Abstract
Triclocarban (3,4,4′-trichlorocarbanilide; TCC), an antimicrobial used in bar soaps, affects endocrine function in vitro and in vivo. This study investigates whether TCC exposure during early life affects the trajectory of fetal and/or neonatal development. Sprague Dawley rats were provided control, 0.2% weight/weight (w/w), or 0.5% w/w TCC-supplemented chow through a series of 3 experiments that limited exposure to critical growth periods: gestation, gestation and lactation, or lactation only (cross-fostering) to determine the susceptible windows of exposure for developmental consequences. Reduced offspring survival occurred when offspring were exposed to TCC at concentrations of 0.2% w/w and 0.5% w/w during lactation, in which only 13% of offspring raised by 0.2% w/w TCC dams survived beyond weaning and no offspring raised by 0.5% w/w TCC dams survived to this period. In utero exposure status had no effect on survival, as all pups nursed by control dams survived regardless of their in utero exposure status. Microscopic evaluation of dam mammary tissue revealed involution to be a secondary outcome of TCC exposure rather than a primary effect of compound administration. The average concentration of TCC in the milk was almost 4 times that of the corresponding maternal serum levels. The results demonstrate that gestational TCC exposure does not affect the ability of dams to carry offspring to term but TCC exposure during lactation has adverse consequences on the survival of offspring although the mechanism of reduced survival is currently unknown. This information highlights the importance of evaluating the safety of TCC application in personal care products and the impacts during early life exposure.
Keywords: triclocarban, neonate survival, endocrine disruptors, cross-fostering, lactation
Introduction
Numerous empirical and epidemiological studies have linked exposure to various compounds found in consumer and personal care products with altered endogenous signaling and/or function of endocrine/reproductive systems.1 Emerging evidence of daily contact with these compounds has raised public concern regarding the potential ecological and human health impacts.2 Widely used as an antimicrobial in personal care products, triclocarban (3,4,4′-trichlorocarbanilide; TCC) is a high production volume antimicrobial, at a mass of up to 1.5% in certain brands of bar soaps.3,4 Once applied, the compound is washed down the drain and enters the wastewater treatment process.5 The removal of TCC through wastewater treatment is insufficient, however, accounting for the pervasive existence of TCC in both United States and international waterways and contributing to its bioaccumulation in aquatic species.6–10 Following wastewater treatment, TCC has a robust propensity to partition to sludge due to its hydrophobic nature (log Kow = 4.9) allowing for potential transfer to the terrestrial environment when a significant proportion of this nutrient-rich sludge is applied as a fertilizer in agriculture use.5,11,12 As a consequence, TCC has been detected at the ppm level in biosolid-amended soil and is environmentally persistent with a reported half-life of 87 to greater than 1000 days.13 These observations raise safety concerns regarding the potential transfer to the food chain. In fact, TCC uptake from biosolid amended soil has been shown in a variety of plants meant for human consumption, including pumpkin, zucchini, and soybean plants.12,14
Triclocarban can be absorbed through the skin during the regular use of TCC-containing personal care products.15,16 Triclocarban has been detected in 35% of human adult urine and 44% of serum samples in the United States.17 Experimentally, a study conducted in a small group of human volunteers demonstrated that peak circulating TCC levels could reach up to 530 nmol/L, 3 hours after a single 15–minute, whole-body shower with soap containing 0.6% TCC.15 It is worth noting that a background TCC level of 285 nmol/L was detected in a volunteer who was a routine user of TCC-containing personal care products, indicating that frequent application of personal care products containing TCC may lead to a significant body burden.15 The widespread existence, high environmental persistence, and the direct human exposure to TCC, therefore, warrants further investigation into its effective biological impact on human health.
Several lines of evidence demonstrate that TCC is a potential endocrine-disrupting chemical with the capacity to modulate androgen and estrogen activities as well as other hormone-mediated biological processes in vitro and in vivo in adult rat and other animal models.18–24 Although the underlying mechanisms of TCC’s action are unclear and could be diverse, collectively, evidence implicates that TCC exposure may adversely impact endogenous hormone action resulting in the deviation from normal homeostatic, physiological control, and therefore adversely affect pregnancy as well as reproductive outcomes.18,19,21,25,26
Timing of exposure is the key to human disease, specifically if the exposure occurs during early life.27,28 Early life development in utero is complex, tightly under endogenous signal control, and susceptive to subtle endogenous/exogenous environmental insult.29,30 The general consensus by the research community suggests that a significant proportion of disease burden among children is due to modifiable environmental factors.27 The so-called “embryo-fetal origins of adult disease” indicates exposure of environmental factors to a developing fetus or infant may have very different consequences from the same exposure to an adult. The interaction between the maternal and the external environment also plays a major role in determining the propensity of an individual to develop a disease or a dysfunction later in life.27 The growing public anxiety regarding the identification of an increasing number of synthetic compounds in biological samples of children further justifies the urgent need to document the adverse effects of early life exposure to these compounds.31
Data with respect to the potential impacts of TCC during early life exposure, however, are scarce. The only published data are available from Nolen and colleagues who reported that chow supplementation in 21- to 23-day-old rats with 0.25% weight/weight (w/w) of a 2:1 mixture of TCC and 3-trifluoromethyl-4,4′-dichlorocarbanilide (TFC) for 8 weeks prior to breeding and continuously throughout gestation reduced the survival rate of neonates.32 As an antimicrobial, TFC is no longer used. Although these data reflect the impact of the mixture on reproductive outcomes, the relatively extended exposure period prior to gestation as well as the fact that TFC is considered slightly more toxic than TCC leaves several fundamental questions regarding toxicity of TCC largely unanswered and prevents the research community, public, and regulatory agencies from obtaining a better understanding of the safety of the compound. This study aims to address 2 primary questions: (1) whether early life TCC exposure alone will alter the trajectory of fetal and/or neonatal development and (2) if it does, what is/are the susceptible windows of exposure for the observed developmental outcomes. In addition, the reproductive end points in surviving F1 offspring were also evaluated. In this report, 3 experiments directed to address these questions were carried out in Sprague Dawley (SD) rats.
Materials and Methods
Animals
Pregnant SD rats (Harlan Laboratory, Dublin, Virginia) were housed individually with Harlan Teklad laboratory grade 7087 soft cob bedding (Harlan Laboratories, Madison, Wisconsin) in clear plastic cages in a room with a 12:12-hour photoperiod, temperature of 20°C to 22°C, and a relative humidity of 40% to 50%. A separate group of animals was used for each experiment. The day after mating was designated as gestational day (GD) 1. On GD 5, dams were weight ranked and randomized to control or treatment groups to produce similar average body weights per group. All randomizations in the report were achieved using a computer random number generator (random.org). Although the treatments were not blinded, the blood/milk chemical analysis and tissue pathological evaluation were both blinded to evaluators. Animals were provided ad libitum access to water and commercial Harlan ground 2020X chow or 2020X supplemented with TCC (purity = 99%, Sigma Aldrich, St Louis, Missouri) at a concentration of 0.2% or 0.5% w/w. This diet is a soy protein-free rodent chow that contains an isoflavone concentration (daidzein + genistein agylcone equivalents) less than 20 mg/kg and is ideal for studying the impacts of xenobiotics on neonatal development and reproductive function since background phytoestrogen levels are minimized. The TCC supplemented chow was prepared weekly by first weighing the correct amount of TCC and mixing the compound with small amounts of powdered chow using a mortar and pestle. This mixture was then added and mixed into a preweighed amount of powdered chow to obtain the required concentration. Fresh supplemented chow was added to feeding containers as needed. Food intake was measured every other day starting on GD 15. Doses were chosen based on previous studies in castrated adult and immature rats as well as a multigeneration TCC exposure study conducted in the rat.18,19,32 Administration of TCC in chow was chosen as the exposure route, which was used in our previous studies.18,19 Exposures by dermal and oral routes lead to similar metabolic profiles in rat and humans, although there is no direct evidence to compare the internal concentrations achieved between rats and humans.32,33. All protocols used in the study were approved by the Animal Use and Care Committee at the University of Tennessee Knoxville, and the studies were conducted in an animal facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Experiment I: Concentration of TCC in Maternal Serum and Amniotic Fluid
Starting on GD 5, dams were fed either with control (n = 4) or with TCC-supplemented chow (0.2% w/w, n = 5 or 0.5% w/w, n = 5). On GD 19, maternal blood was collected between 08:00 am and 12:00 pm prior to sacrifice. At necropsy, amniotic fluid was collected and total number of fetuses and the number of implantation sites were counted. Systemic organs (liver, kidney, and adrenal) and sex organ (ovary) were dissected and weighed. Tissue sections were examined with routine hematoxylin–eosin staining, and histological changes were evaluated by a board-certified histopathologist blinded to the treatment group. Serum and amniotic fluid samples were frozen at −80°C until analysis.
Experiment II: In Utero/Lactational TCC Exposure and Neonate Survival
Experiment IIa: TCC exposure on neonate survival
To determine the consequence of early life TCC exposure, on GD 5, pregnant animals (n = 5 per group) were weight ranked and randomly assigned to groups. Dams were fed either rat chow or chow supplemented with 0.5% w/w TCC from GD 5 until weaning at postnatal day (PND) 21. On the day of delivery (PND 0), total neonate number was recorded and the survival of pups was monitored daily during the study period. Dams were terminated either on PND 21 or on the day when remaining pups died and mammary tissue was removed for histological analysis.
Experiment IIb: TCC exposure during lactation on mammary tissue
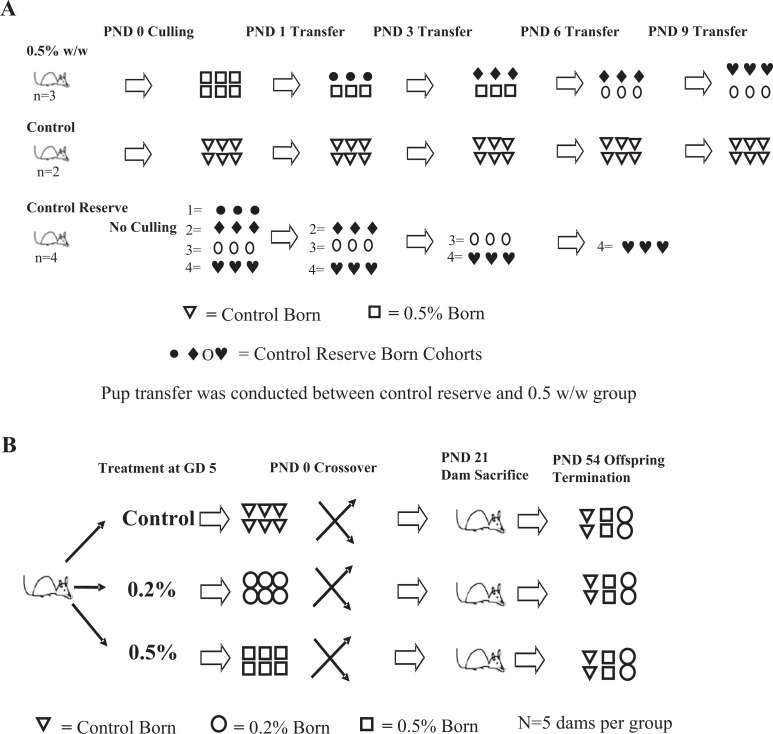
The size of the milk bands indicates an estimate of amount of milk consumed.34 This measure is shown to correlate with stages of deprivation in the rat. Through this assessment, it is possible to determine whether milk has been transferred to the pups, as the bands are visible through the skin.34 To assess whether TCC exposure could directly reduce the lactational capacity of the mammary glands (ie, induce involution) thereby affecting the pup survival, all pups were examined daily for the presence and size of milk bands. Milk bands were rated as described by Ruppert and colleagues.34 Briefly, 0 indicates no band visible; 1, small band visible on the side of pup; 2, small band visible across pup’s abdomen, and 3, large band visible across the pup’s abdomen. Pregnant (GD 5) dams were weight ranked and randomized by body weight into groups fed either rat chow (n = 6) or chow supplemented with 0.5% w/w TCC (n = 3) from GD 5 until PND 14. After delivery at PND 0, litter size was culled to 6 from all 3 of 0.5% w/w-treated dams and only 2 control dams. No culling was conducted for the rest of the control dams (n = 4) which served as reserve controls to provide healthy pups to the treated dams as described subsequently.
Starting on PND 1, healthy age-matched pups (n = 3) born to the 4 reserve control litters were added to replace half (n = 3) the pups raised by TCC-treated dams to maintain normal suckling activity (Figure 1A). Therefore, by PND 1, all 0.5% w/w-treated dams carried 6 pups (3 born to 0.5% w/w TCC-treated dams and 3 born to reserve control dams). On PND 3, the same procedure was conducted as PND 1, except 3 healthy control pups born to the 4 reserve control dams were added to treated dams to replace the pups previously transferred on PND 1 from reserve control dams. Therefore, on PND 3, all 0.5% w/w TCC-treated dams carried 3 of their own pups and 3 new pups transferred from reserve control dams. At PND 6, the procedure was again conducted except that 3 healthy age-matched pups born to the reserve control dams were added to each treated dam to replace the remaining 3 pups originally born to 0.5% w/w-treated dams. After the above-mentioned manipulation on PND 6, pups nursed by the treated dams were all born to reserve control dams. The same substitution procedure was conducted once again on PND 9, and this time the 3 pups transferred from reserve control dams to treated dams on PND 3 were replaced. Milk band quantification comparison was only conducted between control born/raised animals and 0.5% w/w born/raised animals on PNDs 1, 3, and 6, the last day before all the pups born to TCC-treated dams were replaced with pups born to reserve control dams. All dams were terminated on PND 14 and mammary tissue was removed for histological analysis.
Figure 1.
A, Experiment IIb: dams were exposed to either 0.5% w/w TCC-supplemented or control chow from GD 5 to PND 14. On PND 1, healthy age-matched pups (•, n = 3) born to the reserve control litters were added to replace half (□, n = 3) of the pups raised by TCC-treated dams to maintain normal suckling activity. On PND 3, the same procedure was conducted, 3 healthy pups (♦) born to the reserve control dams were added to treated dams to replace the pups (•) previously transferred on PND 1 from reserve control dams. At PND 6, the procedure was again conducted and 3 healthy age-matched pups (O) born to the reserve control dams were added to treated dams to replace the remaining 3 pups (□) originally born to 0.5% w/w-treated dams. The same substitution procedure was conducted once more on PND 9 with 3 pups (♥) transferred from reserve control dams to treated dams replacing the reserve pups (♦) transferred on PND 3. All dams were sacrificed on PND 14. B, Experiment III: cross-fostering design within each dam group (control, 0.2% w/w, and 0.5%w/w; n = 5 dams per group). Pregnant SD rats continued on respective treatment with TCC from GD 5 to PND 21. Crossover was conducted on PND 0. Each dam nursed 2 of their own pups and 2 pups from each of the other 2 treatment groups (∇: pups born to control dams; O: pups born to 0.2% w/w TCC-treated dams; and □: pups born to 0.5% w/w TCC-treated dams). Dams were euthanized on PND 21 or on the date that all pups died. Surviving offspring were continued on respective treatments until PND 54. TCC indicates triclocarban; GD, gestational day; PND, postnatal day; SD, Sprague Dawley.
Experiment IIc: TCC concentration in biological fluids
To measure the concentration of TCC in biological fluid during lactation, starting on GD 5, dams were fed either with control (n = 3) or with TCC-supplemented chow 0.2% w/w (n = 4) or 0.5% w/w (n = 3) until PND 6. Dams were weight ranked and randomly assigned to groups. In addition, a separate population of 3 control dams was used as reserves to provide healthy pups to maintain suckling activity. After delivery, the litter size was culled to 6. No culling was conducted for the 3 reserve control dams. On PND 3, healthy age-matched pups (n = 3) born to reserve control dams were added to each litter of treated dams to replace 3 treated born/raised pups to maintain suckling activity. On PND 5, all dams were individually housed without neonates for 22 hours to increase milk production/accumulation in mammary glands.35 The remaining 3 treated born pups from each TCC-treated dam group were sacrificed on PND 5 and pup blood samples were pooled within each litter and frozen at −80°C for future TCC analysis. On PND 6, all dams were sacrificed and blood samples were collected. At necropsy, mammary tissue/fat pads of dams were carefully separated from the underlying muscles by a cut along the ventral midline. Mammary glands were then opened from inside without penetrating the skin and pooled milk was collected.
Experiment III: In Utero and/or Lactational TCC Exposure on the Survival of F1 Female Rats (Cross-Fostering Study)
Cross-fostering and survival assessment
To identify the susceptive windows of gestational and postnatal TCC exposure to offspring survival, on GD 5 pregnant animals (n = 5 per group) were weight ranked and randomly assigned to groups. Dams were then fed with rat chow or chow supplemented with either 0.2% or 0.5% w/w TCC. Data from experiment II demonstrated that none of the pups could survive when they were nursed by the 0.5% w/w-supplemented dams regardless of their gender and all the pups nursed by control dams survived; however, whether pups raised by 0.2% w/w-supplemented dams could survive was unknown and was one of the primary objectives. To create a manageable workload for the crossover study, only females were used in this experiment. On PND 0, female pups were weighed and sexed based on anogenital distance (AGD). Anogenital distance is defined as the distance between the base of the genital papilla and the rostral end of the anal opening.36 Litter size was culled to 6 females by random removal of pups on PND 0 right after sexing. Specifically, individual pups in each litter were randomly marked with a number using a permanent marker. The numbers were entered into a computer random number generator (random.org) and the order of the numbers was randomized. Pups labeled with the first 6 randomized numbers were kept for the subsequent experiments. After culling, on PND 0, a cross-fostering design was implemented within each litter. Briefly, each dam carried and nursed 2 female pups from their own original litter and fostered 2 female pups from each of the 2 other treatment groups (Figure 1B). In this manner, each control dam raised 2 of their own pups, 2 pups born to 0.2% w/w-treated dams and 2 pups born to 0.5% w/w-treated dams. Each 0.2% w/w-treated dam raised 2 of their own pups, 2 pups born to 0.5% w/w-treated dams, and 2 pups born to control dams. Finally, each 0.5% w/w-treated dam raised 2 of their own pups, 2 pups born to control dams, and 2 pups born to 0.2% w/w-treated dams. The treatment regimen continued from GD 5 throughout lactation until the dams were sacrificed either on weaning/PND 21 or on the same date when all pups died. At PND 3, all pups were reweighed and AGD was measured. Pup mortality was monitored daily throughout the experiment. At PNDs 4 and 5, 3 pups raised by 0.5% w/w-treated dams with greater than a 20% body weight loss over 2 consecutive days were used for pathological assessment.
Vaginal opening and estrous cyclicity assessment
On PND 21, all surviving female offspring from experiment III were weighed, weaned, and AGD was measured. All offspring raised by the same dam were thereafter housed separately with 3 offspring in each cage. The onset of puberty was assessed in female offspring daily from PNDs 30 to 54 for vaginal opening (VO), which is considered as a marker of the onset of puberty in rats.37 All animals were weighed every other day until VO was achieved and the weight of animals on the day of VO was recorded.
All females that displayed VO were assessed for estrous cyclicity by daily vaginal lavage (smears). Vaginal smears were taken between 08:30 am and 10:30 am each morning and examined without stain under light microscopy (×20). The relative abundance of leukocytes, nucleated epithelial cells, and cornified epithelial cells was assessed and cycle stage (day) for each animal was determined.38 The time from VO until the first date of estrus was documented and the cycle stage was recorded until termination on the day of the estrus just prior to or shortly after PND 54. At termination, systemic and sex organs were removed and weighed.
Hormone and TCC Measurements in Biological Samples
Maternal (experiment I on GD 19: control: n = 4; 0.2% w/w: n = 5; 0.5% w/w: n = 5 and experiment IIc on PND 6: control: n = 3; 0.2% w/w: n = 4; 0.5% w/w: n = 3) and neonatal serum (experiment IIc on PND 5: control: n = 3; 0.2% w/w: n = 4; 0.5% w/w: n = 3), and amniotic fluid samples (experiment I on GD 19: control: n = 4; 0.2% w/w: n = 5; 0.5% w/w: n = 5) were analyzed for TCC. First, 50 µL of serum was added into 800 µL of ethyl acetate. Following agitation for 1 hour, 400 µL of liquid was removed from the solution, dried under gentle nitrogen stream, and the residue was redissolved in 100 µL of acetone prior to analysis by liquid chromatography–mass spectrometry (LC-MS-MS). For analysis of TCC from milk, 100 µL of pooled milk sample was mixed with 600 µL of 2-propanol. The mixture was vortexed for 5 minutes at the highest speed followed by centrifugation at 4°C at 5000g for 50 minutes. Supernatant of 300 μL was then removed and mixed with 600 µL of water plus 600 µL of ethyl acetate. The mixture was vortexed for another 5 minutes at highest speed followed by centrifugation for 20 minutes at 4°C at 5000g. After centrifugation, 300 µL of supernatant was collected, dried under nitrogen, and the residue was redissolved in 100 µL of acetone prior to measurement by LC-MS-MS. Triclocarban sample extracts were analyzed on a Dionex UltiMate 3000 UHPLC system coupled to a triple stage quadrupole mass spectrometer (TSQ Quantum Access Max MS/MS, Thermo Scientific, Waltham, Massachusetts). A Hypersil GOLD PFP column (2.1 × 100 mm, 1.9 µm; Thermo Scientific, Waltham, Massachusetts) was used for high-performance liquid chromatography analysis and temperature was held at 38°C for column compartment. The autosampler tray temperature was set at 5°C. The solvent system consists of H2O with 0.02% acetic acid (mobile phase A) and methanol (mobile phase B). The analyte was separated using a gradient program starting with T (minute) = 0, A = 40%, B = 60% at 0.3 mL/min; T = 3, A = 2%, B = 98% at 0.3 mL/min; T = 5.5, A = 2%, B = 98% at 0.3 mL/min; T = 5.6, A = 2%, B = 98% at 0.35 mL/min; T = 12, A = 2%, B = 98% at 0.35 mL/min; T = 12.1, A = 40%, B = 60% at 0.35 mL/min; T = 18.5, A = 40%, B = 60% at 0.35 mL/min; and T = 18.6, A = 40%, B = 60% at 0.3 mL/min. Detection and quantification of TCC were analyzed under negative ion electrospary ionization (ESI−) using selective reaction monitoring and parameters for MS condition were spray voltage (V): −3350; tube lens (V): 215; vaporizer temp: 425°C; capillary temp: 200°C; sheath gas pressure: 20.0 arb units; aux gas pressure: 2.0 arb units; collision gas pressure (mTorr): 1.5; and cycle time (s): 0.45. The m/z 312.718 and 160.000 were used as precursor and product ion, respectively.
For hormone analysis, circulating progesterone, testosterone, total triiodothyronine (T3), and total thyroxine (T4) were measured using commercial radioimmunoassay (RIA) kits (Coat-A-Count, Siemens, Los Angeles, California). 17β-Estradiol levels were measured using ImmunChem Double Antibody RIA kit (MP Biomedicals, Solon, Ohio). Concentrations of thyroid-stimulating hormone were analyzed with an RIA kit specific for rat thyroid-stimulating hormone (TSH; MP Biomedicals, Germany).
Energy Expenditure Assessment
The impact of TCC treatment on energy expenditure of pregnant animals and offspring was monitored using Oxymax Comprehensive Lab Animal Monitoring System (CLAMS, Columbus Instruments, Columbus, Ohio).39 On GD 13, pregnant dams (experiment III) and on PND 41 randomly selected neonates (experiment III) from each respective group were housed individually in a chamber with a 12-hour light/12-hour dark cycle and an ambient temperature of 22°C to 24°C. Animals were acclimated to the system for 12 hours before data were collected. Carbon dioxide production (Vco2) and oxygen consumption (Vo2) were collected every 35 minutes over a 24-hour period. The respiratory exchange ratio (RER) was calculated as Vco2/Vo2 ratio.
Statistical Analysis
Data were presented as group mean ± standard error of the mean. Data were analyzed using SPSS (version 20, IBM, Armonk, New York) by analysis of variance (ANOVA; ie, organ weights, body weight, AGD, TCC, and hormone concentration) or ANOVA with repeat measurements (ie, changes of AGD and body weight over time). In addition, data were analyzed with a covariate of PND 21 body weight (offspring) or pretreatment body weight (dams) when appropriate. Milk band rating was analyzed with a nonparametric Mann-Whitney U test. Mortality measurements were analyzed by Kaplan-Meier survival analysis with JMP Pro 10 (SAS Institute Inc, Cary, North Carolina), followed by pairwise Student-Newman-Keuls post hoc test when appropriate. Statistical significance was considered P <.05. Data were transformed if either normality or the equal variance assumption was invalid. If transformation did not correct normality or equal variance assumption, Kruskal-Wallis one-way ANOVA on ranks was used.
Results
Experiment I: TCC Exposure During Pregnancy
Maternal and fetal compartment TCC concentrations
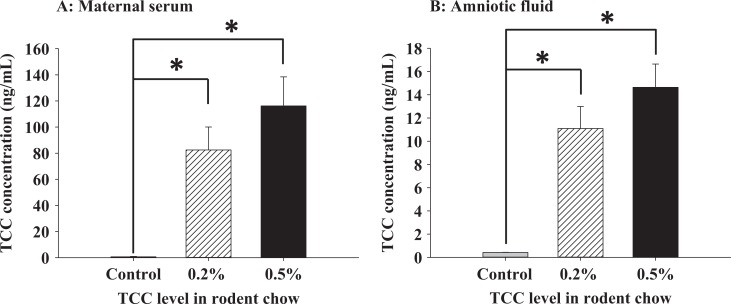
Concentration of TCC in maternal serum and amniotic fluid was measured from samples collected at necropsy on GD 19. The mean concentrations of TCC in the serum collected from TCC-supplemented dams (0.5% w/w: 116.25 ± 22.2; 0.2% w/w: 82.48 ± 17.6 ng/mL) were significantly higher than controls (0.67 ± 0.3 ng/mL, P < .05; Figure 2A). Similarly, significantly higher levels of TCC were detected in amniotic fluid from the TCC-treated dams with a mean concentration of 11.10 ± 1.9 ng/mL detected in the 0.2% w/w TCC-treated group and 14.64 ± 2.0 ng/mL in the 0.5% w/w TCC-treated group compared to 0.42 ± 0.01 ng/mL detected in control dams (P < .05, Figure 2B).
Figure 2.
Experiment I: TCC concentration (ng/mL) on GD 19 from maternal serum (A) and fetal amniotic fluid (B). Pregnant SD rats were treated between GDs 5 and 19 with rat chow supplemented with 0.2% w/w TCC (n = 5, hatched bar), 0.5% w/w TCC (n = 5, dark solid bar), or control food (n = 4, gray solid bar). Data represent mean ± SEM of each group. Data were analyzed with one-way ANOVA followed by Student-Newman-Keuls post hoc test. Statistical significance set at P < .05; * indicates statistical significance between groups; TCC, triclocarban; GD, gestational day; SD, Sprague Dawley; SEM, standard error of the mean; ANOVA, analysis of variance.
Maternal and pregnancy data
The average terminal body weight of 0.5% w/w-treated dams was 6.7% less than that of control dams on GD 19. Maternal body weight gain from GDs 5 to 19 in the 0.5% w/w-treated group was significantly less compared to both control dams and 0.2% w/w TCC-supplemented dams, but there was no statistical difference in body weight gain between the control and the 0.2% w/w TCC-supplemented group (Table 1). Treatment with TCC at any dose had no effect on implantation number. Neither systemic nor sex organ weights at necropsy were statistically different between any group (Table 1). At necropsy on GD 19, circulating levels of estradiol, progesterone, testosterone, T4, and TSH were measured with no statistical difference revealed among any group. Levels of T3 were significantly decreased among dams provided 0.5% w/w TCC-supplemented chow relative to control and 0.2% w/w-treated dams (Table 1). Gross physiological examination and histological evaluation of organs collected at necropsy showed no significant anomaly among treated dams compared to controls (data not shown).
Table 1.
End Points of Dams Exposed to TCC During GDs 5 to 19.a
| End Point | TCC | ||
|---|---|---|---|
| Control | 0.2% w/w | 0.5% w/w | |
| No. of dams | 4 | 5 | 5 |
| Initial body weight, g | 244.9 ± 10.7 | 253.3 ± 1.6 | 249.5 ± 2.8 |
| GD 19 body weight, g | 339.1 ± 11.1 | 337.8 ± 6.1 | 316.3 ± 5.2 |
| Body weight gain (GDs 5-19), g | 94.2 ± 3.7 | 85.0 ± 6.6 | 66.7 ± 4.7b,c |
| Implantation No. | 14.5 ± 1.0 | 14.8 ± 0.4 | 15.2 ± 0.4 |
| Liver, g | 13.3 ± 0.8 | 13.7 ± 0.6 | 12.1 ± 0.4 |
| Kidney, g | 0.77 ± 0.04 | 0.77 ± 0.03 | 0.73 ± 0.01 |
| Adrenal, mg | 30.8 ± 1.0 | 31.5 ± 1.8 | 33.4 ± 1.5 |
| Ovary, mg | 64.7 ± 2.0 | 63.0 ± 3.1 | 64.3 ± 4.9 |
| Estradiol, pg/mL | 101.8 ± 23.0 | 106.8 ± 2.9 | 100.5 ± 10.7 |
| Progesterone, ng/mL | 102.1 ± 11.0 | 111.4 ± 8.7 | 111.3 ± 14.5 |
| Testosterone, ng/mL | 0.29 ± 0.02 | 0.212 ± 0.02 | 0.218 ± 0.07 |
| T3, ng/mL | 0.63 ± 0.05 | 0.52 ± 0.01 | 0.44 ± 0.03b |
| T4, ng/mL | 22.1 ± 4.1 | 20.9 ± 3.0 | 18.1 ± 2.0 |
| TSH, ng/mL | 13.7 ± 1.9 | 16.0 ± 1.1 | 13.1 ± 1.6 |
Abbreviations: ANOVA, analysis of variance; TCC, triclocarban; GD, gestational day; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone.
a ANOVA, P < .05.
b Significant from the control group.
c Significant from the 0.2% w/w TCC group.
Experiment IIa: In Utero/Lactational TCC Exposure
Neonate survival
At birth, no statistical difference in number of live births or average birth weight per litter between groups was noted (data not shown). Although 0.5% w/w TCC treatment did not affect the ability of dams to carry offspring to term, survival analysis revealed that supplementation of 0.5% w/w TCC during gestation and lactation affected neonate survival throughout the experiment (Figure 3). Neonates born to and nursed by 0.5% w/w TCC-treated dams could not survive beyond PND 8; however, all neonates born to and nursed by control dams survived beyond weaning regardless of gender.
Figure 3.
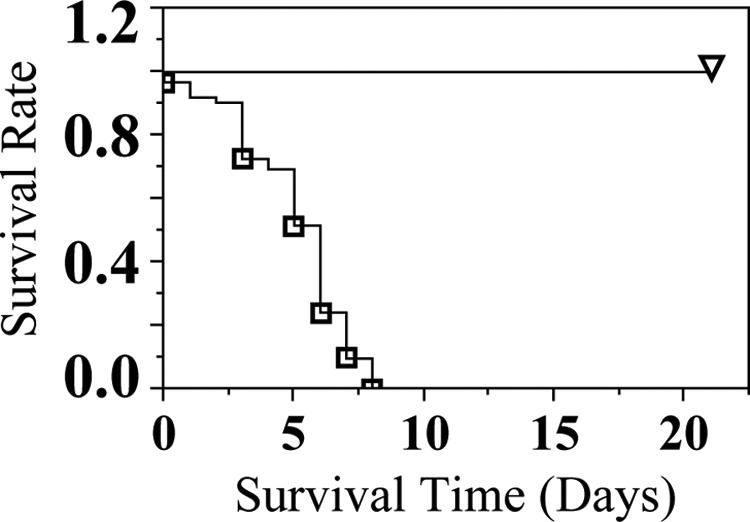
Experiment IIa: survival of neonates raised by dams (n = 5 litters per treatment group) exposed to 0.5% w/w TCC treatment from GD 5 through lactation (∇: born to and raised by control dams and □: born to and raised by 0.5% w/w treated dams). All offspring born to and nursed by control dams survived until weaning. Data were analyzed with Kaplan-Meier survival analysis. Statistical significance was set at P < .05. TCC indicates triclocarban; GD, gestational day.
Maternal data
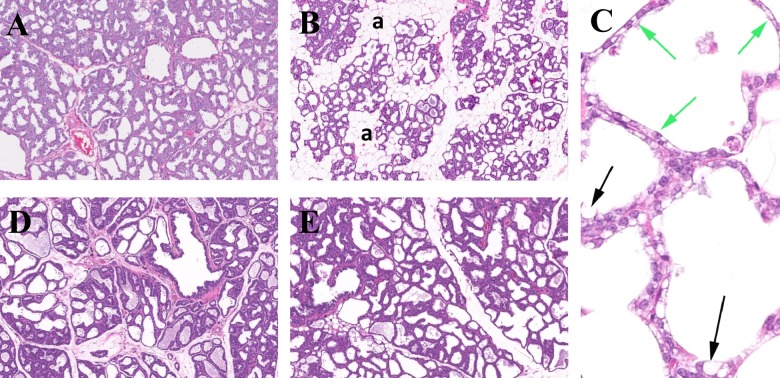
No statistical food intake difference was noted (control: 18.52 ± 1.1 g; 0.5% w/w: 19.06 ± 1.4 g) on GD 19. All TCC-exposed dams were sacrificed right after all pups died (between PNDs 5 and 8). Control dams were sacrificed on PND 21. Milk bands were observed in pups raised by dams treated with 0.5% w/w TCC and histology of mammary tissue collected from both control and 0.5% w/w TCC-supplemented dams revealed evidence of retained secretory material in the tissues. However, the mammary glands collected from 0.5% w/w dams had evidence of involution showing increased lobule separation by interstitial mature fat, thinning epithelial height, and increased epithelial vacuolation with fat (Figure 4, panels A-C).
Figure 4.
Experiment IIb: representative histology of mammary tissue collected from dams at selected time points. Panel A, Normal mammary tissue collected from control dam on PND 21 (H&E 5×). Panel B, Mammary tissue collected from 0.5% w/w exposed dam on PND 8 with moderate involution. Glandular elements are widely separated by adipose tissue (a; H&E 5×). Panel C, Mammary tissue collected from 0.5% w/w exposed dam on PND 8, showing glands with decreased epithelial height (attenuation) indicated by green arrows and vacuolation of epithelial cells with fat (black arrows; H&E 40×). Panel D, Mammary tissue collected from control dam on PND 14 (H&E 5×). Panel E, Mammary tissue collected from 0.5% w/w TCC exposed dam on PND 14 with continuously provided healthy control pups to maintain suckle stimulation (H&E 5×). TCC indicates triclocarban; PND, postnatal day; H&E, hematoxylin–eosin.
Experiment IIb: Effect of TCC exposure on mammary tissue during lactation
In the experiment (Figure 1A) designed to differentiate whether the decreased neonate survival was secondary to the effect of TCC on the reduction in the lactational capacity of the mammary glands (ie, TCC induces involution), milk band scores were similar between PNDs 1 and 3 (median: 3 in 0.5% w/w born/raised pups and control pups). However, the milk band size decreased over time after PND 3. On PND 6, the median milk band score was 0 in 0.5% w/w born/raised pups and 2 among pups born/raised by control dams (Mann-Whitney U test, P < .05). Compared to results from control dams (Figure 4, panel D), histology evaluation revealed that mammary tissue collected from treated dams on PND 14 was not involuted when additional healthy pups were continuously provided on PNDs 3, 6, and 9 to maintain normal suckling activity (Figure 4, panel E).
Experiment IIc: TCC concentration in biological fluids
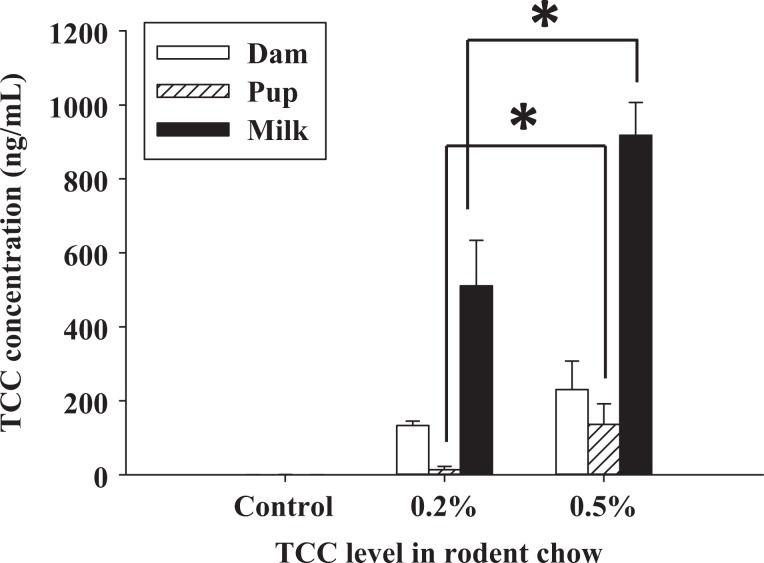
Concentration of TCC on PND 6 was measured from maternal blood and milk. The level of TCC in pooled blood samples collected from neonates on PND 5 was also analyzed (Figure 5). Maternal serum TCC concentration significantly increased with either concentration of TCC in the chow compared to controls at 0.19 ± 0.11 ng/mL in control dams (n = 3), to 134.6 ± 15.4 ng/mL in 0.2% w/w TCC-treated dams (n = 4), and to 230.3 ± 77.3 ng/mL in 0.5% w/w TCC-treated dams (n = 3; P < .05, Figure 5). Following the same pattern, a dose-dependent increase of TCC in maternal milk samples was observed among groups (control [n = 3]: 0.23 ± 0.14 ng/mL; 0.2% w/w [n = 4]: 510.99 ± 122.8 ng/mL; and 0.5% w/w [n = 3]: 917.8 ± 88.9 ng/mL) with significance shown between 0.5% w/w, 0.2% w/w, and control collected milk (P < .05, Figure 5). High levels of TCC were also detected in pooled neonate serum samples raised by TCC-treated dams in both groups compared to controls on PND 5 (0.5% w/w [n = 3]: 136.20 ± 55.86 ng/mL; 0.2% w/w [n = 4]: 13.87 ± 8.5 ng/mL vs 0.56 ± 0.23 ng/mL in controls [n = 3], P < .05, Figure 5); pups raised by 0.5% w/w-treated dams showed significantly higher serum levels of TCC than 0.2% w/w TCC-treated dams raised pups (Figure 5).
Figure 5.
Experiment IIc: TCC concentration (ng/mL) of maternal serum (open bar), maternal milk (solid bar), and neonate serum (hatched bar) collected from control or TCC-exposed dams on PND 6 and neonates raised by control or TCC-exposed dams on PND 5. Dams were exposed to TCC from GD 5 to PND 6 (control: n = 3; 0.2% w/w: n = 4; and 0.5 w/w: n = 3). Neonate sera were collected from pooled neonates raised by each dam group (3 pooled sera from control; 4 pooled sera from the 0.2% w/w group; and 3 pooled sera from the 0.5% w/w group). Data represent mean ± SEM of each group. Data were analyzed with one-way ANOVA followed by Student-Newman-Keuls post hoc test. Statistical significance set at P < .05; * indicates statistical significance between groups. TCC indicates triclocarban; PND, postnatal day; GD, gestational day; SEM, standard error of the mean; ANOVA, analysis of variance.
Experiment III. In Utero/Lactational TCC Exposure (Cross-Fostering Study)
Maternal data
At birth, no statistical difference in number of live births or average birth weight per litter between groups was noted (data not shown). There was no significant difference in RER (0.5% w/w: 0.97 ± 0.01; 0.2% w/w: 0.96 ± 0.01; and control: 0.98 ± 0.01). After birth, dams were continuously exposed to either treated chow or control chow in the manner provided prior to delivery.
F1 female generation data
There was no initial statistical body weight difference in female pups born to control dams or pups born to either group of treated dams prior to culling on PND 0 (control: 5.84 ± 0.17 g; 0.2% w/w: 5.81 ± 0.13 g; and 0.5% w/w: 5.45 ± 0.24 g). After the cross-fostering manipulation, each dam nursed 2 of its own pups and 2 pups from each of the other 2 treatment groups (Figure 1B). All dam groups (n = 5 in each group) raised 30 pups (10 pups born to 0.5% w/w TCC-treated dams, 10 pups born to 0.2% w/w TCC-treated dams, and 10 pups born to control dams). A total of 90 pups were manipulated in experiment III. Average pup body weight in each group after the crossover manipulation at PND 0 was similar among the control, 0.2% w/w TCC, and 0.5% w/w TCC fed groups (Table 2). Starting on PND 3, body weight and AGD were measured every 2 days. Postnatal maternal treatment status significantly affected pup body weight as measured between PNDs 3 and 9. Average body weight was significantly less in pups nursed by TCC-supplemented dams at PND 3 with an average 16% decrease found in pups raised by 0.2% w/w TCC-treated dams and a 25% decrease observed among pups raised by 0.5% w/w TCC-treated dams compared to control raised pups (P < .05, Table 2). Within each dam treatment group, however, no statistical body weight difference was observed among the pups with different in utero exposure status (ie, born to a 0.5% w/w TCC-treated dam, 0.2% w/w TCC-treated dam, or a control dam) at PNDs 3, 6, and 9, respectively (Table 3).
Table 2.
Body Weight (g) and Relative AGDa of Offspring (PNDs 0 to 21) Stratified by Postnatal Expsoure Status.b
| End Point | TCC | ||
|---|---|---|---|
| Control | 0.2% w/w | 0.5% w/w | |
| Litter No. | 5 | 5 | 5 |
| Body weight | |||
| PND 0 | 5.67 ± 0.06 (30) | 5.71 ± 0.06 (30) | 5.66 ± 0.06 (30) |
| PND 3 | 9.19 ± 0.28 (30) | 7.72 ± 0.13 (27)c | 6.89 ± 0.25 (27)c,d |
| PND 6 | 14.23 ± 0.59 (30) | 8.67 ± 0.63 (27)c | ND |
| PND 9 | 21.46 ± 0.84 (30) | 12.06 ± 0.12 (17)c | ND |
| PND 21 | 55.59 ± 0.95 (30) | 29.55 (4)e | ND |
| Relative AGD | |||
| PND 3 | 0.92 ± 0.02 (30) | 1.02 ± 0.05 (27) | 0.92 ± 0.03 (27) |
| PND 6 | 1.03 ± 0.03 (30) | 1.00 ± 0.04 (27) | ND |
| PND 21 | 2.46 ± 0.03 (30) | 2.51 (4)e | ND |
Abbreviations: ANOVA, analysis of variance; AGD, anogenital distance; ND, no offspring survived on that specific PND; TCC, triclocarban; PND, postnatal day.
a Relative AGD: AGD/cube root of body weight on that specific PND.
b ANOVA, P < .05.
c Statistical significance compared to control.
d Statistical significance compared to control and 0.2% w/w groups on that specific PND.
e All the surviving offspring were raised in the same litter. Number in the parentheses indicates the total number of offspring surviving on that specific PND.
Table 3.
Body Weight (g) of Offspring Raised by Control Dams Stratified by In Utero Exposure Status.a
| End Point | In Utero Status | |||
|---|---|---|---|---|
| Control | 0.2% w/w | 0.5% w/w | ||
| Litter No. | 5 | 5 | 5 | |
| Control nursed | PND 0 | 5.81 ± 0.18 (10) | 5.79 ± 0.12 (10) | 5.44 ± 0.17 (10) |
| PND 3 | 9.81 ± 0.40 (10) | 9.11 ± 0.44 (10) | 8.63 ± 0.34 (10) | |
| PND 6 | 15.71 ± 0.56 (10) | 13.82 ± 0.77 (10) | 13.15 ± 0.84 (10) | |
| PND 9 | 23.30 ± 0.64 (10) | 20.80 ± 1.08 (10) | 20.30 ± 1.22 (10) | |
| PND 21 | 58.30 ± 0.89 (10) | 54.83 ± 0.86 (10) | 53.64 ± 1.85 (10) | |
| PND 0 | 5.81 ± 0.19 (10) | 5.83 ± 0.11 (10) | 5.48 ± 0.30 (10) | |
| 0.2% w/w nursed | PND 3 | 8.26 ± 0.32 (9) | 7.74 ± 0.29 (10) | 7.47 ± 0.27 (8) |
| PND 6 | 9.18 ± 0.79 (9) | 8.52 ± 0.65 (10) | 8.40 ± 0.60 (8) | |
| PND 9 | 13.40 ± 0.59 (6) | 12.20 ± 0.49 (6) | 10.10 ± 0.90 (5) | |
| PND 21 | 27.45 ± 7.15 (2) | ND | 31.65 ± 0.35 (2) | |
| 0.5% w/w nursed | PND 0 | 5.84 ± 0.14 (10) | 5.77 ± 0.15 (10) | 5.64 ± 0.13 (10) |
| PND 3 | 7.39 ± 0.17 (10) | 7.07 ± 0.37 (8) | 6.14 ± 0.66 (9) | |
| PND 6 | ND | ND | ND | |
Abbreviations: ND, no offspring survived on that specific PND; PND, postnatal day.
a Number in the parentheses indicates the number of offspring surviving on that specific PND.
Pup mortality was followed throughout the study. A significant reduction in pup number over time was observed between pups raised by 0.5% w/w or 0.2% w/w TCC-treated dams compared to those raised by controls (P < .05, Figure 6A and B). No pups raised by 0.5% w/w TCC-treated dams survived beyond PND 5 regardless of in utero exposure status (n = 30; Figure 6A). The majority of pups (27 of 30) raised by 0.2% w/w TCC-treated dams survived to PND 6, but only 4 animals in this group survived beyond weaning day (all raised in the same litter with 2 offspring born to control dams and 2 offspring born to 0.5% w/w TCC-treated dams; Figure 6B). In contrast, all pups raised by control dams survived throughout the study period regardless of in utero exposure status (n = 30). The abdomens of all pups raised by dams exposed to either TCC concentrations were distended and all pups had diarrhea. Gross pathological examination of randomly selected pups (n = 3) raised by the 0.5% w/w dams on PNDs 4 and 5 showed small acute gastric ulcers and fatty vacuolation of hepatocytes (data not shown). The effect was found in all 3 animals examined; however, the small sample size may not provide a definitive conclusion.
Figure 6.
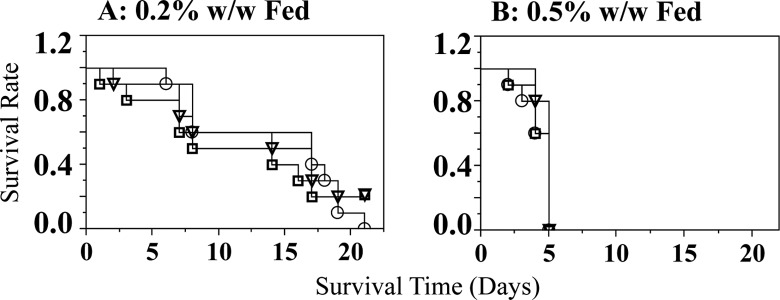
Experiment III: survival of neonates nursed by (A) 0.2% w/w exposed dams (O: born to 0.2% w/w TCC-treated dams; □: pups born to 0.5% w/w TCC dams; and ∇: pups born to control dams) after crossover at PND 0 up to PND 21 and (B) 0.5% w/w TCC supplemented dams (O: born to 0.2% w/w TCC-treated dams; □: pups born to 0.5% w/w TCC dams; and ∇: pups born to control dams). Only 4 offspring survived beyond weaning raised by 0.2% w/w TCC supplemented dams. Data were analyzed with Kaplan-Meier survival analysis followed by a log-rank test for trend to determine individual significance. Statistical significance was set at P < .05. TCC indicates triclocarban; PND, postnatal day.
Because surviving animals in the 0.2% w/w TCC-supplemented group (n = 4) were all raised by the same dam, statistical analysis based on litter could not be conducted. Therefore, only group means were provided for all relevant parameters derived from these 4 surviving offspring. At weaning, the average body weight of the 4 surviving offspring raised by the 0.2% w/w TCC-treated dam was approximately half that of offspring raised by control dams (Table 2). The average RER measured on PND 41 from the 4 surviving offspring raised by 0.2% w/w dams was similar compared to the RER measured from offspring (n = 12) raised by control dams (0.99 and 0.97 ± 0.01, respectively). Among control raised offspring, average RER was similar when analyzed by their respective in utero status (data not shown).
No statistical difference in AGD indexed by cube root of body weight (at the time AGD was acquired) was detected on PND 3 among offspring raised by different dam treatment groups. Similarly, no statistical difference in AGD indexed by cube root of body weight was detected on PND 6 between offspring raised by 0.2% w/w TCC-treated dams compared to those raised by control dams (Table 2). At weaning, the mean relative AGD of the 4 remaining pups was 2.51(mm/3√g) compared to 2.46 (mm/3√g) from offspring born and raised by control dams (Table 3). In utero status had no effect on AGD, VO date, or first date of estrus after VO (data not shown).
The average age of VO in the 4 surviving offspring raised by 0.2% w/w TCC-treated dams was 38.5 days, while the average age of VO from offspring raised by the control dams was 37.17 days. Organ weight indexed by body weight of offspring raised by control dams on the day of sacrifice, categorized by in utero exposure status, is shown in Table 4 with no significant difference noted between any groups for any organ analyzed.
Table 4.
Relative Organ Weight of Offspring Raised by Control Dams Stratified by In Utero Exposure Status.a
| End Point | In Utero Status | ||
|---|---|---|---|
| Control | 0.2% w/w | 0.5% w/w | |
| Litter No. | 5 | 5 | 5 |
| Body weight, g | 181.33 ± 3.91 | 178.25 ± 3.21 | 180.49 ± 6.24 |
| Relative organ weight | |||
| Pituitary | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.08 ± 0.01 |
| Adrenal | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.15 ± 0.01 |
| Kidney | 3.57 ± 0.12 | 3.60 ± 0.03 | 3.61 ± 0.07 |
| Liver | 37.58 ± 1.18 | 38.62 ± 0.88 | 39.86 ± 0.78 |
| Spleen | 2.82 ± 0.03 | 2.80 ± 0.24 | 2.95 ± 0.11 |
| Uterine horn | |||
| Wet | 1.96 ± 0.20 | 2.28 ± 0.54 | 1.97 ± 0.29 |
| Dry | 1.79 ± 0.16 | 1.86 ± 0.18 | 1.67 ± 0.17 |
| Ovary | 0.57 ± 0.023 | 0.49 ± 0.04 | 0.50 ± 0.02 |
Abbreviation: PND, postnatal day.
a Offspring were terminated on estrus day prior to or shortly after PND 54; relative organ weight: organ weight (g) × 1000/body weight (g).
Discussion
Knowledge regarding human and environmental risks to TCC exposure is currently limited, with available data only measuring TCC prevalence and persistence in the environment. Although no long-term TCC exposure studies in humans have been reported, Schebb et al demonstrated that after a 15-minute whole-body shower with 0.6% TCC-containing bar soap, up to 1030 nmol/L of TCC metabolites was detected in the urine of 6 human volunteers.40 The significant excretion suggests that absorbed TCC must be systemically available and thus present in blood.15,40 In fact, after a single 15-minute shower, peak circulating level of TCC was detected within 3 hours with a range of 10 to 530 nmol/L.15 Interestingly, a high TCC background level of 285 ± 5 nmol/L was reported in the circulation of a user who used TCC-containing personal care products regularly compared to other volunteers.15 These data indicate that routine users of TCC-containing products may have a high body burden.
Human exposure through the diet has not been explored. The pharmacokinetic profile of long-term TCC exposure in the chow goes beyond the scope of the current study; nonetheless, after 14 days of oral exposure between GDs 5 and 19, we detected an average circulating level of 82.48 ± 17.6 ng/mL (261.36 nmol/L) and 116.25 ± 22.2 ng/mL (368.37 nmol/L) TCC in 0.2% and 0.5% w/w-treated SD rats, respectively (experiment I, Figure 2), a level within the range of reported human exposure data.15 A similar dose-dependent detection of TCC was identified in the fetal compartments. We detected 11.10 ± 1.9 ng/mL and 14.64 ± 2.0 ng/mL TCC in the amniotic fluid of 0.2% and 0.5% w/w TCC groups, respectively, showing the transplacental transfer of TCC during gestation (Figure 2). The wide range of the TCC concentration in circulation of pregnant rats after exposure (0.2% w/w: 39.85-145.37 ng/mL; 0.5% w/w 71.33-171.85 ng/mL; experiment I, Figure 2) may reflect the interindividual difference in TCC absorption, distribution, and excretion, a similar scenario that has been reported in humans.15
Nolen et al reported that a 2:1 mixture of TCC and another antimicrobial compound TFC compromises reproductive outcomes when rats were fed continuously with 0.25% TCC/TFC mixture in the chow for more than 11 weeks starting 8 weeks prior to pregnancy.32 A significant decrease in the average number of pups born/litter, average number of live pups/litter at PND 4, as well as the number weaned/litter was observed compared to the control group. When exposure was extended to cover the second pregnancy period, only an average of 1 offspring per litter was able to survive beyond weaning. In contrast, when the mixture was administrated only during the organogenesis period (PNDs 6-15), or for an extended period of time but only with 0.2% TCC/TFC mixture, no significant differences were detected by any of the criteria described earlier when compared to the controls. Therefore, Nolen et al conclude that the maximum dietary concentration of the TCC–TFC mixture having no effect on reproduction should be between 0.20% and 0.25%. The authors further concluded that 0.25% concentration of mixture had no effect when fed only during GDs 6 to 15.32 Only the extended exposure with 0.25% w/w TCC–TFC mixture (8 weeks prior to pregnancy plus entire pregnancy period) would affect the survival of neonates.
Although the results of Nolen’s are informative, it raises several critical issues. A 2:1 TCC–TFC mixture rather than an individual compound was tested.32 Subchronic studies indicate TFC is slightly more toxic than TCC; therefore, the possibility that compromised reproductive outcomes observed by Nolen et al may reflect an additive/synergistic effect of the 2 compounds cannot be ruled out. Since the use of TFC as an antimicrobial agent in personal care products has been phased out,32 it is essential to investigate whether the administration of TCC alone would interfere with development and reproduction.
In our study, dose and length of TCC exposure do not appear to affect the ability of the dams to carry pups to term; no statistical difference in number of implantation sites or the number of live births at delivery was observed in either TCC treatment group compared to controls even when 0.5% w/w TCC was administered (experiments I and IIa). Further, we found no statistical difference in reproductive outcomes (AGD, VO, or estrous cycling) of the F1 generation of control raised animals born to different treatment groups (0.5% w/w, 0.2% w/w, or control). However, TCC exposure at 0.5% w/w affected neonate survival, with no survival beyond PND 8 among either male or female pups when the treatment regimen covered both pregnancy and lactation (Figure 3, experiment IIa). Due to the mortality effects of TCC treatment on F1 offspring raised by treated dams, the small number (n = 4) of surviving offspring in the current study prevents a definitive conclusion regarding the examined reproductive outcomes.
To help further examine the potential susceptive windows of TCC exposure (in utero only, in utero plus lactation, or lactation only) that lead to the decline in neonate survival, a cross-fostering design was implemented (Figure 1B, experiment III). Regardless of in utero exposure status, maternal exposure during lactation significantly affected pup body weight (Tables 2 and 3). Compared to controls on PND 3, an average of 16% and 25% body weight reduction was observed in pups raised by 0.2% and 0.5% w/w TCC-treated dams, respectively (Table 2). When control-fed groups were stratified by gestational exposure status, no statistical body weight difference was observed among pups with different in utero exposure status (ie, pups raised by control dams but were born to 0.5% w/w, 0.2% w/w TCC-treated dams, or control dams, Table 3).
All pups raised by control dams survived beyond weaning, regardless of in utero exposure. In contrast, no pups raised by 0.5% w/w TCC-treated dams survived beyond PND 5 regardless of the group they were born to (Figure 6B, experiment III), and only 4 pups raised by 0.2% w/w TCC-treated dams survived beyond weaning (Figure 6A, experiment III). Collectively, these data implicate the critical TCC exposure window for neonate survival occurs during lactation, because even pups with no in utero exposure could not survive when raised by TCC-treated dams and all pups raised by control dams survived even with gestational TCC exposure.
No statistical difference in energy expenditure was observed between any dam treatment groups (experiment III). We observed a 6.7% body weight decrease among 0.5% w/w TCC-treated dams although it was not statistically significant. Treatment with TCC does not appear to affect milk production and transfer. Pathological evaluation of mammary tissue demonstrated involution of the mammary glands in TCC-treated dams when necropsy was conducted between PNDs 5 and 8 after complete litter loss (experiment IIa). To differentiate whether the involution of the mammary gland was due to the TCC treatment (primary) or reduced stimulation on the mammary gland as an outcome of reduced neonate suckling when pups died (secondary), healthy age-matched pups born to control dams were added to the TCC-treated dams at various time points during lactation to maintain normal suckling activity and dams were sacrificed on PND 14 (experiment IIb). Pups born to/raised by 0.5% w/w TCC-treated dams had similar milk band size when compared to control pups on PNDs 1 and 3. The size of milk band was significantly smaller at PND 6 between pups born to/raised by 0.5% w/w TCC-treated dams compared to age-matched pups born to/raised by controls. Microscopic assessment revealed no sign of involution in mammary glands of treated dams that were continuously provided with healthy pup suckling stimulation. Together, our data suggest that the reduced survival in pups raised by TCC-treated dams was unlikely due to the primary impact of TCC on the development and function of mammary glands.
We further compared the concentration of TCC collected from dam and neonate circulation and the milk from dams (experiment IIc). As shown in Figure 5, a similar dose-dependent pattern of TCC concentration was observed in the circulation of dams as well as in the pups that were raised by the treated dams. Interestingly, 510.9 ± 122.8 ng/mL and 917.8 ± 88.9 ng/mL of TCC were detected in the milk of the 0.2% and 0.5% w/w TCC-treated dams, respectively. This level of TCC in the milk was almost 4 times the amount detected in blood circulation from either group. These data imply that TCC concentrates in the milk. Although extrapolation to human exposure still requires further investigation, our data, nevertheless, highlight the potential of high levels of TCC exposure to neonates via lactation.
Several lines of evidence in the current study further support the hypothesis that TCC exposure during lactation influences the survival of the neonates. In treated pups, we observed small, acute gastric ulcers (indicating potential stress) and fatty vacuolation of hepatocytes in pups exposed to TCC during lactation (experiment III). Postmortem evaluation of neonates that died prior to weaning had swollen abdomens, diarrhea, and grossly enlarged, liquid filled ceca, which is consistent with observations in rodents with impaired gut microflora, that is, germ-free mice or rodents orally treated with an excess of antimicrobials.41 In germ-free mice, an enlarged cecum starts during suckling, the appearance of which is postulated to be due to the accumulation of macromolecular, sulfate-containing glycoproteins from the milk that normally are degraded by the microflora of the lower gut.42 These negatively charged macromolecules not only attract water into the cecal lumen but also limit Na+-dependent water transport out of the cecum. The enlarged cecum thus could become a reservoir of pharmacologically active materials that may become blood borne and affect the physiology of the animal.42
The existence of certain intestinal microbes could promote normal mammalian physiology including proper digestion, metabolism, epithelial cell function, angiogenesis, enteric nerve function, and immune system development.43 On the other hand, altered intestinal flora has been reported in patients with inflammatory bowel disease, allergies, or patients with metabolic syndrome, indicating that microbial populations might influence disease pathogenesis although the causality is still unclear.44–46 Imbalances in the composition of intestinal flora diversity could lead to dysfunction and chronic disease state. Antibiotics have been shown to drastically disrupt indigenous microbiota in animals as well as in humans, which could result in a long-term decrease in its overall diversity.43,47–49 Limited information from humans and animals has shown antibiotic treatment can eliminate native intestinal microflora populations that normally compete with or otherwise antagonize invading pathogens or induce the overgrowth of “pathogenic” components of gut microbiota.50,51 The disturbance of microflora therefore could diminish the natural defense mechanisms provided by the colonic microbial ecosystem, making the host vulnerable to infection. Whether high levels of TCC exposure through lactation affects the establishment/colonization of different microflora in the gut of neonates, thereby reducing survival, requires further investigation.
Alternatively, TCC may alter the various processes by which milk components are synthesized and/or secreted or interfere with the delivery of substrate for milk formation and resulting composition.52,53 Results from experiment IIb demonstrated that milk was transferred from dam to pups, however the effect of TCC exposure to the nutritional composition of the milk or its direct toxic effect to the pups is unknown. Artificial feeding methods could be used to control the nutritional composition of the milk and delivery of TCC54 to investigate the mechanisms of reduced survival.
In summary, our study demonstrates that early life exposure of 0.2% w/w and 0.5% w/w TCC affects the survival of neonates. Although the current study by design could not reveal the underlying mechanisms of the reduced survival of F1 offspring during lactation, several lines of suggestive evidence support the hypothesis that TCC exposure during lactation influences the development of the neonates. The susceptive window of exposure is during lactation. Although TCC exposure does not affect the ability of dams to carry offspring to term, few pups can survive beyond weaning if the pups are raised by 0.2% w/w TCC-treated dams and no pups could survive when raised by 0.5% w/w TCC-treated dams, regardless of their in utero exposure status. Collectively, the results of our study demonstrate the need for future research to determine the mechanism of reduced survival during lactation and to evaluate the impact of TCC-containing products on reproductive and developmental health in humans.
There are limitations to prevent full extrapolation of the results derived from animal studies to human exposure scenario. Human exposure to TCC through the use of TCC-containing personal care products is likely sporadic, while the animals in the current study had ad libitum access to the TCC supplemented chow; therefore, animals had constant TCC exposure. If problems occur during breastfeeding and infants failed to thrive, humans can make a decision to use formula, an option that animals do not have. Regardless of these limitations, the animal study data warn the potential risk of TCC exposure during lactation and underscore the importance to assess the levels of TCC exposure in lactating women who are also routine users of TCC-containing products and to evaluate the impact of TCC-containing products to human health.
Acknowledgments
We are in great debt to Dr Kurt Benirschke of Department of Pathology, University of California, San Diego, for assisting with initial pathological evaluation.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Dr. Laura Healy is a paid employee of HistoTox Labs, Inc. Boulder, CO 80301. No other authors have conflicts to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was conducted at The University of Tennessee Knoxville and supported by National Institutes of Environmental Health Sciences to Dr. Jiangang Chen (1R21ES017475-01A1). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS.
References
- 1. Witorsch RJ, Thomas JA. Personal care products and endocrine disruption: A critical review of the literature. Crit Rev Toxicol. 2010;40(suppl 3):1–30. [DOI] [PubMed] [Google Scholar]
- 2. Bergman Å, Heindel JJ, Susan Jobling, Kidd KA, Zoeller RT, eds. State of the Science of Endocrine Disrupting Chemicals - 2012. Geneva: United Nations Environment Programme and the World Health Organization; 2013. [Google Scholar]
- 3. Perencevich EN, Wong MT, Harris AD. National and regional assessment of the antibacterial soap market: a step toward determining the impact of prevalent antibacterial soaps. Am J Infect Control. 2001;29(5):281–283. [DOI] [PubMed] [Google Scholar]
- 4. Baumann A, Lohmann W, Rose T, et al. Electrochemistry-mass spectrometry unveils the formation of reactive triclocarban metabolites. Drug Metab Dispos. 2010;38(12):2130–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kwon JW, Armbrust KL, Xia K. Transformation of triclosan and triclocarban in soils and biosolids-applied soils. J Environ Qual. 2010;39(4):1139–1144. [DOI] [PubMed] [Google Scholar]
- 6. Heidler J, Halden RU. Fate of organohalogens in US wastewater treatment plants and estimated chemical releases to soils nationwide from biosolids recycling. J Environ Monit. 2009;11(12):2207–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sapkota A, Heidler J, Halden RU. Detection of triclocarban and two co-contaminating chlorocarbanilides in US aquatic environments using isotope dilution liquid chromatography tandem mass spectrometry. Environ Res. 2007;103(1):21–29. [DOI] [PubMed] [Google Scholar]
- 8. Zhao JL, Ying GG, Liu YS, Chen F, Yang JF, Wang L. Occurrence and risks of triclosan and triclocarban in the Pearl River system, South China: from source to the receiving environment. J Hazard Mater. 2010;179(1-3):215–222. [DOI] [PubMed] [Google Scholar]
- 9. Coogan MA, Edziyie RE, La Point TW, Venables BJ. Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere. 2007;67(10):1911–1918. [DOI] [PubMed] [Google Scholar]
- 10. Coogan MA, La Point TW. Snail bioaccumulation of triclocarban, triclosan, and methyltriclosan in a North Texas, USA, stream affected by wastewater treatment plant runoff. Environ Toxicol Chem. 2008;27(8):1788–1793. [DOI] [PubMed] [Google Scholar]
- 11. Snyder EH, O'Connor GA, McAvoy DC. Measured physicochemical characteristics and biosolids-borne concentrations of the antimicrobial Triclocarban (TCC). Sci Total Environ. 2010;408(13):2667–2673. [DOI] [PubMed] [Google Scholar]
- 12. Wu C, Spongberg AL, Witter JD, Fang M, Czajkowski KP. Uptake of pharmaceutical and personal care products by soybean plants from soils applied with biosolids and irrigated with contaminated water. Environ Sci Technol. 2010;44(16):6157–6161. [DOI] [PubMed] [Google Scholar]
- 13. Walters E, McClellan K, Halden RU. Occurrence and loss over three years of 72 pharmaceuticals and personal care products from biosolids-soil mixtures in outdoor mesocosms. Water Res. 2010;44(20):6011–6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aryal N, Reinhold D. Phytoaccumulation of antimicrobials by hydroponic Cucurbita pepo. Int J Phytoremediation. 2013;15(4):330–342. [DOI] [PubMed] [Google Scholar]
- 15. Schebb NH, Ahn KC, Dong H, Gee SJ, Hammock BD. Whole blood is the sample matrix of choice for monitoring systemic triclocarban levels. Chemosphere. 2012;87(7):825–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. North-Root H, Demetrulias J, Wester R, Maibach H, Corbin N. Deposition of 3,4,4'-trichlorocarbanilide on human skin. Toxicol Lett. 1984;22(2):235–239. [DOI] [PubMed] [Google Scholar]
- 17. Zhou X, Ye X, Calafat AM. Automated on-line column-switching HPLC-MS/MS method for the quantification of triclocarban and its oxidative metabolites in human urine and serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;881-882:27–33. [DOI] [PubMed] [Google Scholar]
- 18. Chen J, Ahn KC, Gee NA, et al. Triclocarban enhances testosterone action: a new type of endocrine disruptor? Endocrinology. 2008;149(3):1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duleba AJ, Ahmed MI, Sun M, et al. Effects of triclocarban on intact immature male rat: augmentation of androgen action. Reprod Sci. 2011;18(2):119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahn KC, Zhao B, Chen J, et al. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: receptor-based bioassay screens. Environ Health Perspect. 2008;116(9):1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chung E, Genco MC, Megrelis L, Ruderman JV. Effects of bisphenol A and triclocarban on brain-specific expression of aromatase in early zebrafish embryos. Proc Natl Acad Sci U S A. 2011;108(43):17732–17737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hinther A, Bromba CM, Wulff JE, Helbing CC. Effects of triclocarban, triclosan, and methyl triclosan on thyroid hormone action and stress in frog and mammalian culture systems. Environ Sci Technol. 2011;45(12):5395–5402. [DOI] [PubMed] [Google Scholar]
- 23. Zhao B, Baston DS, Hammock B, Denison MS. Interaction of diuron and related substituted phenylureas with the Ah receptor pathway. J Biochem Mol Toxicol. 2006;20(3):103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schebb NH, Buchholz BA, Hammock BD, Rice RH. Metabolism of the antibacterial triclocarban by human epidermal keratinocytes to yield protein adducts. J Biochem Mol Toxicol. 2012;26(6):230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giudice BD, Young TM. The antimicrobial triclocarban stimulates embryo production in the freshwater mudsnail Potamopyrgus antipodarum. Environ Toxicol Chem. 2010;29(4):966–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ankley GT, Jensen KM, Kahl MD, et al. Use of chemical mixtures to differentiate mechanisms of endocrine action in a small fish model. Aquat Toxicol. 2010;99(3):389–396. [DOI] [PubMed] [Google Scholar]
- 27. Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311(6998):171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Skinner MK. Endocrine disruptors and epigenetic transgenerational disease etiology. Pediatr Res. 2007;61(5 pt 2):48R–50R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crinnion WJ. Maternal levels of xenobiotics that affect fetal development and childhood health. Altern Med Rev. 2009;14(3):212–222. [PubMed] [Google Scholar]
- 30. Fligny C, Hatia S, Amireault P, Mallet J, Cote F. Mammalian prenatal development: the influence of maternally derived molecules. Bioessays. 2009;31(9):935–943. [DOI] [PubMed] [Google Scholar]
- 31. Ye X, Zhou X, Wong LY, Calafat AM. Concentrations of bisphenol A and seven other phenols in pooled sera from 3-11 year old children: 2001-2002 national health and nutrition examination survey. Environ Sci Technol. 2012;46(22):12664–12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nolen GA, Dierckman TA. Reproduction and teratogenic studies of a 2:1 mixture of 3,4,4'-trichlorocarbanilide and 3-trifluoromethyl-4,4'-dichlorocarbanilide in rats and rabbits. Toxicol Appl Pharmacol. 1979;51(3):417–425. [DOI] [PubMed] [Google Scholar]
- 33. Hiles RA. Metabolism and toxicity of halogenated carbanilides: absorption, distribution and excretion of radioactivity from 3,4,4'-trichloro[14C]carbanilide (TCC) and 3-trifluoromethyl-4,4'-dichloro[14C]carbanilide (TFC) in rats. Food Cosmet Toxicol. 1977;15(3):205–211. [DOI] [PubMed] [Google Scholar]
- 34. Ruppert PH, Dean KF, Reiter LW. Developmental and behavioral toxicity following acute postnatal exposure of rat pups to trimethyltin. Neurobehav Toxicol Teratol. 1983;5(4):421–429. [PubMed] [Google Scholar]
- 35. Brake SC. Procedures for the collection of milk from the rat dam. Physiol Behav. 1979;22(4):795–797. [DOI] [PubMed] [Google Scholar]
- 36. Hotchkiss AK, Lambright CS, Ostby JS, Parks-Saldutti L, Vandenbergh JG, Gray LE., Jr Prenatal testosterone exposure permanently masculinizes anogenital distance, nipple development, and reproductive tract morphology in female Sprague-Dawley rats. Toxicol Sci. 2007;96(2):335–345. [DOI] [PubMed] [Google Scholar]
- 37. Ojeda SR, Wheaton JE, Jameson HE, McCann SM. The onset of puberty in the female rat: changes in plasma prolactin, gonadotropins, luteinizing hormone-releasing hormone (LHRH), and hypothalamic LHRH content. Endocrinology. 1976;98(3):630–638. [DOI] [PubMed] [Google Scholar]
- 38. Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62(4A):609–614. [DOI] [PubMed] [Google Scholar]
- 39. Lou PH, Yang G, Huang L, et al. Reduced body weight and increased energy expenditure in transgenic mice over-expressing soluble leptin receptor. PLoS One. 2010;5(7): e11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schebb NH, Inceoglu B, Ahn KC, Morisseau C, Gee SJ, Hammock BD. Investigation of human exposure to triclocarban after showering and preliminary evaluation of its biological effects. Environ Sci Technol. 2011;45(7):3109–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reikvam DH, Erofeev A, Sandvik A, et al. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLoS One. 2011;6(3):e17996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wostmann BS. The germfree animal in nutritional studies. Annu Rev Nutr. 1981;1:257–279. [DOI] [PubMed] [Google Scholar]
- 43. Hill DA, Hoffmann C, Abt MC, et al. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 2010;3(2):148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Milani C, Hevia A, Foroni E, et al. Assessing the fecal microbiota: an optimized ion torrent 16 S rRNA gene-based analysis protocol. PLoS One. 2013;8(7):e68739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sokol H, Seksik P, Rigottier-Gois L, et al. Specificities of the fecal microbiota in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12(2):106–111. [DOI] [PubMed] [Google Scholar]
- 46. Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121(6):2126–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manichanh C, Reeder J, Gibert P, et al. Reshaping the gut microbiome with bacterial transplantation and antibiotic intake. Genome Res. 2010;20(10):1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jakobsson HE, Jernberg C, Andersson AF, Sjolund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One. 2010;5(3):e9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lamousé-Smith ES, Tzeng A, Starnbach MN. The intestinal flora is required to support antibody responses to systemic immunization in infant and germ free mice. PloS One. 2011;6(11):e27662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stiefel U, Donskey CJ. The role of the intestinal tract as a source for transmission of nosocomial pathogens. Curr Infect Dis Rep. 2004;6(6):420–425. [DOI] [PubMed] [Google Scholar]
- 51. Donskey CJ. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin Infect Dis. 2004;39(2):219–226. [DOI] [PubMed] [Google Scholar]
- 52. Dostal LA, Weaver RP, Schwetz BA. Transfer of di (2-ethylhexyl) phthalate through rat milk and effects on milk composition and the mammary gland. Toxico Appl Pharmacol. 1987;91(3):315–325. [DOI] [PubMed] [Google Scholar]
- 53. Neville MC, Walsh CT. Effects of xenobiotics on milk secretion and composition. Am J Clin Nutr. 1995;61(3):687S–694S. [DOI] [PubMed] [Google Scholar]
- 54. West JR. Use of pup in a cup model to study brain development. J Nutr. 1993;123(2 suppl):382–385. [DOI] [PubMed] [Google Scholar]