Abstract
The treatment of moving tumors with a scanned ion beam is challenging due to interplay effects and changing beam range. We propose multigating, as a method for 4D-treatment optimization and delivery. In 3D beam tracking, tracking vectors are added during delivery to beam spot positions based on the detected motion phase. This has the disadvantage of dose errors in case of complex motion patterns and an uncertain out-of-target dose distribution. In multigating, the motion phase for each beam spot is predefined, which allows to add the tracking vector prior to beam weight optimization on all motion phases. The synchronization of delivery and target motion is assured by fast gating. The feasibility of the delivery was shown in a film experiment and required only minor software modification to the treatment planning system. In a treatment planning study in 4 lung cancer patients, target coverage could be restored to the level of a static reference plan by multigating (V95 > 99%) but not by standard beam tracking (V95 < 95%). The conformity of the multigating plans was only slightly lower than those of the static plan, with a conformity number of 72.0% (median, range 64.6–76.6%) compared to 75.8% (70.8–81.5%) in spite of target motion of up to 22 mm. In conclusion, we showed the technical feasibility of multigating, a 4D-optimization and delivery method using scanned beams that allows for conformal and homogeneous dose delivery to moving targets also in case of complex motion.
Keywords: Ion beam therapy, Particle therapy, Moving targets, Motion mitigation, 4D-optimization, Treatment planning
Introduction
The physical and biological characteristics of scanned ion beams offer potential advantages over photon therapy in terms of target conformity and sparing of normal tissue. Transferring these possible advantages to the treatment of moving tumors remains challenging due to interplay of beam and target motion and the high dosimetric impact of ion range changes caused by motion.
An approach common to all modalities of radiotherapy is the reduction of target motion. Besides careful patient positioning and immobilization, which is also necessary for static tumors for example of the head and neck region, also dedicated techniques for moving tumors were developed. Abdominal compression can reduce the breathing motion substantially though not completely eliminate it. Deep- inspiration breath hold with cooperative patients (1, 2) or active breath control (3) can lead to extended treatment windows in the absence of motion.
Most methods for the treatment of moving tumors require highly precise tracking of the tumor motion so that an online mitigation of the detected motion is possible. This is a challenging field of research. Direct observation of the tumor motion requires implanted markers and x-ray fluoroscopy, with additional risks to the patient. Numerous methods for the indirect observation of the tumor motion exist, such as via optical tracking of the chest wall, ultrasound, or spirometry (4). The motion monitoring and especially also its mitigation can be supported by training the patient to breath regularly within given parameters, also supported through audiovisual feedback (5). For the scope of this study, motion monitoring is considered as given and ideal, with the impact of uncertainty in this aspect reviewed in the discussion.
Several concepts for the treatment of moving tumors by scanned ion beam therapy have been published, such as rescanning, gating, and beam tracking (6). All of these concepts offer distinct advantages and disadvantages. Rescanning is technically comparably simple to deliver but requires an increased target volume including motion and range changes, leading to an increased out-of-target dose. Gating spares the normal tissue but elongates the treatment time substantially. Beam tracking results in the highest dose conformity, but requires dedicated equipment to adjust the beam range (7). Beam tracking is optimized for a reference phase in 3D only with a lookup table of tracking vectors for all possible combinations of beam positions and motion phases (8). The actual 4D delivery of the beam spots is not controlled, i.e. it is unknown prior to irradiation which beam spots will be delivered to which motion phase. This leads to the following issues:
Complex rotational or deformable motion of the target leads to dose aberrations in the target or in the beam entry channel in front of the target (9).
Differential motion of the target and the entry channel leads to hot and cold spots in the entry channel (inverse interplay) that makes the dose to normal tissue and especially organs at risk in that region essentially uncertain prior to delivery (10).
We propose the concept of multigating, which is inherently a 4D optimized extension of a standard beam tracking strategy. The basic principle is to assign beam spots to specific motion phases already during treatment planning. The treatment delivery must then be synchronized to the target motion to ensure this prescribed distribution of beam spots. This is achieved by defining an appropriate beam spot sequence, and by applying fast gating during delivery if the beam spot does not belong to the detected motion phase. Conceptually this is similar to a gating treatment, but the dose is not delivered completely to a single gating window, but rather in controlled fractions to all phases of the breathing cycle, hence the name multigating.
Similar 4D optimization methods have been proposed for nearly a decade for photon treatment, where also the treatment plan was optimized in all phases of a 4DCT (11, 12). In contrast to photon treatment, ion beam therapy could benefit even more from this approach, as not only the target motion is extracted from the 4DCT but also the highly relevant beam range information.
Materials and Methods
Beam Tracking
A standard beam tracking plan in TRiP98, the GSI treatment planning system (13, 14), is based on a plan optimized for the 3D reference phase (typically end-exhale) of a 4DCT. Vector fields describing the transition from all motion phases to the reference phase are computed by deformable image registration (DIR). From this data, a lookup table (LUT) containing tracking vectors for each beam spot is calculated based on the DIR. This LUT contains vectors from the reference phase to each phase of the 4DCT. During beam delivery, the appropriate tracking vector for a given motion phase is selected based on online motion monitoring. As this motion is unknown prior to delivery, also the actual set of tracking vectors used cannot be determined a priori, and will also not be the same for example in different fractions of a treatment. The tracking vectors typically consist of a lateral and a distal component in beam’s eye view. While the lateral component is easily applied using the scanning magnets, the distal component requires an energy change (8). At GSI, this can be achieved through a moving wedge system mounted close to the target in the beam path (7). This mechanical system has several drawbacks such as a delay and additional scattering.
For certain types of motion, tracking does not adequately compensate dose errors, e.g., if the relative position of the entry channels of the beam spots deviate from the static reference plan for rotations, or if the relative beam spot position is altered due to deformations (9). Even if the motion permits adequate target coverage, the dose in the entry channel is not known exactly as it depends on the motion sequence. Thus, planned dose constraints to normal tissue might be violated. A potential solution to this problem was proposed by Luchtenborg et al. (9) by online adaptation of the intensity of beam spots with lower energies. This approach lead to good results but also has some disadvantages, as complex computations have to be carried out during delivery. The compensation also works for the target only and can potentially aggravate the uncertainty in the dose to the entry channel.
Multigating
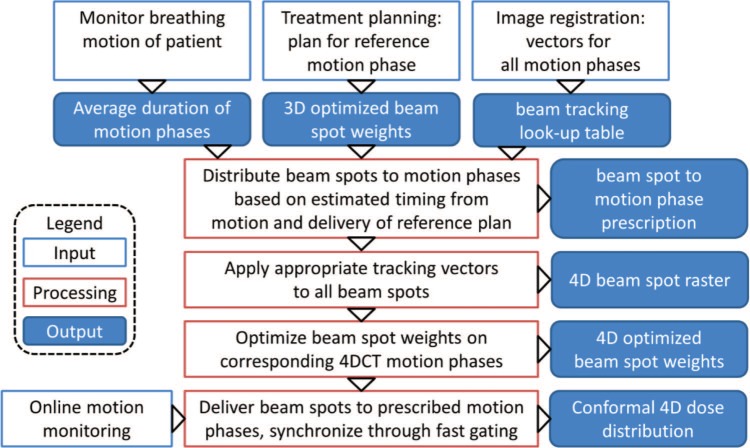
Multigating should alleviate the stated limitations of beam tracking by re-optimizing the particle numbers of a tracking plan on the whole 4DCT. This 4D-optimization permits to consider complex motions and also the accompanying range changes directly from the individual 4DCT phases. To this end, specific motion phases for each pencil beam are predefined. The workflow of multigating treatment planning and delivery is shown in Figure 1. The required inputs are a standard beam tracking plan, consisting of a 4DCT with corresponding tracking LUT and a plan optimized for the reference phase to estimate delivery timing based on spot weights and accelerator parameters. In addition, a breathing motion curve of the patient is needed to measure the approximate breathing period.
Figure 1:
Flow chart of the multigating treatment planning and delivery process, illustrating how a standard tracking plan is distributed to defined motion phases and re-optimized on the corresponding 4DCT phases. Finally, the delivery is synchronized to the online detected patient motion via fast gating.
As with all 4D-optimization methods, the beam delivery has to be synchronized to the target motion. In methods developed for photon treatment, this was achieved by changing the gantry rotation speed (15). Here, fast beam gating is applied after each motion phase as needed, i.e. a beam spot is only irradiated during its intended motion phase. A schematic depiction of the synchronization strategy is given in Figure 2.
Figure 2:
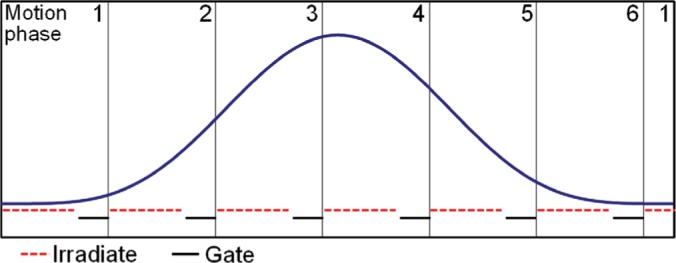
Schematic depiction of the intended synchronized irradiation. Beam spots are assigned to motion phases so that they are likely to be irradiated within this motion phase. To account for uncertainties in the estimated timing of motion and irradiation, a short gating window is foreseen at the end of each motion phase.
The motion phases have to be predefined in a sequence that permits a high duty cycle. Therefore, an estimate of the timing of both motion and beam delivery is extracted from the given motion curve and the beam weights of the static treatment plan. From this, the sequence of beam spots is defined so that the delivery of each set of spots foreseen for a certain motion phase can expected to be delivered during this motion phase. This is a critical issue, as a failure to deliver the spots within the prescribed motion phases either leads to dose errors or to a considerable delay while the beam is gated until the required breathing phase occurs again. The timing can be better predicted if the accelerator provides online intensity control and, in the case of a synchrotron, a flexible spill time structure. Both is currently not available at GSI, but e.g. at HIMAC (16) or recently also at HIT (17). These timing issues are easily controlled in cyclotron-based facilities.
To facilitate the timing, a safety pause is planned at the end of each motion phase, such that only 70% of the estimated duration of a phase is used for delivery, see Figure 2. Thus, if either motion or delivery shows a delay, the risk of not completing delivery to a motion phase is reduced. The fraction of this pause can be varied to increase the probability of a smooth delivery at the cost of a longer best case scenario.
Due to the predefined beam spot distribution to the motion phases, the tracking vectors to be used are known. They are thus applied to the beam spots, and particle numbers are re-optimized on each corresponding 4DCT phase using the deformation field to collect all contributions to the biologically effective dose in the reference phase. This allows considering also changed beam entry channel paths caused by complex motion, as well as changed distances between beam spots due to deformations. Also, changes in the waterequivalent length (WEL) no longer require online adaptation but can be assigned to the closest standard iso-energy slice. The remaining WEL error as well as rotations and deformations can be compensated by optimizing particle numbers accordingly on each 4DCT phase.
Feasibility of Treatment Delivery by Multigating
The resulting beam spot raster has the same format as a standard 3D raster, meaning that each beam spot position is irradiated once. Its sequence is fixed in an optimized scanpath, though the positions of the beam spots have been altered by the tracking vectors. The motion phase in which the pencil beams should be irradiated in is defined in an accompanying lookup table given to the modified treatment control system.
Using online motion monitoring (see bottom row of Figure 1), the current motion phase is detected. If the current beam spot is to be irradiated in the detected phase, the beam is turned on and otherwise gated. If the estimation of motion and delivery timing is correct, this results in a brief beam gate towards the end of each motion phase when all beam spots intended for this phase have been irradiated, see Figure 2. If the motion phase ends before all beam spots were delivered in spite of the safety pause, the beam will be gated for an entire breathing cycle until the motion phase is detected again.
To show the feasibility of the delivery, a simple film experiment was conducted with a single 12C beam energy of 200 MeV/u. A reference static irradiation of a 30 mm circle was compared to irradiations were the film was mounted on a sinusoidal sliding table moving 20 mm left-right perpendicular to the beam for both multigating and without motion compensation (interplay) for comparison. A laser distance sensor was used for motion monitoring. The synchronization of beam delivery and target motion was performed through RF knockout extraction (18), to enable multiple, fast beam gating. Due to the lack of intensity control, it was expected that the delivery timing estimate was imprecise.
The gamma coefficient (19) (3 mm/3%) of the optical density of the film was computed in comparison to the static reference irradiation.
Patient Treatment Planning Study
A treatment planning study was conducted in 4 lung cancer patients; with 4D-CTs and contours provided by M.D. Anderson Cancer Center, Houston, Tx (MDACC). The target motion range was 5.6 to 22.2 mm. The motion phases of the 4DCTs were connected by deformable image registration using Plastimatch (20).
The plans, similar to a clinically used protocol from NIRS, Chiba, Japan (21), consisted of 4 fields in a transverse plane oriented +20°, -20°, -70°, and -110° from the AP-axis, delivered in 4 fractions of uniform 8.2 Gy (RBE) each. The relative biological effectiveness (RBE) for lung tumor control with carbon ions was computed using the Local Effect Model IV (22). 4D dose results in the reference phase are reported as the sum of all fractions (23). Besides multigating plans, also a standard tracking plan, a plan without motion compensation (interplay) and a static reference plan on the end-exhale phase were computed. A Lujan motion with a period of 5 sec was used for tracking and interplay together with the simulated delivery timing of the GSI SIS18 to compute 4D doses (14). For multigating, the prescribed 4D delivery sequence was assumed in the dose calculation. In no case, residual motion within a motion phase was considered.
We analyzed the (4D) CTV dose coverage by V95, the overdose as V107, and the homogeneity of the dose distribution within the CTV by D5-D95. The conformity was assessed by the conformity number CN (24).
Results
Experiments
The multigating plan could be delivered successfully, but took up to 150 sec instead of 40 sec for the static delivery. The main reason for delay was incomplete delivery to a motion phase, so that an entire motion period had to be gated.
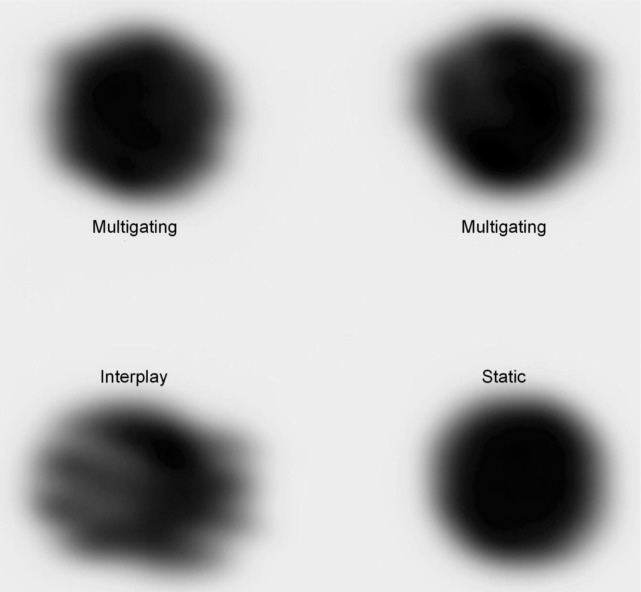
The irradiated film was digitized (DosimetryPro, Vidar) and transformed into optical densities, see Figure 3. Some dose deviation remained due to neglected residual motion within the phases (up to 3 mm). The gamma analysis resulted in agreement of 79.6 and 84.5% between multigating and the static reference, in comparison to 34.4% for interplay.
Figure 3:
Result of the film experiment. For the static irradiation, the film was stopped, while for all other irradiations the film was moving with a 20 mm peak-to-peak sinusoidal motion. Both upper irradiations are the result of an identical multigating plan.
Patient Treatment Planning Study
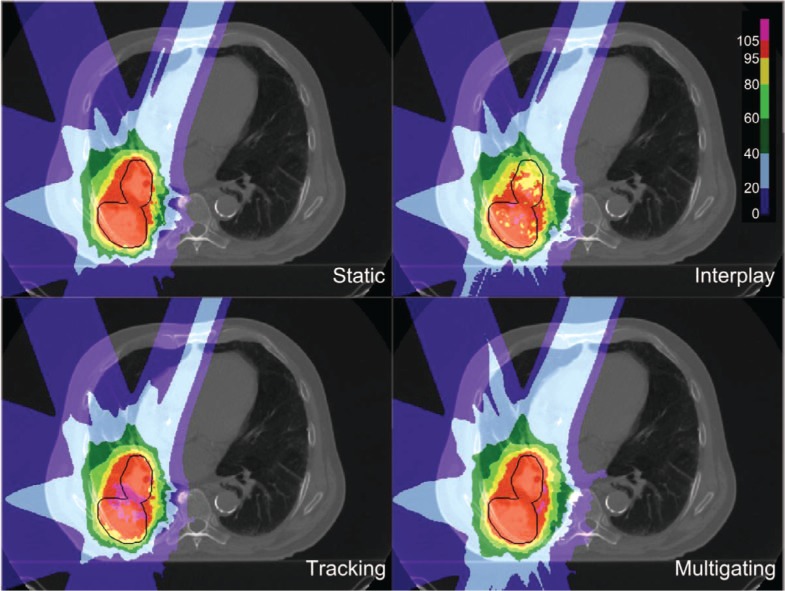
Dose cuts for one example patient with a complex GTV shape and a peak-to-peak target motion of 12.6 mm are shown in Figure 4, with the corresponding DVH in Figure 5. In this patient target coverage lost in interplay could not be completely restored by beam tracking. Multigating results in good target coverage with a slight increase in dose outside the target. Comparable results were achieved for all patients, as depicted in Figure 6. Again, tracking could partly but not completely regain dose coverage, with V95 ranging from 89.5 to 95%, while multigating achieved more than 99.7%. The conformity number for multigating was slightly lower at 72.0% compared to tracking (73.4%) and static reference (75.8%), so that at least a part of the 4D dose coverage was achieved through increased dose to the normal tissue.
Figure 4:
Dose cuts for one patient for a static reference plan calculated for end-exhale, and 4D-dose calculations for no motion mitigation (interplay), 3D-optimized tracking, and 4D-optimized multigating.
Figure 5:
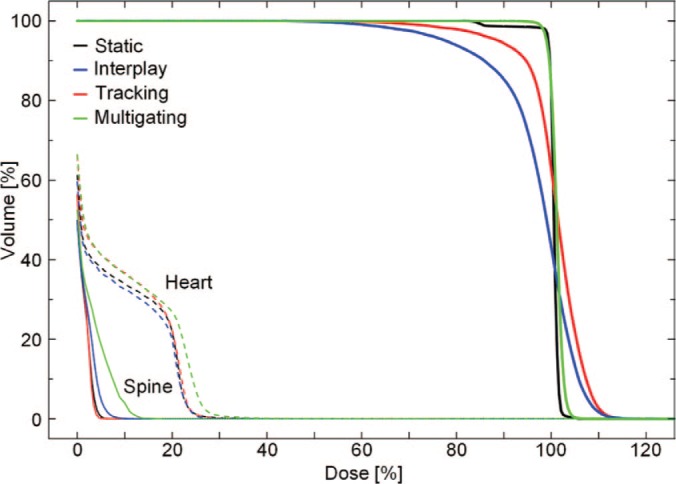
DVH of the same patient as shown in Figure 4. The DVHs of the target are shown as bold lines, of the heart as dashed, and of the spine as thin lines.
Figure 6:
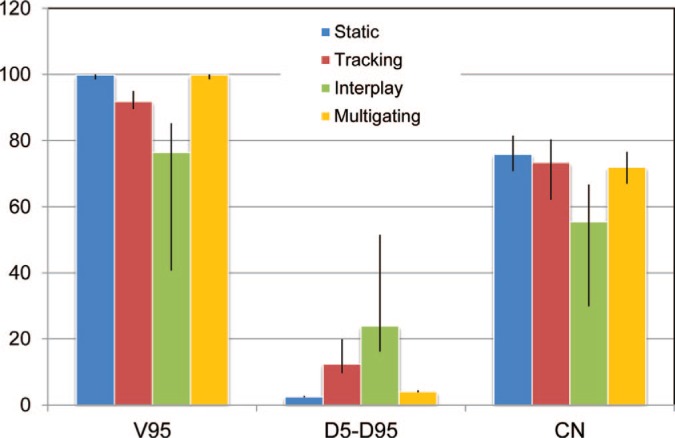
DVH results (median and range) of the treatment planning study, with dose coverage (V95), homogeneity (D5-D95), and conformation (CN). All data is given as percent of the target dose or volume as appropriate. Multigating restored dose coverage to the level of the static dose at the cost of slightly worse conformity.
Discussion
In this study, a practical method for 4D-optimized treatment planning and delivery in scanned ion beam therapy was developed and tested in patient treatment planning studies as well as an experiment. It lead to homogeneous target coverage in moving targets with only a slight reduction in conformity in comparison to a static irradiation to the reference phase. In future studies, intensity modulated particle therapy including OAR constraints could be used to further improve conformity.
The delivery of the multigating plan in a film experiment required only minor software modifications to the treatment control system, in contrast to e.g. beam tracking which requires dedicated hardware for the online adjustment of beam range. The implementation of multigating in existing systems could thus be accomplished with comparably low effort. The experiment also revealed that the residual motion within the motion phases proves to be a challenge, which could be countered by rescanning. As intensity control would provide a large benefit for multigating due to improved timing, implementation of rescanning would automatically result in a flavor of breath-sampled rescanning (25) or phase-controlled rescanning (26). Both result in improved homogenization at a low number of rescans. The lack of intensity control lead to long gating periods, when the irradiation of a motion phase was not finished before a phase change in the experiment. The rigid spill structure at GSI aggravated this problem, as the needed motion phase could occur in a spill pause, resulting in a large increase of nearly a factor of 4 in treatment duration. Synchrotrons dedicated to medical treatment offer more flexible timing such that the synchronization would be easier (27).
The distribution of beam spots to motion phases in this study was not part of the optimization process. It was performed in a forward iteration based on a standard beam tracking plan to assure complete target coverage while at the same time aiming at a reasonable timing of the motion phases. In principle this distribution could also be used for a number of additional effects, such as a reduction of integral dose, lower number of iso-energy slices, or avoidance of OARs, all through exploiting the target motion. Especially in case of differential motion to other tissues, the out-of-target dose distribution could be efficiently shaped. This optimization process appears highly complex, as it has to assure the multiple criteria of target coverage, homogeneous dose distribution and delivery timing for each phase, and finally also out-of-target dose. The distribution of raster spots directly influences the optimization of particle numbers, and thus the two optimization processes cannot be separated. Though such an optimization offers exciting possibilities, it is beyond the scope of this study.
Multigating requires precise intra-fractional tumor motion monitoring. Uncertainty in the detection of the motion phases results into dose errors as the synchronization of delivery and motion can no longer be upheld. The requirements in this respect are comparable to 3D beam tracking. Real-time motion monitoring is a topic of ongoing research with a variety of available methods, including x-ray fluoroscopy, optical systems, RF tags, strain sensors, or ultrasound. All offer specific advantages and disadvantages with respect to invasiveness, additional dose, direct or indirect measurement of the tumor motion, accuracy, and detection speed (28).
This study demonstrated the technical feasibility of multigating. For clinical applicability, uncertainties that were ignored here have to be considered. No margins were included in treatment planning to form a PTV, which is clearly necessary for a robust delivery. The 4D-optimization relies on the accuracy of the 4DCT, which represents only a few breathing cycles imaged days before the actual treatment. A good option for the investigation of motion uncertainty in the 4DCT would be the simulated 4DCTs on the basis of 4DMRI as proposed by Boye et al. (29). The use of MRI permits to study varying breathing motion over a longer time period. This kind of 4DCT could be either used in forward dose calculation for the assessment of robustness but also included into the inverse planning process as additional motion phases. In these studies also the uncertainty of specific motion monitoring methods can be considered through simulated mismatch of the prescribed and detected motion phases. The introduction of rescanning as discussed above would also add to robustness with respect to all variable errors such as motion detection. Ultimately, more complex experiments are needed, including more realistic phantom geometries, complex 3-D motions and clinical motion tracking systems, similar to previous experiments performed at GSI (30).
A simple option for increased robustness would be to exclude the transition motion phases between end-exhale and endinhale which exhibit a higher motion speed. This would reduce the time available for treatment, but also decrease potential errors from residual motions within the motion phases.
In conclusion, multigating permits a conformal irradiation of moving targets also for complex motion. The delivery requires only minor software modifications to existing treatment control systems but multigating requires 4D optimization capability in the treatment planning system. Further studies are required to assess robustness and clinical applicability.
Abbreviations:
- 4DCT:
Time-resolved computed tomography
- CN:
Conformity number, describing dose conformation
- CTV:
Clinical target volume
- D5-D95:
Dose homogeneity, steepness of the DVH
- DIR:
Deformable image registration
- DVH:
Dose-volume-histogram
- LUT:
Look-up table
- V95:
Target coverage, target volume receiving at least 95% of the target dose
- V107:
Overdose, target volume receiving at least 107% of the target dose
- WEL:
Water equivalent length, radiological depth of a voxel/structure
Footnotes
Conflict of Interest: All authors certify that his manuscript has not been published in whole or in part nor is it being considered for publication elsewhere. The authors have no conflicts of interest to declare.
References
- 1.Hanley J, Debois MM, Mah D, Mageras GS, Raben A, Rosenzweig K, Mychalczak B, Schwartz LH, Gloeggler PJ. & Lutz W. Deep inspiration breath-hold technique for lung tumors: the potential value of target immobilization and reduced lung density in dose escalation. Int J Radiat Oncol 45, 603–611, (1999). PMID: 10524412. [DOI] [PubMed] [Google Scholar]
- 2.Kontrisova K, Stock M, Dieckmann K, Bogner J, Potter R. & Georg D. Dosimetric comparison of stereotactic body radiotherapy in different respiration conditions: a modeling study. Radiother Oncol 81, 97–104, (2006). DOI: 10.1016/j.radonc.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Wong JW, Sharpe MB, Jaffray DA, Kini VR, Robertson JM, Stromberg JS. & Martinez AA. The use of active breathing control (ABC) to reduce margin for breathing motion. Int J Radiat Oncol 44, 911–919, (1999). PMID: 10386650. [DOI] [PubMed] [Google Scholar]
- 4.Riboldi M, Orecchia R. & Baroni G. Real-time tumour tracking in particle therapy: technological developments and future perspectives. Lancet Oncol 13, e383–e391 (2012). DOI: 10.1016/S1470-2045(12)70243-7. [DOI] [PubMed] [Google Scholar]
- 5.Nelson C, Starkschall G, Balter P, Fitzpatrick MJ, Antolak JA, Tolani N. & Prado K. Respiration-correlated treatment delivery using feedback-guided breath hold: a technical study. Med Phys 32, 175–181, (2005). PMID: 15719968. [DOI] [PubMed] [Google Scholar]
- 6.Bert C. & Durante M. Motion in radiotherapy: particle therapy. Phys Med Biol 56, R113–R144 (2011). DOI: 10.1088/0031-9155/56/16/R01. [DOI] [PubMed] [Google Scholar]
- 7.Saito N, Bert C, Chaudhri N, Gemmel A, Schardt D. & Rietzel E. Speed and accuracy of a beam tracking system for treatment of moving targets with scanned ion beams. Phys Med Biol 54, 4849–4862, (2009). DOI: 10.1088/0031-9155/54/16/001. [DOI] [PubMed] [Google Scholar]
- 8.Bert C, Saito N, Schmidt A, Chaudhri N, Schardt D. & Rietzel E. Target motion tracking with a scanned particle beam. Med Phys 34, 4768–4771, (2007). PMID: 18196804. [DOI] [PubMed] [Google Scholar]
- 9.Lüchtenborg R, Saito N, Durante M. & Bert C. Experimental verification of a real-time compensation functionality for dose changes due to target motion in scanned particle therapy. Med Phys 38, 5448–5458, (2011). DOI: 10.1118/1.3633891. [DOI] [PubMed] [Google Scholar]
- 10.Bert C, Gemmel A, Saito N, Chaudhri N, Schardt D, Durante M, Kraft G. & Rietzel E. Dosimetric precision of an ion beam tracking system. Radiat Oncol 5, 61 (2010). DOI: 10.1186/1748-717X-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keall PJ, Siebers JV, Joshi S. & Mohan R. Monte Carlo as a four-dimensional radiotherapy treatment-planning tool to account for respiratory motion. Phys Med Biol 49, 3639–3648, (2004). PMID: 15446794. [DOI] [PubMed] [Google Scholar]
- 12.Trofimov A, Rietzel E, Lu HM, Martin B, Jiang S, Chen GTY. & Bortfeld T. Temporo-spatial IMRT optimization: concepts, implementation and initial results. Phys Med Biol 50, 2779–2798, (2005). PMID: 15930602. [DOI] [PubMed] [Google Scholar]
- 13.Krämer M. & Scholz M. Treatment planning for heavy-ion radiotherapy: calculation and optimization of biologically effective dose. Phys Med Biol 45, 3319–3330, (2000). PMID: 11098906. [DOI] [PubMed] [Google Scholar]
- 14.Richter D, Schwarzkopf A, Trautmann J, Kramer M, Durante M, Jakel O. & Bert C. Upgrade and benchmarking of a 4D treatment planning system for scanned ion beam therapy. Med Phys 40, 051722 (2013). DOI:10.1118/1.4800802. [DOI] [PubMed] [Google Scholar]
- 15.Nohadani O, Seco J. & Bortfeld T. Motion management with phase-adapted 4D-optimization. Phys Med Biol 55, 5189–5202, (2010). DOI: 10.1088/0031-9155/55/17/019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato S, Furukawa T. & Noda K. Dynamic intensity control system with RF-knockout slow-extraction in the HIMAC synchrotron. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment 574, 226–231, (2007). DOI: 10.1016/j.nima.2007.01.174. [Google Scholar]
- 17.Schömers C, Feldmeier E, Haberer T, Naumann J, Panse R. & Peters A. Implementation of an intensity feedback loop for an ion therapy synchtrotron. IPAC; 2011, p. 2851. [Google Scholar]
- 18.Noda K, Kanazawa M, Itano A, et al. Slow beam extraction by a transverse RF field with AM and FM. Nucl Instrum Meth A 374, 269–277, (1996). [Google Scholar]
- 19.Low DA, Harms WB, Mutic S. & Purdy JA. A technique for the quantitative evaluation of dose distributions. Med Phys 25, 656–661, (1998). PMID: 9608475. [DOI] [PubMed] [Google Scholar]
- 20.Shackleford JA, Kandasamy N. & Sharp GC. On developing B-spline registration algorithms for multi-core processors. Phys Med Biol 55, 6329–6351, (2010). DOI: 10.1088/0031-9155/55/21/001. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto T, Yamamoto N, Nishimura H, et al. Carbon ion radiotherapy for stage I non-small cell lung cancer. Radiother Oncol 66, 127–140, (2003). PMID: 12648784. [DOI] [PubMed] [Google Scholar]
- 22.Elsässer T, Weyrather WK, Friedrich T, et al. Quantification of the relative biological effectiveness for ion beam radiotherapy: direct experimental comparison of proton and carbon ion beams and a novel approach for treatment planning. Int J Radiat Oncol Biol Phys 78, 1177–1183, (2010). DOI: 10.1016/j.ijrobp.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Wölfelschneider J. Fraktionierte Bestrahlung bewegter Tumoren mit gescannten Schwerionen Technische Hochschule Mittelhessen; 2011.
- 24.van't Riet A, Mak AC, Moerland MA, Elders LH. & van der Zee W. A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: application to the prostate. Int J Radiat Oncol Biol Phys 37, 731–736, (1997). PMID: 9112473. [DOI] [PubMed] [Google Scholar]
- 25.Seco J, Robertson D, Trofimov A. & Paganetti H. Breathing interplay effects during proton beam scanning: simulation and statistical analysis. Phys Med Biol 54, N283–N294 (2009). DOI: 10.1088/0031-9155/54/14/N01. [DOI] [PubMed] [Google Scholar]
- 26.Furukawa T, Inaniwa T, Sato S, Shirai T, Mori S, Takeshita E, Mizushima K, Himukai T. & Noda K. Moving target irradiation with fast rescanning and gating in particle therapy. Med Phys 37, 4874–4879, (2010). PMID: 20964205. [DOI] [PubMed] [Google Scholar]
- 27.Tsunashima Y, Vedam S, Dong L, Umezawa M, Sakae T, Bues M, Balter P, Smith A. & Mohan R. Efficiency of respiratory-gated delivery of synchrotron-based pulsed proton irradiation. Phys Med Biol 53, 1947–1959, (2008). DOI: 10.1088/0031-9155/53/7/010. [DOI] [PubMed] [Google Scholar]
- 28.Riboldi M, Orecchia R. & Baroni G. Real-time tumour tracking in particle therapy: technological developments and future perspectives. Lancet Oncol 13, e383–e391 (2012). DOI: 10.1016/S1470-2045(12)70243-7. [DOI] [PubMed] [Google Scholar]
- 29.Boye D, Lomax T. & Knopf A. Mapping motion from 4D-MRI to 3D-CT for use in 4D dose calculations: a technical feasibility study. Med Phys 40, 061702 (2013). DOI: 10.1118/1.4801914. [DOI] [PubMed] [Google Scholar]
- 30.Seregni M, Kaderka R, Fattori G, et al. Tumor tracking based on correlation models in scanned ion beam therapy: an experimental study. Phys Med Biol 58, 4659–4678, (2013). DOI: 10.1088/0031-9155/58/13/4659. [DOI] [PubMed] [Google Scholar]