution of chromium and cobalt ions in various blood fractions after resurfacing hip arthroplasty. J Arthroplasty 2008;23:814–21 [DOI] [PubMed] [Google Scholar]
- 41. Davda K, Lali FV, Sampson B, et al. An analysis of metal ion levels in the joint fluid of symptomatic patients with metal-on-metal hip replacements. J Bone Joint Surg Br 2011:93:738–45 [DOI] [PubMed] [Google Scholar]
- 42. Brune D, Aitio A, Nordberg G, Vesterberg O, Gerhardsson L. Normal concentrations of chromium in serum and urine a TRACY project. Scand J Work Environ Health 1993;19(Suppl 1):39–44 [PubMed] [Google Scholar]
- 43. Torra M, Rodamilanst M, Corbella J, et al. Blood chromium determination in assessing reference values in an unexposed Mediterranean population. Biol Trace Elem Res 1999;70:183–9 [DOI] [PubMed] [Google Scholar]
- 44. Changa F-H, Wang S-L, Huang Y-L, et al. Biomonitoring of chromium for residents of areas with a high density of electroplating factories. J Expo Sci Environ Epidemiol 2006;16:138–46 [DOI] [PubMed] [Google Scholar]
- 45. Dobbs HS, Minski MJ. Metal ion release after total hip replacement. Biomaterials 1980;1:193–8 [DOI] [PubMed] [Google Scholar]
- 46. Maezawa K, Nozawa M, Yuasa T, et al. Seven years of chronological changes of serum chromium levels after Metasul metal-on-metal total hip arthroplasty. J Arthroplasty 2009;24:549–53 [DOI] [PubMed] [Google Scholar]
- 47. Luetzner J, Krummenauer F, Lengel AM, Ziegler J, Witzleb W-C. Serum metal ion exposure after total knee arthroplasty. Clin Orthop Relat Res 2007;461:136–42 [DOI] [PubMed] [Google Scholar]
- 48. Goldberg SH, Urban RM, Jacobs JJ, et al. Modes of wear after semiconstrained total elbow arthroplasty. J Bone Joint Surg Am 2008;90:609–19 [DOI] [PubMed] [Google Scholar]
- 49. McPhee IB, Swanson CE. Metal ion levels in patients with stainless steel spinal instrumentation. Spine 2007;32:1963–8 [DOI] [PubMed] [Google Scholar]
- 50. Gutensohn K, Beythien2 C, Bau J, et al. In vitro analyses of diamond-like carbon coated stents: reduction of metal ion release, platelet activation, and thrombogenicity. Thromb Res 2000;99:577–85 [DOI] [PubMed] [Google Scholar]
- 51. Petoumenou E, Arndt M, Keilig L, et al. Nickel concentration in the saliva of patients with nickel-titanium orthodontic appliances. Am J Orthod Dentofacial Orthop 2009;135:59–65 [DOI] [PubMed] [Google Scholar]
- 52. Zaffe D, Bertoldi C, Consolo U. Accumulation of aluminium in lamellar bone after implantation of titanium plates, Ti-6Al-4V screws, hydroxyapatite granules. Biomaterials 2004;25:3837–44 [DOI] [PubMed] [Google Scholar]
- 53. Patton MS, Lyon TDB, Ashcroft GP. Levels of systemic metal ions in patients with intramedullary nails. Acta Orthop 2008;79:820–25 [DOI] [PubMed] [Google Scholar]
- 54. Case CP, Langkamer VG, James C, et al. Widespread dissemination of metal debris from implants. J Bone Joint Surg Br 1994;76-B:701–12 [PubMed] [Google Scholar]
- 55. Urban RM, Jacobs JJ, Tomlinson MJ, et al. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am 2000;82:457–77 [DOI] [PubMed] [Google Scholar]
- 56. Hart AJ, Quinn PD, Sampson, et al. The chemical form of metallic debris in tissues surrounding metal-on-metal hips with unexplained failure. Acta Biomater 2010;6:4439–46 [DOI] [PubMed] [Google Scholar]
- 57. Khan M, Takahashi T, Kuiper JH, et al. Current in vivo wear of metal-on-metal bearings assessed by exercise-related rise in plasma cobalt level. J Orthop Res 2006;24:2029–35 [DOI] [PubMed] [Google Scholar]
- 58. Khan M, Kuiper J-H, Richardson JB. The exercise-related rise in plasma cobalt levels after metal-on-metal hip resurfacing arthroplasty. J Bone Joint Surg Br 2008;90-B:1152–7 [DOI] [PubMed] [Google Scholar]
- 59. Heisel C, Silva M, Skipor AK, et al. The relationship between activity and ions in patients with metal-on-metal bearing hip prostheses. J Bone Joint Surgery Am 2005;87A:781–7 [DOI] [PubMed] [Google Scholar]
- 60. Pattyn CA, Lauwagie SN, Verdonk RC. Whole blood metal ion concentrations in correlation with activity level in three different metal-on-metal bearings. J Arthroplasty 2011;26:58–64 [DOI] [PubMed] [Google Scholar]
- 61. Morlock MM, Bishop N, Zustin J, et al. Modes of implant failure after hip resurfacing: morphological and wear analysis of 267 retrieval specimens. J Bone Joint Surg Am 2008;90:89–95 [DOI] [PubMed] [Google Scholar]
- 62. Langton DJ, Jameson SS, Joyce TJ, et al. The effect of component size and orientation on the concentrations of metal ions after resurfacing arthroplasty of the hip. J Bone Joint Surg Br 2008;90-B:1143–51 [DOI] [PubMed] [Google Scholar]
- 63. De Haan R, Pattyn C, Gill HS, et al. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J Bone Joint Surg Br 2008;90-B:1291–7 [DOI] [PubMed] [Google Scholar]
- 64. Williams S, Leslie I, Isaac G, Jin Z, Ingham E, Fisher J. Tribology and wear of metal-on-metal hip prostheses: influence of cup angle and head position. J Bone Joint Surg Am 2008;90(Suppl 3):111–7 [DOI] [PubMed] [Google Scholar]
- 65. Anderson RA. Chromium, glucose intolerance and diabetes. J Am Coll Nutr 1998;17:548–55 [DOI] [PubMed] [Google Scholar]
- 66. Rajagopalan KV. Molybdenum: an essential trace element in human nutrition. Ann Rev Nutr 1988;8:401–27 [DOI] [PubMed] [Google Scholar]
- 67. Yokoi K, Uthus EO, Nielsen FH. Nickel deficiency diminishes sperm quantity and movement in rats. Biol Trace Elem Res 2003;93:141–53 [DOI] [PubMed] [Google Scholar]
- 68. Barceloux DG. Vanadium. Clin Toxicol 1999;37:265–78 [DOI] [PubMed] [Google Scholar]
- 69. Sunderman FW Jr. A review of the carcinogenicities of nickel, chromium and arsenic compounds in man and animals. Prev Med 1976;5:279–94 [DOI] [PubMed] [Google Scholar]
- 70. Lauwerys LR. Mutagenicity, carcinogenicity and teratogenicity of cobalt metal and cobalt compounds. Mut Res/Rev Genet Toxicol 1990;239:17–27 [DOI] [PubMed] [Google Scholar]
- 71. Heath JC, Freeman MAR, Swanson SAV. Carcinogenic properties of wear particles from prostheses made in cobalt-chromium alloy. Lancet 1971;297:564–6 [DOI] [PubMed] [Google Scholar]
- 72. McGregor DB, Baan RA, Partensky C, et al. Evaluation of the carcinogenic risks to humans associated with surgical implants and other foreign bodies – a report of an IARC Monographs Programme Meeting. Eur J Cancer 2000;36:307–13 [DOI] [PubMed] [Google Scholar]
- 73. Keegan GM, Learmonth ID, Case CP. Orthopaedic metals and their potential toxicity in the arthroplasty patient – review of current knowledge and future strategies. J Bone Joint Surg Br 2007;89-B:567–73 [DOI] [PubMed] [Google Scholar]
- 74. Merritt K, Brown SA. Release of chromium with a valence of 6 from corrosion of stainless steel and cobalt-chromium alloys. J Biomed Mater Res 1995;29:627–33 [DOI] [PubMed] [Google Scholar]
- 75. Hart AJ, Hester T, Sinclair K, et al. The association between metal ions from hip resurfacing and reduced T-cell counts. J Bone Joint Surg Br 2006;88:449–54 [DOI] [PubMed] [Google Scholar]
- 76. Hart AJ, Skinner JA, Winship P, et al. Circulating levels of cobalt and chromium from metal-on-metal hip replacement are associated with CD8+ T-cell lymphopenia. J Bone Joint Surg Br 2009;91-B:835–42 [DOI] [PubMed] [Google Scholar]
- 77. Whittingham-Jones PM, Dunstan E, Altaf H, et al. Immune responses in patients with metal-on-metal hip articulations: a long-term follow-up. J Arthroplasty 2008;23:1212–8 [DOI] [PubMed] [Google Scholar]
- 78. Papageorgiou I, Yin Z, Ladon D, et al. Genotoxic effects of particles of surgical cobalt chrome alloy on human cells of different age in vitro . Mut Res/Fundam Mol Mech Mutagen 2007;619:45–58 [DOI] [PubMed] [Google Scholar]
- 79. Parry MC, Bhabra G, Sood A, et al. Thresholds for indirect DNA damage across cellular barriers for orthopaedic biomaterials. Biomaterials 2010;31:4477–83 [DOI] [PubMed] [Google Scholar]
- 80. Savarino L, Granchi D, Ciapetti G, et al. Effects of metal ions on white blood cells of patients with failed total joint arthroplasties. J Biomed Mater Res 1999;47:543–50 [DOI] [PubMed] [Google Scholar]
- 81. Granchi D, Savarino L, Ciapetti G, et al. Immunological changes in patients with primary osteoarthritis of the hip after total joint replacement. J Bone Joint Surg Br 2003;85-B:758–64 [PubMed] [Google Scholar]
- 82. Curtis JR, Goode GC, Herrington J, Urdaneta LE. Possible cobalt toxicity in maintenance hemodialysis patients after treatment with cobaltous chloride: a study of blood and tissue cobalt concentrations in normal subjects and patients with terminal and renal failure. Clin Nephrol 1976;5:61–5 [PubMed] [Google Scholar]
- 83. Pehrsson K, Lins L-E. Cobalt in uræmic cardiomyopathy. Lancet 1978;312:51–2 [DOI] [PubMed] [Google Scholar]
- 84. Karovic O, Tonazzini I, Rebola N, et al. Toxic effects of cobalt in primary cultures of mouse astrocytes. Similarities with hypoxia and role of HIF-1alpha. Biochem Pharmacol 2007;73:694–708 [DOI] [PubMed] [Google Scholar]
- 85. Licht A, Oliver M, Rachmilewitz EA. Optic atrophy following treatment with cobalt chloride in a patient with pancytopenia and hypercellular marrow. Isr J Med Sci 1972;8:61–6 [PubMed] [Google Scholar]
- 86. Meecham HM, Humphrey P. Industrial exposure to cobalt causing optic atrophy and nerve deafness: a case report. J Neurol Neurosurg Psychiatry 1991;54:374–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Steens W, von Foerster G, Katzer A. Severe cobalt poisoning with loss of sight after ceramic-metal pairing in a hip – a case report. Acta Orthop 2006;77:830–2 [DOI] [PubMed] [Google Scholar]
- 88. Rizzetti MC, Liberini P, Zarattini G, et al. Loss of sight and sound. could it be the hip? Lancet 2009;373:1052 [DOI] [PubMed] [Google Scholar]
- 89. Oldenburg M, Wegner R, Baur X. Severe cobalt intoxication due to prosthesis wear in repeated total hip arthroplasty. J Arthroplasty 2009;24:825 [DOI] [PubMed] [Google Scholar]
- 90. Meeker JD, Rossano MG, Protas B, et al. Cadmium, lead, and other metals in relation to semen quality: human evidence for molybdenum as a male reproductive toxicant. Environ Health Perspect 2008;116:1473–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Brodner W, Grohs JG, Bancher-Todesca D, et al. Does the placenta inhibit the passage of chromium and cobalt after metal-on-metal total hip arthroplasty? J Arthroplasty 2004;19(Suppl 1):102–6 [DOI] [PubMed] [Google Scholar]
- 92. Novak CC, Della Valle CJ, Skipor AK, et al. Metal ion levels in maternal and placental blood following metal-on-metal Arthroplasty. J Arthroplasty 2010;25:e54 [PubMed] [Google Scholar]
- 93. Revell PA. The combined role of wear particles, macrophages and lymphocytes in the loosening of total joint prostheses. J R Soc Interface 2008;5:1263–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jacobs JJ, Hallab NJ, Urban RM, Wimmer MA. Wear particles. J Bone Joint Surg Am 2006;88-A(Suppl 2):99–102 [DOI] [PubMed] [Google Scholar]
- 95. National Joint Registry. NJR 5th Annual report 2009:78. See http://www.njrcentre.org.uk/njrcentre/AbouttheNJR/Publicationsandreports/Annualreports/tabid/86/Default.aspx (last checked 19 September 2008)
- 96. Toms AP, Marshall TJ, Cahir J, et al. MRI of early symptomatic metal-on-metal total hip arthroplasty: a retrospective review of radiological findings in 20 hips. Clin Radiol 2008;63:49–58 [DOI] [PubMed] [Google Scholar]
- 97. Willert HG, Bucchorn GH, Fayyazi A, et al. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints: a clinical and histomorphological study. J Bone Joint Surg Am 2005;87-A:28–36 [DOI] [PubMed] [Google Scholar]
- 98. Hur CI, Yoon TR, Cho SG, et al. Serum ion level after metal-on-metal THA in patients with renal failure. Clin Orthop Relat Res 2008;466:696–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sabah SA, Mitchell AW, Henckel J, et al. Magnetic resonance imaging findings in painful metal-on-metal hips. A prospective study. J Arthroplasty 2011;26:71–6 [DOI] [PubMed] [Google Scholar]