Abstract
The purpose of the present study was to explore the outcome, cumulative dose in tumor and organs at risk and toxicity after extra-cranial stereotactic re-irradiation. Twenty-seven patients were evaluated who had been re-irradiated with stereotactic body radiotherapy (SBRT) after conventional radiotherapy (CRT). The dose summation of the SBRT and CRT plans was done by dose point calculations accounting for fraction size by the linear-quadratic model. Efficacy and toxicity was scored by looking at the reduction in tumor size, pain and bleeding. Symptomatic response was observed in 96% of the patients. The median maximum SBRT dose to the tumor was 90 Gy3 (range: 42-420 Gy3). The median cumulative dose for the rectum, bowel and bladder resulted in 104 Gy3, 98 Gy3 and 113 Gy3, respectively. No grades 5, 4 and 3 acute and late toxicity was observed. In conclusion: re-irradiation to the same region using extra-cranial stereotactic radiotherapy is feasible and resulted in a 96% symptomatic response with low toxicity.
Keywords: Re-irradiation, Stereotactic radiotherapy, Toxicity
Introduction
Despites the progress in local and combined systemic treatments in patients with cancer, metastasis or local failure can occur. Re-irradiation should be considered, if surgery or chemotherapy is not an option. Re-irradiation, however, is often limited by dose constraints to anatomical structures surrounding the tumor. The potential toxicity to surrounding tissues such as spinal cord, bowel, bladder and other organs limits the high dose needed to treat the tumor adequately. For organs in the abdominal and pelvic region, the spinal cord with its nerves is probably the most important organ. On the other hand, bowel, rectum and bladder and sometimes the kidneys are also dose limiting structures.
Throughout the years, various studies reported the maximum doses that are acceptable for normal tissues. Clinical re-irradiation studies of the myelum showed little incidence of radiation myelopathy (1-4) and animal studies showed that retreatment tolerance of the myelum increases with increasing interval from the first radiation treatment. An assumption of a median of 50% repair of the myelum after 6 to 12 months is feasible (5-9). Re-irradiation studies regarding recurrences from rectum cancer, showed a cumulative medium dose of 85 Gy with a maximum cumulative dose of 119 Gy (10-13). The surrounding tissue like bladder and bowel were in the re-irradiation field, but unfortunately the cumulative doses were not reported. There were no grade 4 acute and late toxicities and there was a small number of grade 3 toxicity.
So, it seems to be feasible to re-irradiate if other treatment options are ineffective or not possible. However, it remains difficult to retreat previous irradiated fields with conventional external beam radiation due to the high doses given to the organs at risk. Radiation treatment by stereotactic body radiotherapy (SBRT) could have an advantage in some oncology patients compared with conventional external beam radiation therapy (EBRT). SBRT has the advantage of delivering many noncoplaner beams from a wider solid angle. This result in delivering high doses of radiation to the target volumes combined with sub-millimetre accuracy. The expose of radiation dose to the surrounding healthy tissues is minimized, due to steep decrease in dose gradients. Extra-cranial use of SBRT in patients with malignancies has been evaluated in different regions. However there are only a few reports on the efficacy of SBRT re-irradiation in the abdomen and pelvis. Patients with recurrent spinal tumors, who received initial median radiation dose of 35 Gy, showed a median survival of 10.5 months after SBRT re-irradiation. The re-irradiation dose given was 21.05 Gy in 3 fractions (14). SBRT re-irradiation doses given to 5 patients with gynaecological recurrences were between 15 Gy and 24 Gy, given in 3 fractions. The initial treatment in some patients was external beam radiation 45 Gy in 25 fractions, with or without brachytherapy (15).
The available studies about SBRT re-irradiation involved little group of patients, without clear information about the maximum given dose to the organs at risk.
Therefore, the purpose of the present study was to explore the outcome, cumulative dose in tumor and organs at risk, and toxicity after abdominal or pelvic stereotactic body radiotherapy in 27 patients who were treated at our department.
Methods and Materials
Patient Characteristics
Between March 2005 and July 2009, 33 patients have been re-irradiated in the pelvic region, by stereotactic body radiotherapy (SBRT) using the CyberKnife (Accuray, Sunnyvale). The re-irradiated region had a partial or complete overlap with the previous irradiated region. Six patients were excluded from the analyses. Four of them had their first radiation treatment in another hospital. The conventional localization films of the radiation fields of the other 2 patients were not available. For these 6 patients it was not possible to determine the precise cumulative dose in the points of interest. The remaining 27 patients were included in the analysis. There were 13 females (48%) and 14 males (52%). The patient characteristics are shown in Table I. Most of the patients had rectal cancer as primary tumor. Patient age at re-irradiation by stereotactic radiotherapy ranged from 27-80 years (median: 59 years). The median SBRT dose was 34 Gy (range: 8-60 Gy) in 1-10 fractions (Table II). To evaluate whether the outcome of stereotactic re-irradiation was dose dependent, the patients were classified in a group receiving a total recalculated dose of less than 60 Gy10, and another group receiving more than 60 Gy10 or more. Seven patients were treated with curative intention.
Table I.
Patient characteristics.
| No. of patients | % | ||
|---|---|---|---|
| Primary tumor | Rectum cancer | 13 | 48 |
| Cervix cancer | 6 | 22 | |
| Ovarian cancer | 2 | 7 | |
| Sarcoma | 2 | 7 | |
| Other cancer | 4 | 15 | |
| Re-irradiation region | Pelvis | 21 | 78 |
| Intra-abdominal | 6 | 22 | |
| Gender | Female | 13 | 48 |
| Male | 14 | 52 |
Table II.
Cyberknife schedules.
| Second radiation | First radiation | ||||
|---|---|---|---|---|---|
| Patient | Fraction | Fraction dose (Gy) | EQD2 dose (Gy10) | Prescripted isodose line (%) | EQD2 dose (Gy10) |
| 1 | 3 | 20 | 150 | 80 | 50 |
| 2 | 3 | 15 | 94 | 85 | 46 |
| 3 | 6 | 8 | 72 | 80 | 48 |
| 4 | 6 | 8 | 72 | 80 | 50 |
| 5 | 6 | 7 | 60 | 80 | 46 |
| 6 | 6 | 7 | 60 | 80 | 46 |
| 7 | 6 | 7 | 60 | 70 | 46 |
| 8 | 6 | 6 | 48 | 80 | 66 |
| 9 | 6 | 6 | 48 | 75 | 46 |
| 10 | 6 | 6 | 48 | 80 | 72 |
| 11 | 6 | 6 | 48 | 65 | 39 |
| 12 | 4 | 8 | 48 | 80 | 46 |
| 13 | 6 | 6 | 48 | 80 | 50 |
| 14 | 6 | 6 | 48 | 75 | 66 |
| 15 | 5 | 6 | 40 | 80 | 83 |
| 16 | 4 | 7 | 40 | 75 | 70 |
| 17 | 5 | 6 | 40 | 79 | 31 |
| 18 | 3 | 8 | 36 | 80 | 71 |
| 19 | 3 | 6 | 24 | 80 | 76 |
| 20 | 3 | 6 | 24 | 80 | 46 |
| 21 | 2 | 8 | 24 | 80 | 80 |
| 22 | 2 | 8 | 24 | 80 | 42 |
| 23 | 3 | 6 | 24 | 80 | 46 |
| 24 | 2 | 8 | 24 | 80 | 87 |
| 25 | 3 | 6 | 24 | 85 | 48 |
| 26 | 3 | 6 | 24 | 80 | 48 |
| 27 | 2 | 8 | 24 | 70 | 78 |
Dose Summation and Organ Constraints
Different doses and fractionation schemes were used during the treatment course. Therefore, all treatment schedules were recalculated to a biologically equivalent schedule of 2-Gy fractions. The dose was recalculated by the formula: EQD2 = D * (d + α/β) / (2.0 + α/β) with D is the total dose of a treatment course, d is the fraction dose, and α/β = 3 Gy for the organs at risk and α/β = 10 Gy for the tumor. The organs at risk were delineated in the planning CT scan.
The treatment planning system used for SBRT was On Target (version 3.4.1). All patients were treated in a vacuum cast. Prior to treatment, markers were placed in or near the tumor for position verification. The margins from GTV to PTV were 2 or 3 mm. The first radiation treatment of 26 patients was performed by conventional external beam radiotherapy. Twenty-three patients were planned with the CadPlan treatment planning system (Varian, version R.6.4.7) and 3 patients with the XIO planning system (Elekta-CMS, version 4.3.3.12). One patient was initially treated with SBRT. Dose summation poses many challenges. These challenges are not only related to technical issues, such as the absence of standardized output of dose matrices, but also to more fundamental issues such as the deformed patient’s anatomy between the 1st and 2nd treatment course. Here, dose summation was carried out by using a simple approximation: dose point calculation. The maximum dose of the critical organ of interest was traced in the planning CT scan of the first radiation treatment. Subsequently, the corresponding slice of the planning CT scan of the 2nd treatment was identified and the maximum dose inside the organ at that slice was added to the maximum dose of the 1st treatment.
To keep the toxicity to a minimum, constraints for organs at risk were used during treatment planning of the re-irradiation treatment. For rectum and bowel, the cumulative maximum dose was 110 Gy3. A maximum volume of 10 cc bowel or rectum was allowed to receive a higher dose. The cumulative maximum dose to the bladder was 120 Gy3, where 10 cc of bladder was allowed to get a higher dose. However, if the tumor was located inside the rectum, bowel or bladder, then a larger volume was allowed to receive a dose higher than the allowed maximum dose. For each organ, the volumes receiving more dose than the prescribed constraints were determined. The total dose given to an organ at risk with external beam radiation in the overlapping treatment volume was calculated in EQD2 (e.g.: 50 Gy in 2 Gy/fraction is 50 Gy EQD2). This dose was subtracted from the maximum dose allowed by the constraint (e.g.: 110 Gy3 for the bowel, in our example: 110 Gy-50Gy3 is 60Gy3, so 60Gy3 is left). This dose in EQD2 was recalculated to a dose per fraction and the total dose in the SBRT plan (e.g. for a patient who will receive 6 fractions, a total dose of 34.2 Gy3 equals 60 Gy3 EQD2). The OAR in the SBRT plan was contoured only in the overlapping area. The percentage of the organs at risk receiving this dose (in our example 34.2 Gy3) was looked up in the On Target planning system. By multiplying this percentage with the volume of the delineated organ at risk in the overlapping treatment volume, the volumes of the organ at risk receiving a cumulative maximum dose higher than the constraint was derived.
Toxicity and Efficacy
The toxicity and efficacy of the re-irradiation was scored for each patient, by collecting response, time of response and complications from patient’s hospital medical records. Twenty-one patients were followed by diagnostic scans every 3 to 6 months. In six patients there was no diagnostic scan, because of short survival after the treatment or due to progression of the disease. The response to the radiation in patients treated with palliative intention was scored by looking at the reduction in tumor size, pain and bleeding. In the patients treated with curative intention, the response was scored by recurrence free interval. Toxicity was scored with the RTOG toxicity criteria (16). Patients were followed by the radiation oncologist, 3 months after the radiation. Three patients were lost to follow up. The median follow up after SBRT re-irradiation was 15 months (range: 2-52 months).
Results
Efficacy and Survival
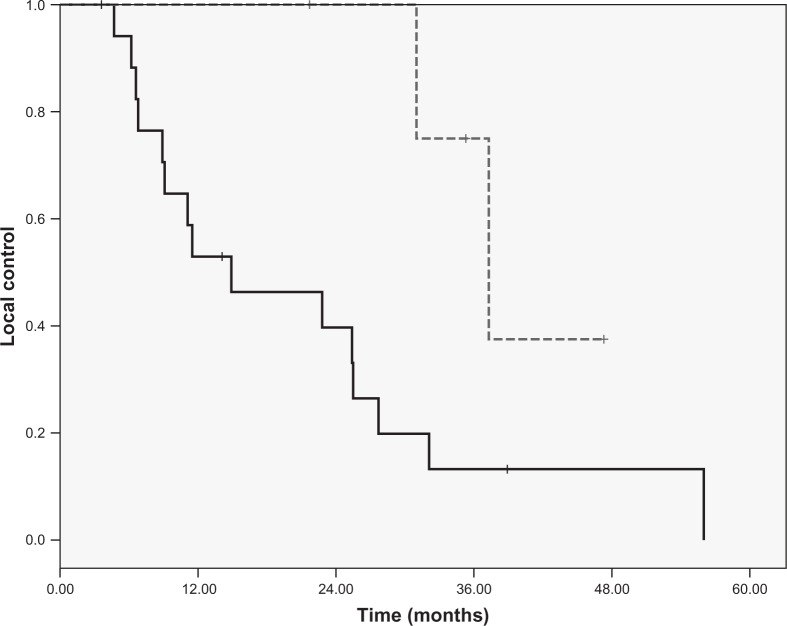
Ninety-six percent of the patients responded to the stereotactic re-irradiation. Reduction in tumor size and pain reduction were the main effects after stereotactic re-irradiation, seen in respectively 100% and 95% of the patients. In 75% of the patients there was a reduction in bleeding. The 1-year and 2-year local control rate for the whole group was 64% and 53%, respectively. Looking at the two different groups with patients classified by the dose given by SBRT, the 1-year and 2-year local control rate for the group of patients receiving 60 Gy or less was 53% and 40%, respectively. Whereas the 1-year and 2-year local control rate for the group of patients who received more than 60 Gy was 100% (Figure 1). This difference was significant (p = 0.04). The median overall survival for all patients was 14 months (range: 2-56 months). The 1-year and 2-years overall survival was 52% and 37%, respectively. The 1-year overall survival of patients treated with 60 Gy or less was 45%. Whereas the 1-year overall survival of patients treated with doses higher than 60 Gy was 71%. The difference in overall survival was not significant (p = 0.2).
Figure 1:
Local control of patients treated with high (-) (>60 Gy) and low (--) (#60 Gy).
Radiation Dose
The median maximum SBRT re-irradiation dose, converted to an equivalent dose in 2-Gy fractions, was 90 Gy3 (range: 42-420 Gy3). The median PTV size was 154 cc (range: 6.7-1114.5 cc). The median maximum cumulative dose (1st + 2nd treatment) was 152 Gy3 (range: 93-468 Gy3). This resulted for the rectum and bowel in a median cumulative dose of 104 and 98 Gy3, respectively (Table III). The rectum and bowel of 7 patients received more than 110 Gy. The median volume of normal tissue that received a dose above 110 Gy3 was 11.6 cc (range: 0.7-99 cc). The constraint that no more than 10 cc should receive more than 110 Gy3 was exceeded in 3 of 7 patients. For these 3 patients, the volume of bowel or rectum receiving more than 110 Gy was 36 cc, 38 cc and 99 cc. The 3 patients had a local recurrence in the pelvis, close to or growing in the bowel/rectum. The median cumulative dose for bladder was 113 Gy3. Five patients received a dose above 120 Gy3 on the bladder. The median volume of the bladder tissue receiving a dose above 120 Gy3 was 1.7 cc (range: 0.0-17 cc).
Table III.
Cumulative dose (EQD2 / α/β = 3 Gy) given to the organs at risk after stereotactic re-irradiation.
| Median | Mean | Min | Max | No. of patients | |
|---|---|---|---|---|---|
| Bowel | 98 | 97 | 56 | 144 | 18 |
| Rectum | 104 | 103 | 65 | 129 | 10 |
| Bladder | 113 | 116 | 79 | 235 | 13 |
Toxicity
There were no patients with grade 5, 4 or 3 acute toxicity after re-irradiation by stereotactic radiotherapy (Table IV). Grade 2 acute pain was seen in 7% of the patients. Seven percent of the patients had grade 2 acute skin toxicity. Grade 2 acute nausea was seen in 11% of the patients. Grade 2 vomiting and diarrhea was seen in 4% of the patients. Grade 1 acute pain was seen in 15% of the patients. Seven percent of the patients had grade 1 acute skin toxicity. Grade 1 dysphagia, nausea and diarrhea was seen in 4% of the patients.
Table IV.
Toxicity.
| Acute toxicity (%) | Late toxicity (%) | ||||
|---|---|---|---|---|---|
| RTOG | grd 1 | grd 2 | RTOG | grd 1 | grd 2 |
| Pain | 15 | 7 | Pain | 0 | 4 |
| Skin | 7 | 7 | Skin | 0 | 4 |
| Diarrhea | 4 | 4 | Diarrhea | 4 | 0 |
| Proctitis | 0 | 0 | Abdominal pain | 0 | 0 |
| Nausea | 4 | 11 | Nausea | 0 | 0 |
| Vomiting | 0 | 4 | Incontinence | 0 | 0 |
| Dysuria | 4 | 0 | Dysuria | 11 | 4 |
| Dysphagia | 4 | 0 | Nerve complaints | 15 | 4 |
| Limb dysfunction | 4 | 7 | |||
*RTOG: Radiation therapy oncology group.
None of the patients experienced clinically grade 5, 4 or 3 late toxicity. Grade 2 pain, skin, nerve complaints and dysuria was seen in 4% of the patients. Seven patients experienced grade 2 limb dysfunction. These patients had moderate stiffness and pain of their joint due to the radiation, which was controlled by pain medication. Grade 1 nerve complaints were seen in 15% of the patients. They experienced mild numbness in a limb. Grade 1 dysuria was seen in 11% of the patients.
Four out of seven patients who received a total re-irradiation dose above 110 Gy3 on the bowel and rectum experienced low grade toxicities: one patient experienced grade 1 acute pain toxicity. This patient also experienced grade 2 late nerve complaints. The other three patients experienced late grade 1 and 2 dysuria and acute grade 1 nausea and diarrhea.
Discussion
In our study, 96% of the patients responded on the stereotactic re-irradiation. Tumor reduction and pain reduction were the main effect, in 100% and 95% of the patients, respectively. This effect of re-irradiation with SBRT corresponds with the results of previous studies on re-irradiation with conventional radiotherapy. Lingareddy et al. (13) reported high symptomatic response in patients with recurrent rectal cancer. The bleeding was palliated in all patients. Pain and reduction of tumor mass was seen in respectively 65% and 24% of the patients (17). The same effect was seen in the long term results. The palliative effect of re-irradiation was 100% for bleeding. Pain and reduction of tumor mass was seen in respectively 55% and 25% of the patients (18). Juffermans et al. (10) evaluated that good or complete palliative effect was achieved in 72% of the patients with recurrent colorectal carcinoma, also after conventional radiotherapy (19). To evaluate if the dose of the SBRT re-irradiation has impact on the outcome, the patients were divided in 2 groups. One group of patients was treated with a high stereotactic re-irradiation dose of more than 60 Gy3. The second group of patients was treated with a low stereotactic re-irradiation dose 60 Gy3 or less. The group of patients treated with high SBRT re-irradiation dose the 1-year and 2-year local control rate control rate was 100%. The second group of patients had a 1-year and 2-year local control rate of 53% and 40%, respectively. Although the number of patients in the both patients group were not equally, the local control in patients treated with higher stereotactic re-irradiation dose is significant better than in patients treated with lower stereotactic re-irradiation dose. The one year overall survival rate in the group treated with high SBRT re-irradiation dose was 71%, whereas the one year overall survival in the group treated with low SBRT re-irradiation dose was 45%. These differences in overall survival weren’t significant. Although there aren’t many reports on SBRT re-irradiation, the found local control for the patients with high stereotactic radiation dose corresponds with other studies evaluating the use of stereotactic radiotherapy for re-irradiation. Roh et al. (20) evaluated stereotactic re-irradiation in head and neck cancer patients. All patients had previously been treated with full-dose irradiation ranging between 39.6 to 134.4 Gy. The reirradiation dose ranged from 30 to 40 Gy delivered in 3 to 5 fractions. The 1 and 2 year local recurrencefree survival rates were 61% and 52%, respectively. The 1 and 2 years overall survival rates were 52% and 31%, respectively. The response rate was 43%. There was no grade 4 acute toxicity reported. In 8% of the patients there was grade 4 late toxicity (20).
In this study the median maximum SBRT re-irradiation dose given was 90 Gy3.The median maximum cumulative dose given after external beam radiation and SBRT radiation was 154 Gy3. This resulted in a median cumulative dose given to the rectum and bowel of 104 Gy3 and 98 Gy3, respectively. In previous studies on conventional radiotherapy re-irradiation, the reported dose to the rectum is around 100 Gy3 (10, 17, 18, 21). Because of the low toxicity reported and the use of SRT with the steep dose fall off and the small volumes, we used a constraint of a cumulative maximum dose of 110 Gy3 with a maximum of 10 cc tissue for rectum and bowel. Seven patients received a dose above 110 Gy3 on the rectum or bowel. Three of these patients exceeded the maximum volume of tissue, respectively 35.8 cc, 38 cc and 99 cc. In all these three patients the tumor was a local recurrence in the pelvis, close to the bowel or in the rectum. Because no other good treatment options existed for these patients with tumor in the organs at risk and with clinically a lot of pain, this was accepted by patient and physician. The bladder received a median cumulative dose of 114 Gy3. From animal studies and daily practice, it is know that the tissue of the bladder can handle a higher dose than the tissue of rectum and bowel. Therefore, the constraint for the bladder was set on a cumulative maximum dose of 120 Gy3 with a maximum of 10 cc tissue. Five patients received doses above 120 Gy3. Two of them exceeded slightly the maximum volume constraint for normal tissue (15 cc and 16 cc) with a good coverage of the tumor, so this was accepted.
Despite the high cumulative dose given with SBRT reirradiation after an initial external beam radiation, the toxicity was rather low. There was no grade 4 or 3 acute or late toxicity. Only grade 2 acute toxicity was seen. Although 89% of the patients had a longer follow than 6 months, the question raises if there would have been more late toxicity if the survival of the whole group was longer than 2 years. On the other hand, it corresponds with the low percentage of grade 3 and 4 acute and late toxicity reported in other studies with conventional external beam radiation (10, 17, 18, 22, 23). Gagnon et al. (14) evaluated the effects of SBRT on pain and quality-of-life outcomes in patients with spinal tumors mainly previously treated with conventional radiotherapy to a median dose of 35 Gy. There were no grade 3 or 4 acute toxicities and 2 patients experienced grade 4 late toxicity after a re-irradiation dose of 21.05 Gy in 3 fractions (14).
In our study we have shown that stereotactic re-irradiation has a high response with low toxicity. This could be explained by the capability of the stereotactic radiation device to deliver many non-coplanar beams from a wider solid angle, resulting in a rather higher degree of dose fall-off gradient outside the PTV. This leads to less normal tissue being included in the high-dose region which leads to lesser toxicity.
However, the design of this study can be discussed, because of the retrospective character and the small number of patients. Another point of discussion could be the dose summation of both irradiation treatments, which was done by a point-dose addition. Non-rigid registration techniques that yield an anatomical coherent correspondence between tissue of the 1st and 2nd CT are required to accumulate dose in three dimensions. The development of those non-rigid registration techniques is in progress (24-26). However, validation is still challenging. To compensate for the different fraction doses used in this study, the physical dose was converted to a biologically equivalent dose using the linear-quadratic model (LQ-model). Many studies used the LQ-model to convert relatively high fraction doses (27-30). Some studies conclude that this model may have limitations if the dose per fraction is too high, other conclude that it could be used looking at the survival data. Several groups have tried to design a new model (30, 31). All these studies do not implicate the dose summation in re-irradiation. Besides a validated alternative model has not yet been established.
In conclusion, re-irradiation to the same region using extracranial stereotactic radiotherapy is feasible and resulted in a 96% symptomatic response with low toxicity.
Footnotes
Conflict of Interest: There is no conflict of interest.
Reference
- 1.Rades D., Rudat V., Veninga T., Stalpers L. J., Hoskin P. J., Schild S. E. Prognostic factors for functional outcome and survival after reirradiation for in-field recurrences of metastatic spinal cord compression. Cancer 11, 1090–1096 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Nieder C., Grosu A. L., Andratschke N. H., Molls M. Update of human spinal cord reirradiation tolerance based on additional data from 38 patients. Int J Radiat Oncol Biol Phys 66, 1446–1449 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Morris D. E. Clinical experience with retreatment for palliation. Semin Radiat Oncol 10, 210–221 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Nieder C., Grosu A. L., Andratschke N. H., Molls M. Proposal of human spinal cord reirradiation dose based on collection of data from 40 patients. Int J Radiat Oncol Biol Phys 61, 851–855 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Stewart F. A., van der Kogel A. J. Retreatment Tolerance of Normal Tissues. Semin Radiat Oncol 4, 103–111 (1994). [DOI] [PubMed] [Google Scholar]
- 6.Stewart F. A. Re-treatment after full-course radiotherapy: Is it a viable option? Acta Oncologica 38, 855–862 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Nieder C., Milas L., Ang K. K. Tissue tolerance to reirradiation. Semin Radiat Oncol 10, 200–209 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Ruifrok A. C., Kleiboer B. J., van der Kogel A. J. Reirradiation tolerance of the immature rat spinal cord. Radiother Oncol 23, 249–256 (1992). [DOI] [PubMed] [Google Scholar]
- 9.Abusaris H., Storchi P. R., Brandwijk R. P., Nuyttens J. J. Second re-irradiation: Efficacy, dose and toxicity in patients who received three courses of radiotherapy with overlapping fields. Radiother Oncol 99, 253–259 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Juffermans J. H., Hanssens P. E., van Putten W. L., van Rhoon G. C., van Der Z. J. Reirradiation and hyperthermia in rectal carcinoma: a retrospective study on palliative effect. Cancer 98, 1759–1766 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Das P., Delclos M. E., Skibber J. M., Rodriguez-Bigas M. A., Feig B. W., Chang G. J., Eng C., Bedi M., Krishnan S., Crane C. H. Hyperfractionated Accelerated Radiotherapy for Rectal Cancer in Patients with Prior Pelvic Irradiation. Int J Radiat Oncol Biol Phys 77, 60–65 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Mohiuddin M., Marks G., Marks J. Long-term results of reirradiation for patients with recurrent rectal carcinoma. Cancer 95, 1144–1150 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Lingareddy V., Ahmad N. R., Mohiuddin M. Palliative reirradiation for recurrent rectal cancer. Int J Radiat Oncol Biol Phys 38, 785–790 (1997). [DOI] [PubMed] [Google Scholar]
- 14.Gagnon G. J., Nasr N. M., Liao J. J., Molzahn I., Marsh D., McRae D., Henderson F. C., Sr. Treatment of spinal tumors using cyberknife fractionated stereotactic radiosurgery: pain and quality-of-life assessment after treatment in 200 patients. Neurosurgery 64, 297–306 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Kunos C., Chen W. H., Debernardo R., Waggoner S., Brindle J., Zhang Y., Williams J. R., Einstein D. B. Stereotactic Body Radiosurgery for Pelvic Relapse of Gynecologic Malignancies. Technol Cancer Res Treat 8, 393–400 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Cox J. D., Stetz J., Pajak T. Toxicity criteria of the radiation therapy oncology group (RTOG) and the european organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys 31, 1341–1346 (1995). [DOI] [PubMed] [Google Scholar]
- 17.Lingareddy V., Ahmad N. R., Mohiuddin M. Palliative reirradiation for recurrent rectal cancer. Int J Radiat Oncol Biol Phys 38, 785–790 (1997). [DOI] [PubMed] [Google Scholar]
- 18.Mohiuddin M., Marks G., Marks J. Long-term results of reirradiation for patients with recurrent rectal carcinoma. Cancer 95, 1144–1150 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Juffermans J. H. M., Hanssens P. E. J., van Putten W. L. J., Van Rhoon G. C., van der Zee J. Reirradiation and hyperthermia in rectal carcinoma – A retrospective study on palliative effect. Cancer 98, 1759–1766 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Roh K. W., Jang J. S., Kim M. S., Sun D. I., Kim B. S., Jung S. L., Kang J. H., Yoo E. J., Yoon S. C., Jang H. S., Chung S. M., Kim Y. S. Fractionated stereotactic radiotherapy as reirradiation for locally recurrent head and neck cancer. Int J Radiat Oncol Biol Phys 74, 1348–1355 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Das P., Declos M., Skibber J., Rodriguez-Bigas M., Feig B., Chang G., Eng C. Hyperfractionated accellerated radiotherapy for rectal cancer in patients with prior pelvic irradiation. Int J Radiat Oncol Biol Phys 77, 60–65 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Ebara T., Noriko T., Etoh T., Schischi I., Honda A., Nakajima N. Palliative Re-irradiation for In-field Recurrence after Definitive Radiotherapy in Patients with Primary Lung Cancer. Anticancer Res 27, 531–534 (2007). [PubMed] [Google Scholar]
- 23.Okamoto Y., Murakami M., Yoden E., Sasaki R., Okuno Y., Nakajima T., Kuroda Y. Reirradiation for locally recurrent lung cancer previously treated with radiation therapy. Int J Radiat Oncol Biol Phys 52, 390–396 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Bondar L., Hoogeman M. S., Osorio E. M. Vasquez, Heijmen B. J. A Symmetric nonrigid registration method to handle large organ deformations in cervical cancer patients. Med Phys 37, 3760–3772 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Osorio E. M. Vasquez, Hoogeman M. S., Bondar L., Levendag P. C., Heijmen B. J. A novel flexible framework with automatic feature correspondence optimization for nonrigid registration in radiotherapy. Med Phys 36, 2848–2859 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Osorio E. M. Vasquez, Hoogeman M. S., Teguh D. N., Al-Mamgani A., Kolkman-Deurloo I. K., Bondar L., et al. Three-Dimensional Dose Addition of External Beam Radiotherapy and Brachytherapy for Oropharyngeal Patients Using Nonrigid Registration. Int J Radiat Oncol Biol Phys 80, 1268–1277 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Fowler J. F., Tome W. A., Fenwick J. D., Mehta M. P. A challenge to trational radiation oncologie. Int J Radiat Oncol Biol Phys 60, 1241–1256 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Kirkpatrick J. P., Meyer J. J., Marks L. B. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol 18, 240–243 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Brenner D. J. The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction. Semin Radiat Oncol 18, 234–239 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L., Bassano D. A., Prasad S. C., Hahn S. S., Chung C. T. The linear-quadratic model and fractionated stereotactic radiotherapy. Int J Radiat Oncol Biol Phys 57, 827–832 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Wang J. Z., Huang Z., Lo S. S., Yuh W. T., Mayr N. A. A generalized linear-quadratic model for radiosurgery, stereotactic body radiation therapy, and high-dose rate brachytherapy. Sci Transl Med 2, 39–48 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Guerrero M., Li X. A. Extending the linear-quadratic model for large fraction doses pertinent to stereotactic radiotherapy. Phys Med Biol 49, 4825–4835 (2004). [DOI] [PubMed] [Google Scholar]