Abstract
Background:
Very few studies have evaluated the expression of homeobox A10 (HOXA10) and steroid (estrogen and progesterone) receptors exclusively in deep endometriosis. Conclusions drawn from studies evaluating peritoneal and ovarian endometriosis are usually generalized to explain the pathogenesis of the disease as a whole. We aimed to evaluate the expression of HOXA10, estrogen receptor α (ER-α), progesterone receptor (PR), and PR-B in rectosigmoid endometriosis (RE), a typical model of deep disease.
Methods:
We used RE samples from 18 consecutive patients to construct tissue microarray blocks. Nine patients each were operated during the proliferative and secretory phases of the menstrual cycle. We quantified the expressions of proteins by immunohistochemistry using the modified Allred score.
Result:
The HOXA10 was expressed in the stroma of nodules during the secretory phase in 5 of the 18 patients. Expression of ER-α (in 16 of 18 patients), PR (in 17 of 18 patients), and PR-B (17 of 18 patients) was moderate to strong in the glands and stroma of nodules during both phases. Expression of both PR (P = .023) and PR-B (P = .024) was significantly greater during the secretory phase.
Conclusion:
The HOXA10 is expressed in RE, where it likely imparts the de novo identity of endometriotic lesions. The ER-α, PR, and PR-B are strongly expressed in RE, which differs from previous studies investigating peritoneal and ovarian lesions. This suggests different routes of pathogenesis for each of the 3 types of endometriosis.
Keywords: HOXA10, estrogen receptor, progesterone receptor, rectosigmoid endometriosis, deep endometriosis pathogenesis
Introduction
Endometriosis is defined as the presence of a tissue that is histologically similar to the endometrium (glandular and/or stromal tissue) at ectopic sites.1 Peritoneal, ovarian, and deep adenomyotic rectovaginal lesions represent the 3 distinct clinical forms of the disease.2 Deep endometriosis (DE) is present in 5% to 10% of women.3 It is the most significant form of endometriosis and is responsible for pain and infertility associated with endometriosis.4,5 Despite its clinical importance, there are few studies that focused on DE lesions exclusively when investigating the pathogenesis of the disease.6,7 Most research has focused on peritoneal and ovarian endometriosis, and the conclusions drawn from those studies were generalized to explain the pathogenesis of the disease as a whole.8–11 To further investigate DE specifically, rectosigmoid endometriosis (RE) is an acceptable model because it has many characteristics of DE lesions, such as being composed of scanty glands and stroma among smooth muscle exhibiting extensive hyperplasia and metaplasia.
The homeobox (Hox in nonhuman vertebrates, HOX in humans) genes are master regulators of anteroposterior corporal segmentation in animals12 and are related to the pathogenesis of endometriosis.13,14 The HOX genes are responsible for imparting identity to undifferentiated tissues and have well-organized spatiotemporal expression patterns during embryogenesis and adult life. For example, the posterior HOX genes HOXA9, HOXA10, HOXA11, and HOXA13 regulate morphogenesis of the oviduct, uterus, lower uterine segment/cervix, and upper vagina, respectively.15 The HOXA10 gene, specifically, is expressed in the space between the oviduct–uterine transition and the uterine isthmus in both embryos and adults.15 It plays an important role in endometrial differentiation and uterine receptivity.15–17 For unknown reasons, the HOXA10 gene is abnormally expressed outside its spatial domain in different types of endometriotic lesions, such as ovarian endometriosis, peritoneal endometriosis, pulmonary endometriosis, and DE.13,14 Interestingly, van Langendonckt et al14 demonstrated different expression patterns of HOXA10 and HOXA13 in DE nodules compared to peritoneal endometriosis, suggesting that each lesion has a unique etiology. It is possible that HOXA10 is necessary for de novo endometrial development at sites of endometriosis,13 but its role in the pathogenesis of DE needs to be further evaluated. It is also unknown whether HOXA10 is expressed in RE, which would represent another instance of spatially abnormal expression.
There are few known regulators of HOXA10.18 Estrogens and progesterone are known contributors to the development of endometriosis.15,16 Binding of these hormones to their nuclear receptors activates HOXA10 transcription. Different isoforms of each receptor give rise to different responses for each of the hormones.19 The estrogen receptor α (ER-α) and the progesterone receptor B (PR-B) have been shown to be potent activators of gene transcription.20,21 There are at least 2 estrogen responsive elements in the HOXA10 gene that are equally activated by the estrogen receptor isoforms ER-α and ER-β.22 Also, genital tract morphogenesis during embryogenesis is driven by ER-α-activating HOXA10 expression.23 Regarding progesterone regulation of HOXA10, the porcine homolog to HOXA10 contains a promoter region that can be bound by the PR.24 Additionally, maximal expression of HOXA10 occurs during the window of implantation that coincides with high progesterone levels.16 Therefore, HOXA10, estrogens, and progesterones are fundamentally linked, and many of the hormonal effects in the female genital tract are dependent on activation of HOXA10.
Despite the importance of estrogen and PR isoforms in the pathogenesis of endometriosis, there are few studies that focus on them in DE. Most of what is known about the roles of each receptor isoform in endometriosis is derived from studies of peritoneal and ovarian lesions. Based on these studies, it is suggested that PR-B is weakly expressed in endometriosis due to hypermethylation of its promoter region.10 However, the role of PR-B in DE has not been thoroughly evaluated and remains largely unknown. It is also suggested that the reduced expression of ER-α and lower ER-α–ER-β ratio demonstrated in ovarian endometriosis would be responsible for PR suppression and the subsequent progesterone resistance observed in the disease. Contrary to these studies, Samartzis et al25 found variable levels of ER-α and PR expression in peritoneal, ovarian, and adenomyotic nodules of endometriosis. This study demonstrated high expression of ER-α in DE, which further suggests pathogenesis for each type of endometriosis is different. In the present study, we evaluated the protein expression of HOXA10 gene products, ER-α, PR, and PR-B in RE by immunohistochemistry (IHC) using tissue microarray (TMA).
Materials and Methods
We obtained surgical specimens from 18 consecutive patients who attended a private fertility center in Sao Paulo, Brazil, and had been treated for RE by laparoscopic rectosigmoidectomy. Surgeries took place between June 2003 and July 2007. All patients were infertile and had clear indications for surgery based on pain and/or infertility. Patients gave informed consent to participate in the study, which was approved by the local ethics committee. None of the patients received any hormonal treatment for at least 90 days preceding surgery. Patients were classified as having surgery during the proliferative or secretory phase of the menstrual cycle based on medical records. This was further confirmed by endometrial histological analyses according to the criteria of Noyes et al.26
Tissue Microarray Construction
A pathologist identified areas of RE on hematoxylin/eosin-stained sections, and the corresponding regions in the paraffin blocks were dissected for TMA analysis as described previously.27,28 Briefly, we used a precision instrument (Beecher Instrument, Sun Prairie, Wisconsin) to create circular perforations in donor blocks and extracted cylindrical cores that were 0.6 mm in diameter. We then inserted the cores into recipient blocks and sliced TMA sections that were 2.5-μm thick and placed onto slides for IHC analysis.
Immunohistochemistry
We used monoclonal antibodies against the following antigens for IHC: HOXA10 (1:200 dilution, clone sc-17159; Santa Cruz Biotechnology, Santa Cruz, California), ER-α (1:200 dilution, Product No. M3634; Dako, Glostrup, Denmark), PR (1:50 dilution, Product No. M3569; Dako), and PR-B (1:100 dilution, clone hPRa7; Neomarkers, Fremont, California). Normal endometrial tissue was used as a positive control for expression of HOXA10, while normal breast tissue and breast cancer tissue were used as positive controls for expression of ER and PR. Staining without primary antibodies was performed as the negative control for all groups.
Immunohistochemistry for HOXA10, ER-α, PR, and PR-B was performed on TMA blocks. Antigen retrieval was performed by incubating slides in 0.21% citric acid (pH 6.0) in a pressure cooker for 8 minutes. After 20 minutes of cooling at room temperature, the slides were washed in distilled water before blocking endogenous peroxidase and immersed them in phosphate-buffered saline (PBS). All slides were incubated with primary antibodies diluted in PBS. Staining was visualized by incubating in a chromogen substrate solution, diaminobenzine (Sigma-Aldrich, Saint Louis, Missouri), protected from light, and counterstained with Harris hematoxylin (Merck, Whitehouse Station, New Jersey).
Scoring
Two independent pathologists were blinded and selected glandular and stromal samples for analysis. The IHC staining was quantified according to the modified Allred score.29 The intensity of protein expression was scored as follows: 0 (negative), 1 (weak), 2 (moderate), or 3 (strong). Then, the frequency of expression was measured and scored as follows: 0 (<1%), 1 (1%-10%), 2 (11%-50%), 3 (51%-90%), or 4 (>90%). A final score ranging from 0 to 12 was calculated for each sample. The final score was the product of intensity and frequency scores. Final expression scores were categorized as follows: negative (0-1), weak (2-4), moderate (5-8), and strong (9-12).
Statistical Analyses
Means and standard deviations were calculated for each protein during each phase of the menstrual cycle. Poisson distribution tests were used to identify patterns of expression for each protein, and Bonferroni tests were used to compare means between the phases. The Spearman rank correlation test was used for correlation calculations. A P value of <.05 was considered statistically significant.
Results
The clinical characteristics of patients enrolled in the study are listed in Table 1. All patients had stage IV DE according to the revised classification of endometriosis by the American Society for Reproductive Medicine.30 Of the 18 patients, 14 achieved 17 gestations after surgery, including 9 spontaneous pregnancies.
Table 1.
Characteristics of Patients Who Underwent Laparoscopic Resection of DE, Including Rectosigmoidectomy.
| Proliferative Phase | Secretory Phase | P Value | |
|---|---|---|---|
| Na | 9 | 9 | |
| Age, years | 32 ± 2.5 | 33.7 ± 3.9 | .3 |
| Previous surgeries for endometriosis | 1.2 ± 0.8 | 0.9 ± 1.1 | .1 |
| Previous IVF attempts | 1.2 ± 1.7 | 0.6 ± 1.1 | .3 |
| Nodules per patient | 1.4 ± 0.5 | 1.3 ± 0.5 | .7 |
| Nodule size, cm | 2.2 ± 0.9 | 2.3 ± 0.8 | .4 |
| Symptoms | |||
| Dysmenorrhea | 8 | 9 | |
| Deep dyspareunia | 4 | 6 | |
| Chronic pelvic pain | 4 | 6 | |
| Constipation | 2 | 4 | |
| Cyclic intestinal bleeding | 1 | 1 | |
Abbreviations: DE, deep endometriosis; IVF, in vitro fertilization.
aNumber of patients.
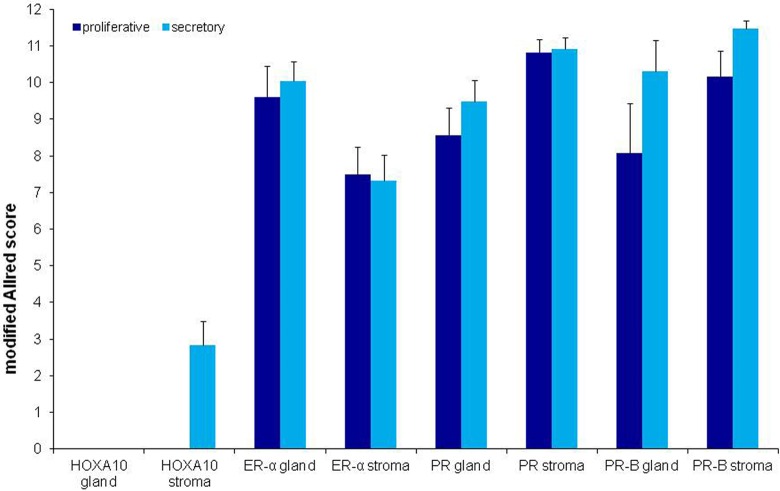
Of the 18 patients in the study, 5 had samples positive for expression of HOXA10, 16 for expression of ER-α, 17 for expression of PR, and 17 for expression of PR-B. All patients had representative samples for at least 1 of the analyses. We found weak expression of HOXA10 in the stroma of RE during the secretory phase of the menstrual cycle. There was moderate to strong expression of ER-α, PR, and PR-B in both the glands and the stroma of RE during both phases of the menstrual cycle (Figures 1 and 2). Positive IHC staining was restricted to the nuclei of glandular and stromal cells for all proteins.
Figure 1.
Expression of homeobox A10 (HOXA10), estrogen receptor (ER)-α, progesterone receptor (PR), and PR-B proteins in the glands and stroma of rectosigmoid endometriosis during the proliferative and secretory phases of the menstrual cycle. All values are represented as the mean ± standard deviation (SD).
Figure 2.
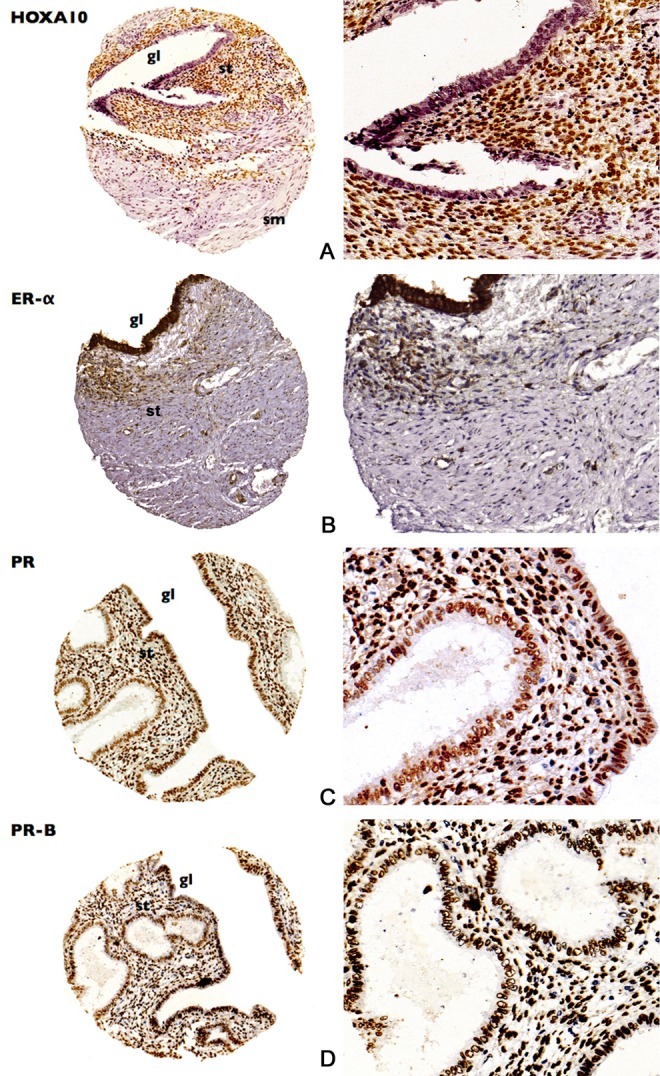
Tissue microarray (TMA) samples demonstrating the expressions of (A) homeobox A10 (HOXA10), (B) estrogen receptor(ER)-α, (C) progesterone receptor (PR), and (D) PR-B proteins in rectosigmoid endometriosis. Samples in the right column magnified 200 times. gl indicates gland; st, stroma; sm, smooth muscle.
Expression of both PR (P = .023) and PR-B (P = .024) was significantly increased in RE during the secretory phase of the menstrual cycle. HOXA10 was not expressed during the proliferative phase, and there was no difference in the expression of ER-α between the proliferative and the secretory phases.
Direct and inverse relationships between expression patterns of the proteins are shown in Table 2. There were direct correlations between the expression of ER-α in the glands and ER-α in the stroma (r = .447; P = .003), PR and PR-B in the glands (r = .6; P = .001), and PR and PR-B in the stroma (r = .32; P = .036). Inverse relationships were found for the expression of ER-α in the glands and PR in the stroma (r = −.402; P = .028) as well as ER-α in the stroma and PR in the glands (r = −.373; P = .032).
Table 2.
Correlation Between Expression of HOXA10, ER-α, PR, and PR-B Proteins in Rectosigmoid Endometriosis.
| Correlation | HOXA10 Stroma | ER-α Gland | ER-α Stroma | PR Gland | PR Stroma | PR-B Gland |
|---|---|---|---|---|---|---|
| ER-α gland | ||||||
| r a | – | |||||
| P | – | |||||
| Nb | 2 | |||||
| ER-α stroma | ||||||
| r | – | .447 | ||||
| P | – | .003 | ||||
| N | 2 | 41 | ||||
| PR gland | ||||||
| r | 1 | −.092 | −.373 | |||
| P | – | .634 | .032 | |||
| N | 4 | 29 | 33 | |||
| PR stroma | ||||||
| r | 0.395 | −.402 | −.022 | −.281 | ||
| P | 0.510 | .028 | .902 | .051 | ||
| N | 5 | 30 | 35 | 49 | ||
| PR-B gland | ||||||
| r | – | −.184 | −.333 | .600 | −.052 | |
| P | – | −.496 | .192 | .001 | .777 | |
| N | 2 | 16 | 17 | 27 | 32 | |
| PR-B stroma | ||||||
| r | .544 | .116 | −.085 | −.162 | .320 | −.077 |
| P | .456 | .606 | .692 | .351 | .036 | .663 |
| N | 4 | 22 | 24 | 35 | 43 | 34 |
Note. Significant values (p < .05) are in bold. Abbreviations: ER-α, estrogen receptor α; HOX, homeobox; PR, progesterone receptor.
aEstimate of correlation.
bNumber of samples.
Discussion
This is the first study to profile the expression of HOXA10 in conjunction with ER and PR exclusively in RE, a representative model of DE. Peritoneal, ovarian, and adenomyotic nodules are suggested to be different clinical manifestations of endometriosis.2 However, studies typically investigate a single type of lesion8–10 and generalize conclusions to all types of endometriosis, despite the fact that histological features and expression of genes and proteins are strikingly different in each.14,25,31 There are few studies that have investigated steroid receptors in DE lesions exclusively,6,7 and this is the first study to report on expression of HOXA10 in RE.
Although weakly expressed, the demonstration that HOXA10 protein is expressed in RE is significant because it confirms that HOXA10 is ubiquitously expressed in different types of endometriotic lesions.13,14 Expression of HOXA10 in RE and other endometriotic lesions is noteworthy because it occurs outside its normal spatial domain, namely the endometrium and myometrium.15 The HOXA10 gene is not known to be involved in the development or differentiation of the rectosigmoid or other portions of the gastrointestinal tract.32 Therefore, its expression in the rectosigmoid would not be expected.
In mice, expression of HOXA10 was observed in the distal intestine, a segment that corresponds to the rectosigmoid in humans.15 It is also interesting to note that ectopic endometrium has been found in the rectal muscular wall during human development as early as 18 weeks of gestation.33 Taken together, it is plausible that HOXA10 plays a key role in the pathogenesis of RE and possibly other DE lesions. HOXA10 could function to differentiate embryonic tissues to endometrial tissue during development, as Hox/HOX genes primarily function to regulate cell differentiation and impart tissue identity.
This hypothesis is reinforced by the fact that endometriosis in the large intestine is spatially well defined. Endometriosis of the bowel occurs in rectosigmoid, appendix, terminal ileum, and cecum. This strict localization might resemble the typical spatial expression of Hox/HOX genes observed at the female genital tract. This is exemplified by the action of the teratogen diethylbestrol (DES) in the embryo. The DES causes a posterior shift in the expression of Hox/HOX genes in the developing Müllerian system. After DES exposure, HOXA9 is expressed in the uterus instead of the Fallopian tubes, expression of HOXA10 is decreased in the uterine fundus, and expression of HOXA11 is decreased in the entire uterus.34 These changes in expression cause the characteristic “T”-shaped uterus and other uterine malformations commonly associated with DES. Ectopic expression of HOXA10 and HOXA11 could also explain the related cases of vaginal adenosis, a situation where endometrial glands are found ectopically in the vagina. Therefore, it would be interesting to investigate whether Hoxa10/HOXA10 is expressed at other ectopic sites in the pelvis and intestine during embryogenesis. If so, it would be possible to correlate intestinal and other extrauterine sites of HOXA10 expression during development with sites of endometriosis in adulthood.
In our study, expression of HOXA10 was restricted to the stromal tissue of RE, which agrees with previous studies of HOXA10 in other DE lesions.14 We suggest 2 hypotheses to explain this finding. First, HOX genes are expressed in different populations of stromal stem cells, including those from bone marrow, dental pulp, and the colon itself.35 In these locations, HOX genes regulate the differentiation of stromal cells. It is possible that HOXA10 plays the same role in RE, regulating the differentiation of stromal cells. A second explanation concerns embryogenesis. There is a clear shift of HOXA10 expression over time in the endometrium during embryogenesis. It is initially expressed in the glands but later is exclusively expressed in the stroma. Therefore, it is possible that stromal expression of HOXA10 in RE during adulthood would mirror the pattern of expression that occurs during early development.
Another important finding of our study was the strong expression of ER-α in the glands and stroma of RE during both phases of the menstrual cycle. Samartzis et al25 also found strong expression of ER-α in the glands and stroma of DE in 22 of the 23 samples. This is the only study we were able to find which evaluated expression of ER-α in DE lesions specifically. Those findings25 and ours contradict previous findings that expression of ER-α is weak or absent in endometriosis, based on studies of ovarian and peritoneal lesions.8,11,36 A possible explanation is that hormones and their receptors may have different actions in different cellular environments,19,37 and this reinforces the possibility that pathogenesis in each of the 3 clinical forms of endometriosis is different.
We were unable to determine whether there is a direct relationship between ER-α and HOXA10 in RE because of the low number of samples that were positive for HOXA10. Estradiol regulates Müllerian morphogenesis in the embryo through ER-α23 and activates expression of HOXA10 in the adult endometrium.16 We hoped to evaluate this relationship in RE, but further studies measuring a greater number of samples are necessary. Strong expression of ER-α in RE, however, confirms estrogen is the most important mitogen also in DE.
We found that both PR and PR-B were strongly expressed in the glands and stroma of RE. The greatest expression occurred during the secretory phase of the menstrual cycle. As with ER-α, we were unable to establish a relationship between expression of PR, PR-B, and HOXA10 in RE due to the low number of HOXA10-positive samples. Expression of PR and PR-B was greatest during the secretory phase in RE, which reflects the known cyclical action of progesterone in DE.
To our knowledge, this is the first study to evaluate the expression of the PR-B isoform in DE lesions specifically. Its expression was strong and clearly evident in RE as opposed to peritoneal and ovarian lesions, where its expression is weak or absent.9,10 The PR-B isoform is the most potent inducer of gene transcription,38 and its strong expression in RE contradicts previous findings that PR-B could be repressed in endometriosis.9,10
It has been suggested that PR-B repression in endometriosis could explain the clinically observed resistance of the disease to progesterone.10 However, our finding of a strong expression of PR-B in RE contradicts this hypothesis, most likely because peritoneal and ovarian lesions were evaluated in other studies.9 , 10 In fact, dienogest, which is a progestin, is gaining worldwide acceptance for the control of painful symptoms related to endometriosis. Probably, the positive effect of the drug observed in some patients could be related to the reduction in inflammatory substances in the lesion, caused by an agonist (and more specific) response mediated by both PR-A and PR-B. The smooth muscle metaplasia of DE lesions, however, is not modified by progesterone. Therefore, DE lesions could not be completely “resistant” to progesterone, although the drug cannot eradicate them.
Discrepancies between studies could also be due to different methodologies. Attia et al9 used Western blot analyses, while Wu et al10 used laser capture microdissection and polymerase chain reaction to measure receptor RNA levels. We used IHC following TMA to evaluate receptor protein expression. Certainly, future studies on expression of PR-B RNA in DE should be performed to corroborate our findings of PR-B protein expression. Tissue microarray is a relatively new technique employed in endometriosis research and in need of improvements. Although it is a well-established tool in cancer research, there are few instances where TMA has been used to study steroid receptors in endometriosis,26,31,39 and no studies have used it to measure HOXA10. Despite this, the technique has been validated to study ER and PR in peritoneal and ovarian endometriosis39 and we were able to obtain representative samples from all of the patients for at least 1 of the analyses. Therefore, we understand our results are valid.
In conclusion, we provide evidence that HOXA10, ER-α, PR, and PR-B are expressed in RE, a typical model of DE. Demonstrating expression of HOXA10 in RE and other types of endometriotic lesions suggests that HOXA10 might be necessary for de novo endometrial development in endometriosis, which may occur during embryonic development when HOXA10 is critical for cellular differentiation. Strong expression of ER-α and PR-B contradicts previous findings from studies that investigated peritoneal and ovarian endometriosis. This reinforces the hypothesis that different types of endometriotic lesions represent different diseases and should be evaluated separately in research.
Acknowledgments
We are grateful to Filomena Carvalho, MD, PhD, José Luiz Guerra, MD, PhD, and Bernardo Almeida MD, PhD, for performing the pathologic analysis of the specimens. We are also grateful do Marcia Riboldi, BSc, PhD, for scientific support.
Footnotes
Authors’ Note: This research was performed at Huntington Reproductive Medicine Center and São Paulo University School of Medicine, Sao Paulo, Brazil.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Clement PB. The pathology of endometriosis: a survey of the many faces of a common disease emphasizing diagnostic pitfalls and unusual and newly appreciated aspects. Adv Anat Pathol. 2007;14 (4):241–260. [DOI] [PubMed] [Google Scholar]
- 2. Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68 (4):585–596. [DOI] [PubMed] [Google Scholar]
- 3. Koninckx PR, Kennedy SH, Barlow DH. Endometriotic disease: the role of peritoneal fluid. Hum Reprod Update. 1998;4 (5):741–751. [DOI] [PubMed] [Google Scholar]
- 4. Bianchi PH, Pereira RM, Zanatta A, Alegretti JR, Motta EL, Serafini PC. Extensive excision of deep infiltrative endometriosis before in vitro fertilization significantly improves pregnancy rates. J Minim Invasive Gynecol. 2009;16 (2):174–180. [DOI] [PubMed] [Google Scholar]
- 5. Koninckx PR, Ussia A, Adamyan L, Wattiez A, Donnez J. Deep endometriosis: definition, diagnosis, and treatment. Fertil Steril. 2012;98 (3):564–571. [DOI] [PubMed] [Google Scholar]
- 6. Noel JC, Chapron C, Bucella D, et al. Estrogen and progesterone receptors in smooth muscle component of deep infiltrating endometriosis. Fertil Steril. 2009;93 (6):1774–1777. [DOI] [PubMed] [Google Scholar]
- 7. Kitano T, Matsumoto T, Takeuchi H, et al. Expression of estrogen and progesterone receptors in smooth muscle metaplasia of rectovaginal endometriosis. Int J Gynecol Pathol. 2007;26 (2):124–129. [DOI] [PubMed] [Google Scholar]
- 8. Brandenberger AW, Lebovic DI, Tee MK, et al. Oestrogen receptor (ER)-alpha and ER-beta isoforms in normal endometrial and endometriosis-derived stromal cells. Mol Hum Reprod. 1999;5 (7):651–655. [DOI] [PubMed] [Google Scholar]
- 9. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85(8):2897–2902. [DOI] [PubMed] [Google Scholar]
- 10. Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1(2):106–111. [DOI] [PubMed] [Google Scholar]
- 11. Trukhacheva E, Lin Z, Reierstad S, Cheng YH, Milad M, Bulun SE. Estrogen receptor (ER) beta regulates ERalpha expression in stromal cells derived from ovarian endometriosis. J Clin Endocrinol Metab. 2009;94 (2):615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68 (2):283–302. [DOI] [PubMed] [Google Scholar]
- 13. Browne H, Taylor H. HOXA10 expression in ectopic endometrial tissue. Fertil Steril. 2006;85 (5):1386–1390. [DOI] [PubMed] [Google Scholar]
- 14. van Langendonckt A, Luyckx M, Gonzalez MD, Defrere S, Donnez J, Squifflet J. Differential expression of genes from the homeobox A cluster in deep endometriotic nodules and peritoneal lesions. Fertil Steril. 2010;94 (6):1995–2000. [DOI] [PubMed] [Google Scholar]
- 15. Taylor HS, Vanden Heuvel GB, Igarashi P. A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod. 1997;57 (6):1338–1345. [DOI] [PubMed] [Google Scholar]
- 16. Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101 (7):1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zanatta A, Rocha AM, Carvalho FM, et al. The role of the Hoxa10/HOXA10 gene in the etiology of endometriosis and its related infertility: a review. J Assist Reprod Genet. 2010;27 (12):701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daftary GS, Taylor HS. Endocrine regulation of HOX genes. Endocr Rev. 2006;27 (4):331–355. [DOI] [PubMed] [Google Scholar]
- 19. Watanabe T, Inoue S, Ogawa S, et al. Agonistic effect of tamoxifen is dependent on cell type, ERE-promoter context, and estrogen receptor subtype: functional difference between estrogen receptors alpha and beta. Biochem Biophys Res Commun. 1997;236 (1):140–145. [DOI] [PubMed] [Google Scholar]
- 20. McDonnell DP, Goldman ME. RU486 exerts antiestrogenic activities through a novel progesterone receptor A form-mediated mechanism. J Biol Chem. 1994;269 (16):11945–11949. [PubMed] [Google Scholar]
- 21. Kulakosky PC, McCarty MA, Jernigan SC, Risinger KE, Klinge CM. Response element sequence modulates estrogen receptor alpha and beta affinity and activity. J Mol Endocrinol. 2002;29 (1):137–152. [DOI] [PubMed] [Google Scholar]
- 22. Akbas GE, Song J, Taylor HS. A HOXA10 estrogen response element (ERE) is differentially regulated by 17 beta-estradiol and diethylstilbestrol (DES). J Mol Biol. 2004;340 (5):1013–1023. [DOI] [PubMed] [Google Scholar]
- 23. Couse JF, Dixon D, Yates M, et al. Estrogen receptor-alpha knockout mice exhibit resistance to the developmental effects of neonatal diethylstilbestrol exposure on the female reproductive tract. Dev Biol. 2001;238 (2):224–238. [DOI] [PubMed] [Google Scholar]
- 24. Wu D, Song D, Li X, Yu M, Li C, Zhao S. Molecular characterization and identification of the E2/P4 response element in the porcine HOXA10 gene. Mol Cell Biochem. 2013;374 (1-2):213–222. [DOI] [PubMed] [Google Scholar]
- 25. Samartzis N, Samartzis EP, Noske A, et al. Expression of the G protein-coupled estrogen receptor (GPER) in endometriosis: a tissue microarray study. Reprod Biol Endocrinol. 2012;10 (1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. [DOI] [PubMed] [Google Scholar]
- 27. Kallioniemi OP, Wagner U, Kononen J, Sauter G. Tissue microarray technology for high-throughput molecular profiling of cancer. Hum Mol Genet. 2001;10 (7):657–662. [DOI] [PubMed] [Google Scholar]
- 28. Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4 (7):844–847. [DOI] [PubMed] [Google Scholar]
- 29. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11 (2):155–168. [PubMed] [Google Scholar]
- 30. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–821. [DOI] [PubMed] [Google Scholar]
- 31. Calcagno A, Grassi T, Mariuzzi L, et al. Expression patterns of Aurora A and B kinases, Ki-67 and the estrogen and progesterone receptors determined using an endometriosis tissue microarray model. Hum Reprod. 2011;26 (10):2731–2741. [DOI] [PubMed] [Google Scholar]
- 32. Yu YY, Pan YS, Zhu ZG. Homeobox genes and their functions on development and neoplasm in gastrointestinal tract. Eur J Surg Oncol. 2007;33 (2):129–132. [DOI] [PubMed] [Google Scholar]
- 33. Signorile PG, Baldi F, Bussani R, D'Armiento M, De FM, Baldi A. Ectopic endometrium in human foetuses is a common event and sustains the theory of mullerianosis in the pathogenesis of endometriosis, a disease that predisposes to cancer. J Exp Clin Cancer Res. 2009;28:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Block K, Kardana A, Igarashi P, Taylor HS. In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing mullerian system. FASEB J. 2000;14(9):1101–1108. [DOI] [PubMed] [Google Scholar]
- 35. Picchi J, Trombi L, Spugnesi L, et al. HOX and TALE signatures specify human stromal stem cell populations from different sources. J Cell Physiol. 2013;228 (4):879–889. [DOI] [PubMed] [Google Scholar]
- 36. Xue Q, Lin Z, Cheng YH, et al. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod. 2007;77 (4):681–687. [DOI] [PubMed] [Google Scholar]
- 37. Burns KA, Rodriguez KF, Hewitt SC, Janardhan KS, Young SL, Korach KS. Role of estrogen receptor signaling required for endometriosis-like lesion establishment in a mouse model. Endocrinology. 2012;153 (8):3960–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McDonnell DP, Shahbaz MM, Vegeto E, Goldman ME. The human progesterone receptor A-form functions as a transcriptional modulator of mineralocorticoid receptor transcriptional activity. J Steroid Biochem Mol Biol. 1994;48 (5-6):425–432. [DOI] [PubMed] [Google Scholar]
- 39. Kamat AA, Younes PS, Sayeeduddin M, Wheeler TM, Simpson JL, Agoulnik AI. Protein expression profiling of endometriosis: validation of 2-mm tissue microarrays. Fertil Steril. 2004;82 (6):1681–1683. [DOI] [PubMed] [Google Scholar]