Abstract
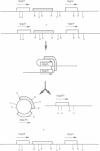
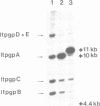
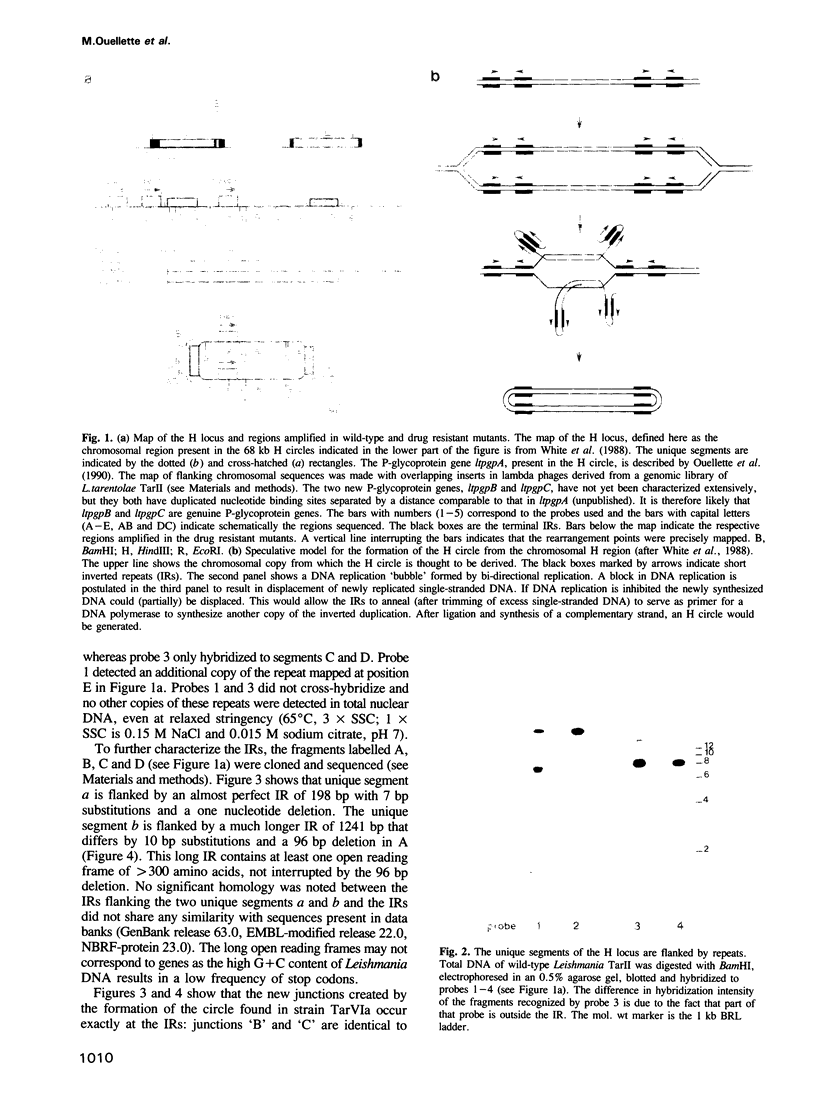
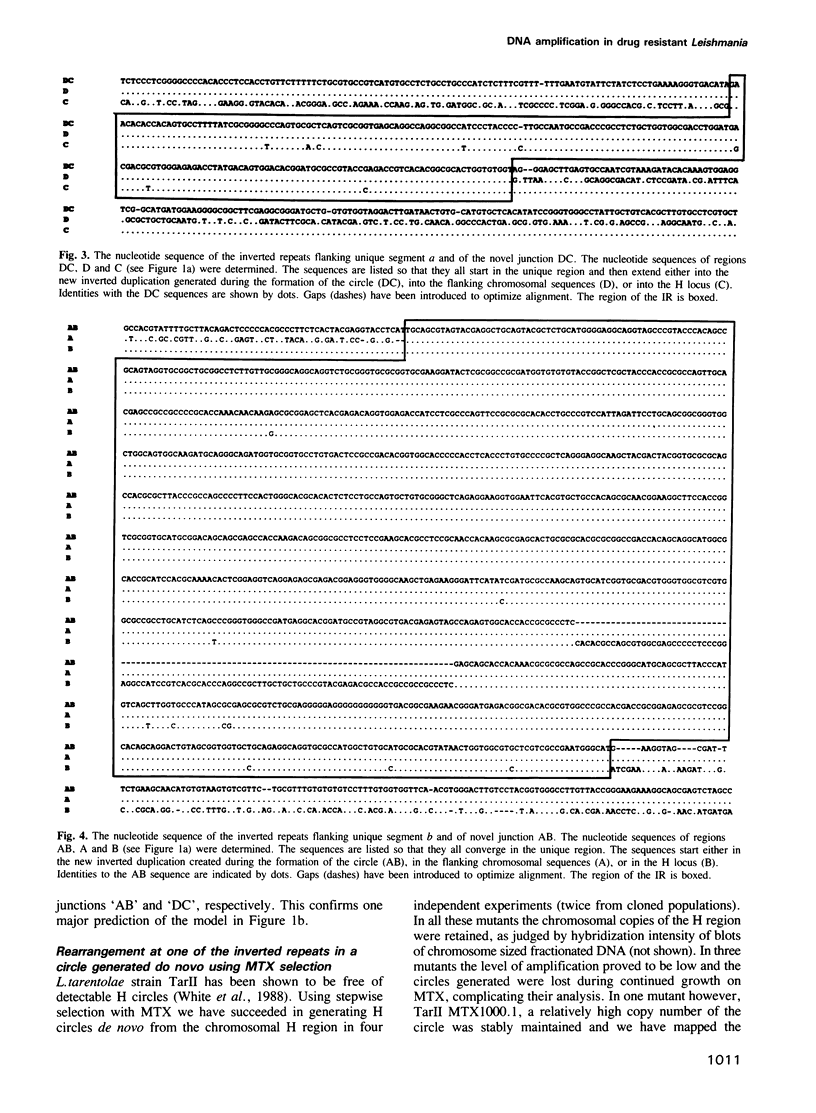
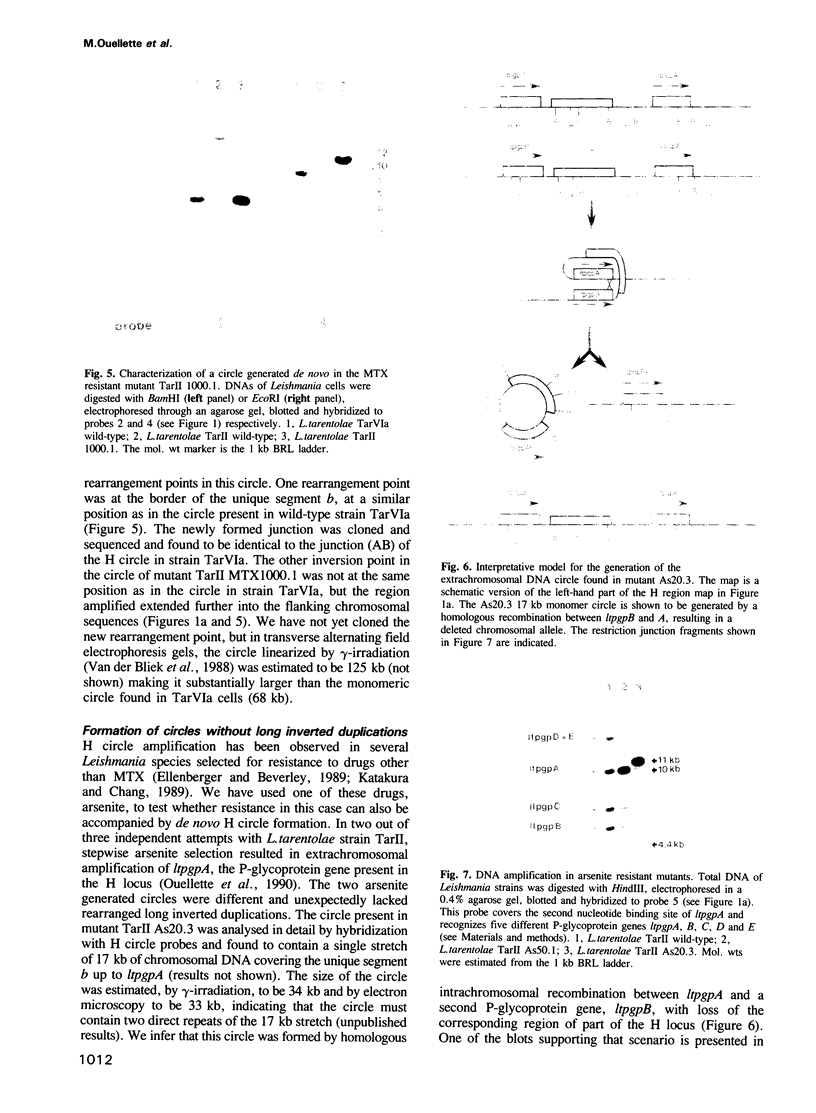
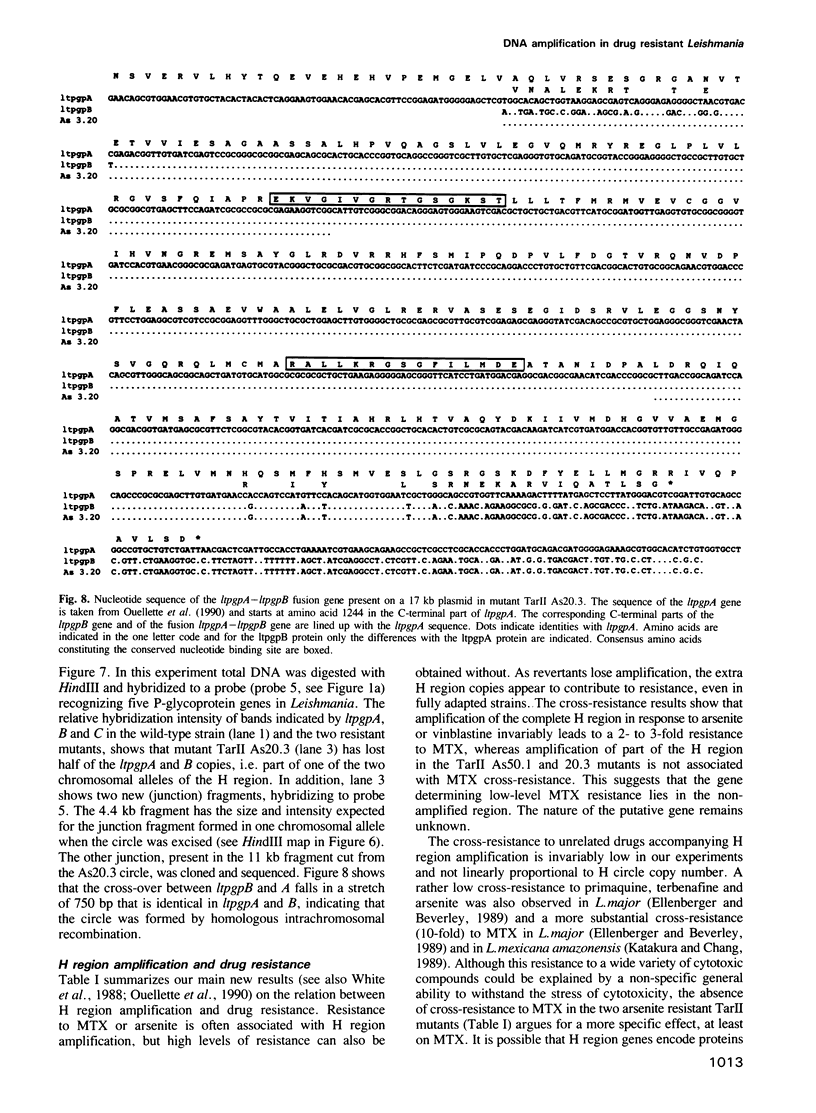
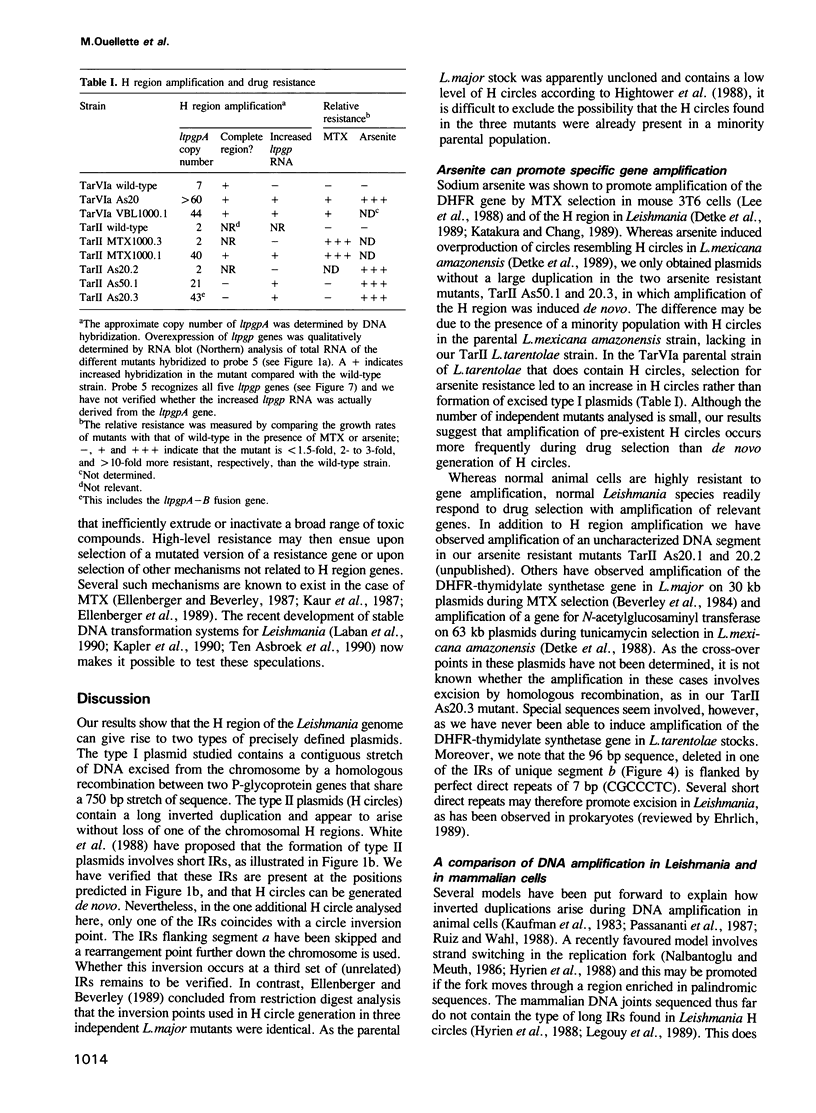
The H circle of Leishmania species contains a 30 kb inverted duplication separated by two unique DNA segments, a and b. The corresponding H region of chromosomal DNA has only one copy of the duplicated DNA. We show here that the chromosomal segments a and b are flanked by inverted repeats (198 and 1241 bp) and we discuss how these repeats could lead to formation of H circles from chromosomal DNA. Selection of Leishmania tarentolae for methotrexate resistance indeed resulted in the de novo formation of circles with long inverted duplication, but two mutants selected for arsenite resistance contained new H region plasmids without such duplications. One of these plasmids appears due to a homologous recombination between two P-glycoprotein genes with a high degree of sequence homology. Our results show how the same DNA region in Leishmania may be amplified to give plasmids with or without long inverted duplications and apparently by different mechanisms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverley S. M., Coderre J. A., Santi D. V., Schimke R. T. Unstable DNA amplifications in methotrexate-resistant Leishmania consist of extrachromosomal circles which relocalize during stabilization. Cell. 1984 Sep;38(2):431–439. doi: 10.1016/0092-8674(84)90498-7. [DOI] [PubMed] [Google Scholar]
- Carroll S. M., DeRose M. L., Gaudray P., Moore C. M., Needham-Vandevanter D. R., Von Hoff D. D., Wahl G. M. Double minute chromosomes can be produced from precursors derived from a chromosomal deletion. Mol Cell Biol. 1988 Apr;8(4):1525–1533. doi: 10.1128/mcb.8.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre J. A., Beverley S. M., Schimke R. T., Santi D. V. Overproduction of a bifunctional thymidylate synthetase-dihydrofolate reductase and DNA amplification in methotrexate-resistant Leishmania tropica. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2132–2136. doi: 10.1073/pnas.80.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz A., Beverley S. M. Gene replacement in parasitic protozoa. Nature. 1990 Nov 8;348(6297):171–173. doi: 10.1038/348171a0. [DOI] [PubMed] [Google Scholar]
- Detke S., Chaudhuri G., Kink J. A., Chang K. P. DNA amplification in tunicamycin-resistant Leishmania mexicana. Multicopies of a single 63-kilobase supercoiled molecule and their expression. J Biol Chem. 1988 Mar 5;263(7):3418–3424. [PubMed] [Google Scholar]
- Detke S., Katakura K., Chang K. P. DNA amplification in arsenite-resistant Leishmania. Exp Cell Res. 1989 Jan;180(1):161–170. doi: 10.1016/0014-4827(89)90220-6. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger T. E., Beverley S. M. Multiple drug resistance and conservative amplification of the H region in Leishmania major. J Biol Chem. 1989 Sep 5;264(25):15094–15103. [PubMed] [Google Scholar]
- Ellenberger T. E., Beverley S. M. Reductions in methotrexate and folate influx in methotrexate-resistant lines of Leishmania major are independent of R or H region amplification. J Biol Chem. 1987 Oct 5;262(28):13501–13506. [PubMed] [Google Scholar]
- Ellenberger T. E., Wright J. E., Rosowsky A., Beverley S. M. Wild-type and drug-resistant Leishmania major hydrolyze methotrexate to N-10-methyl-4-deoxy-4-aminopteroate without accumulation of methotrexate polyglutamates. J Biol Chem. 1989 Sep 25;264(27):15960–15966. [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Foote S. J., Kyle D. E., Martin R. K., Oduola A. M., Forsyth K., Kemp D. J., Cowman A. F. Several alleles of the multidrug-resistance gene are closely linked to chloroquine resistance in Plasmodium falciparum. Nature. 1990 May 17;345(6272):255–258. doi: 10.1038/345255a0. [DOI] [PubMed] [Google Scholar]
- Ford M., Fried M. Large inverted duplications are associated with gene amplification. Cell. 1986 May 9;45(3):425–430. doi: 10.1016/0092-8674(86)90328-4. [DOI] [PubMed] [Google Scholar]
- Heartlein M. W., Latt S. A. Amplified inverted duplications within and adjacent to heterologous selectable DNA. Nucleic Acids Res. 1989 Feb 25;17(4):1697–1716. doi: 10.1093/nar/17.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower R. C., Ruiz-Perez L. M., Wong M. L., Santi D. V. Extrachromosomal elements in the lower eukaryote Leishmania. J Biol Chem. 1988 Nov 15;263(32):16970–16976. [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hunt J. D., Valentine M., Tereba A. Excision of N-myc from chromosome 2 in human neuroblastoma cells containing amplified N-myc sequences. Mol Cell Biol. 1990 Feb;10(2):823–829. doi: 10.1128/mcb.10.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyrien O., Debatisse M., Buttin G., de Saint Vincent B. R. The multicopy appearance of a large inverted duplication and the sequence at the inversion joint suggest a new model for gene amplification. EMBO J. 1988 Feb;7(2):407–417. doi: 10.1002/j.1460-2075.1988.tb02828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapler G. M., Coburn C. M., Beverley S. M. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol. 1990 Mar;10(3):1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapler G. M., Coburn C. M., Beverley S. M. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol. 1990 Mar;10(3):1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakura K., Chang K. P. H DNA amplification in Leishmania resistant to both arsenite and methotrexate. Mol Biochem Parasitol. 1989 May 1;34(2):189–191. doi: 10.1016/0166-6851(89)90010-8. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A., Latt S. A. Evolution of chromosomal regions containing transfected and amplified dihydrofolate reductase sequences. Mol Cell Biol. 1983 Apr;3(4):699–711. doi: 10.1128/mcb.3.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur K., Coons T., Emmett K., Ullman B. Methotrexate-resistant Leishmania donovani genetically deficient in the folate-methotrexate transporter. J Biol Chem. 1988 May 25;263(15):7020–7028. [PubMed] [Google Scholar]
- Laban A., Tobin J. F., Curotto de Lafaille M. A., Wirth D. F. Stable expression of the bacterial neor gene in Leishmania enriettii. Nature. 1990 Feb 8;343(6258):572–574. doi: 10.1038/343572a0. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Tanaka N., Lamb P. W., Gilmer T. M., Barrett J. C. Induction of gene amplification by arsenic. Science. 1988 Jul 1;241(4861):79–81. doi: 10.1126/science.3388020. [DOI] [PubMed] [Google Scholar]
- Legouy E., Fossar N., Lhomond G., Brison O. Structure of four amplified DNA novel joints. Somat Cell Mol Genet. 1989 Jul;15(4):309–320. doi: 10.1007/BF01534970. [DOI] [PubMed] [Google Scholar]
- Looney J. E., Hamlin J. L. Isolation of the amplified dihydrofolate reductase domain from methotrexate-resistant Chinese hamster ovary cells. Mol Cell Biol. 1987 Feb;7(2):569–577. doi: 10.1128/mcb.7.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbantoglu J., Meuth M. DNA amplification--deletion in a spontaneous mutation of the hamster aprt locus: structure and sequence of the novel joint. Nucleic Acids Res. 1986 Nov 11;14(21):8361–8371. doi: 10.1093/nar/14.21.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette M., Fase-Fowler F., Borst P. The amplified H circle of methotrexate-resistant leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J. 1990 Apr;9(4):1027–1033. doi: 10.1002/j.1460-2075.1990.tb08206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passananti C., Davies B., Ford M., Fried M. Structure of an inverted duplication formed as a first step in a gene amplification event: implications for a model of gene amplification. EMBO J. 1987 Jun;6(6):1697–1703. doi: 10.1002/j.1460-2075.1987.tb02420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauletti G., Lai E., Attardi G. Early appearance and long-term persistence of the submicroscopic extrachromosomal elements (amplisomes) containing the amplified DHFR genes in human cell lines. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2955–2959. doi: 10.1073/pnas.87.8.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrillo-Peixoto M. L., Beverley S. M. Amplified DNAs in laboratory stocks of Leishmania tarentolae: extrachromosomal circles structurally and functionally similar to the inverted-H-region amplification of methotrexate-resistant Leishmania major. Mol Cell Biol. 1988 Dec;8(12):5188–5199. doi: 10.1128/mcb.8.12.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz J. C., Wahl G. M. Chromosomal destabilization during gene amplification. Mol Cell Biol. 1990 Jun;10(6):3056–3066. doi: 10.1128/mcb.10.6.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz J. C., Wahl G. M. Formation of an inverted duplication can be an initial step in gene amplification. Mol Cell Biol. 1988 Oct;8(10):4302–4313. doi: 10.1128/mcb.8.10.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito I., Stark G. R. Charomids: cosmid vectors for efficient cloning and mapping of large or small restriction fragments. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8664–8668. doi: 10.1073/pnas.83.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured cells. J Biol Chem. 1988 May 5;263(13):5989–5992. [PubMed] [Google Scholar]
- Stark G. R., Debatisse M., Giulotto E., Wahl G. M. Recent progress in understanding mechanisms of mammalian DNA amplification. Cell. 1989 Jun 16;57(6):901–908. doi: 10.1016/0092-8674(89)90328-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M. The importance of circular DNA in mammalian gene amplification. Cancer Res. 1989 Mar 15;49(6):1333–1340. [PubMed] [Google Scholar]
- Wellems T. E., Panton L. J., Gluzman I. Y., do Rosario V. E., Gwadz R. W., Walker-Jonah A., Krogstad D. J. Chloroquine resistance not linked to mdr-like genes in a Plasmodium falciparum cross. Nature. 1990 May 17;345(6272):253–255. doi: 10.1038/345253a0. [DOI] [PubMed] [Google Scholar]
- White T. C., Fase-Fowler F., van Luenen H., Calafat J., Borst P. The H circles of Leishmania tarentolae are a unique amplifiable system of oligomeric DNAs associated with drug resistance. J Biol Chem. 1988 Nov 15;263(32):16977–16983. [PubMed] [Google Scholar]
- Yen P. H., Li X. M., Tsai S. P., Johnson C., Mohandas T., Shapiro L. J. Frequent deletions of the human X chromosome distal short arm result from recombination between low copy repetitive elements. Cell. 1990 May 18;61(4):603–610. doi: 10.1016/0092-8674(90)90472-q. [DOI] [PubMed] [Google Scholar]
- ten Asbroek A. L., Ouellette M., Borst P. Targeted insertion of the neomycin phosphotransferase gene into the tubulin gene cluster of Trypanosoma brucei. Nature. 1990 Nov 8;348(6297):174–175. doi: 10.1038/348174a0. [DOI] [PubMed] [Google Scholar]
- van der Bliek A. M., Borst P. Multidrug resistance. Adv Cancer Res. 1989;52:165–203. doi: 10.1016/s0065-230x(08)60213-4. [DOI] [PubMed] [Google Scholar]
- van der Bliek A. M., Lincke C. R., Borst P. Circular DNA of 3T6R50 double minute chromosomes. Nucleic Acids Res. 1988 Jun 10;16(11):4841–4851. doi: 10.1093/nar/16.11.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]