Abstract
Our objective was to determine if protons allow for the expansion of treatment volumes to cover high-risk nodes in patients with regionally advanced non-small-cell lung cancer. In this study, 5 consecutive patients underwent external-beam radiotherapy treatment planning. Four treatment plans were generated for each patient: 1) photons (x-rays) to treat positron emission tomography (PET)-positive gross disease only to 74 Gy (XG); 2) photons (x-rays) to treat high-risk nodes to 44 Gy and PET-positive gross disease to 74 Gy (XNG); 3) protons to treat PET-positive gross disease only to 74 cobalt gray equivalent (PG); and 4) protons to treat high-risk nodes to 44 CGE and PET-positive gross disease to 74 CGE (PNG). We defined high-risk nodes as mediastinal, hilar, and supraclavicular lymph nodal stations anatomically adjacent to the foci of PET-positive gross disease. Four-dimensional computed tomography was utilized for all patients to account for tumor motion. Standard normal-tissue constraints were utilized. Our results showed that proton plans for all patients were isoeffective with the corresponding photon (x-ray) plans in that they achieved the desired target doses while respecting normal-tissue constraints. In spite of the larger volumes covered, median volume of normal lung receiving 10 CGE or greater (V10Gy/CGE), median V20Gy/CGE, and mean lung dose were lower in the proton plans (PNG) targeting gross disease and nodes when compared with the photon (x-ray) plans (XG) treating gross disease alone. In conclusion, proton plans demonstrated the potential to safely include high-risk nodes without increasing the volume of normal lung irradiated when compared to photon (x-ray) plans, which only targeted gross disease.
Keywords: Proton therapy, Normal-tissue sparing, Non-small-cell lung cancer, Elective nodal irradiation
Introduction
Overall survival and local disease control are poor in regionally advanced non-small-cell lung cancer. Radiation dose escalation is one strategy for improved disease control. Another strategy involves treatment of the regional nodes that are at high risk for harboring subclinical disease. Patients with regionally advanced non-small-cell lung cancer often have compromised pulmonary function related to chronic obstructive pulmonary disease. Consequently, normal-tissue constraints related to both radiation dose and volume of lung irradiated often compel the clinician to reduce the radiation dose and/or volume. Specifically, the radiation oncologist may feel pressured to choose between radiation dose escalation to the site of gross disease and treatment of high-risk regional lymph nodes. Current thought favors dose escalation to the site of gross disease over the elective treatment of high-risk nodes (1–5). Although clinically identified nodal failure in surviving patients is rare, only 9% in the Memorial Sloan Kettering series (2), surgical data indicates that current clinical staging modalities, including positron emission tomography (PET) imaging, may understage the mediastinal, hilar, and supraclavicular nodal stations in 39% of stage III patients (6). Since patients with failure in these nodal stations may go on to develop disseminated disease before such regional failure becomes evident, it is reasonable to believe that if elective nodal irradiation (ENI) to a dose adequate to eradicate subclinical disease (40 to 45 Gray [Gy]) could be delivered without undue risk of normal-tissue damage and without compromising dose to the site of gross disease, local-regional-disease control and overall survival might be improved.
Protons, by virtue of their unique dosimetric properties, may offer an opportunity to safely achieve both strategies of dose escalation to the primary tumor and adequate coverage of high-risk regional nodes. Currently, conventional photon (x-ray) dose distributions are optimized within a given volume by the delivery of multiple beams of x-rays that intersect over the target, but do not overlap non-targeted tissue. This strategy is used in most contemporary treatment protocols, such as three-dimensional conformal radiotherapy (3DCRT) and intensity-modulated radiotherapy (IMRT), and results in a high dose to the target and a low dose to a large volume of non-targeted tissue. When protons are used, dose distributions can be further optimized by exploiting the “spread-out Bragg peak.” Unlike x-rays, protons are particles that can be accelerated to penetrate to a specific depth in tissue where they stop and discharge most of their energy in what is called a “Bragg peak,” after which there is no exit dose. Additionally, there is a lower relative entrance dose with protons than with x-rays. The improved conformality of dose distribution to the target may be particularly important in lung cancer where the target is proximate to the esophagus, heart, and spinal cord, which are all organs with relevant dose-related toxicities, and, more importantly, surrounded by an exquisitely radiosensitive normal structure: the lung.
The purpose of this study was to compare dose distributions with conventional radiotherapy (RT) to proton therapy in an effort to determine if the dosimetric advantages of protons can be leveraged to permit both dose escalation and elective regional node irradiation in advanced non-small-cell lung cancer.
Materials and Methods
Five consecutive patients referred for treatment of regionally advanced non-small-cell lung cancer underwent external-beam RT treatment planning. All patients signed an informed consent to participate in clinical research on the IRB- approved University of Florida Proton Therapy Institute Outcomes Tracking Protocol. Four patients presented with stage IIIA disease and 1 with an isolated right hilar recurrence 2 years after successful stereotactic RT for a T1N0 squamous cell cancer of the right lung. The presenting characteristics for the 5 patients are shown in Table I.
Table I.
Pretreatment characteristics.
| Patient No. | AJCC stage | Primary tumor location | Primary tumor maximum size | Nodal location(s) | Maximum nodal size |
|---|---|---|---|---|---|
| 1 | IIIA (T3N2) | Right middle lobe | 5.5 cm | Right hilar; paratracheal; subcarinal | 2cm |
| 2 | IIIA (T2N2) | Left upper lobe | 4 cm | Left hilar; left paratracheal | 2 cm |
| 3 | IIIA (T2N2) | Left upper lobe | 4.7 cm | Left hilar; AP window; left paratracheal | 4.7 cm (contiguous with primary) |
| 4 | Right hilar recurrence | Right hilum | NA | Right hilar | 2.0 cm |
| 5 | IIIA (T1N2) | Right lower lobe | 2.0 cm | Right hilar/ paratracheal | 1.5 cm |
Four-dimensional imaging was utilized in the planning process for all patients to account for tumor motion. Patients were imaged on a Philips Brilliance large bore computed tomography (CT) scanner with a 60-cm field of view and 1-mm slices (Philips Healthcare, Amsterdam, the Netherlands). Free-breathing CT (FBCT) images with intravenous contrast were captured as well as a 10-phase image set to assess tumor motion through the full respiratory cycle. PET CT imaging was available for all patients although images were not fused with the planning CTs. Gross tumor volume (GTV), corresponding to PET-positive deposits of gross disease was contoured on the FBCT image set. An internal GTV (IGTV) was established by expanding the initial GTV to include the GTVs for each phase of the breathing cycle. The high-risk clinical target volume (CTV) was then based on the IGTV plus a 6-mm expansion into adjacent lung parenchyma. There was no expansion of the CTV into bone, chest wall, or mediastinal structures.
A standard-risk CTV was also established consisting of the internal CTV of the high-risk regional lymph nodes for each patient. We defined high-risk nodes as mediastinal, hilar, and/or supraclavicular lymph nodal stations anatomically adjacent to foci of PET-positive gross disease. The University of Michigan CT atlas was used to define the nodal stations (6). A 5-mm PTV expansion was placed on all CTV targets to account for setup uncertainty.
Optimized three-dimensional conformal photon (x-ray) plans were generated on an ADAC Pinnacle planning computer.
Double-scattered proton plans were generated on the Varian Eclipse planning system (Varian Medical Systems Inc., Palo Alto, CA). For the proton plans, the aperture margins covered the PTV plus 1 cm. Distal and proximal spread-out Bragg peak expansions of 5 mm and 8 mm smearing margins were utilized for each beam. Beam angles were selected to either avoid the treatment table completely or to enter the table in an en face or slightly oblique position. Gantry angles, which intersected the table edge, were avoided.
Photon (x-ray) doses are stated in Gy, while proton doses are stated in Cobalt Gray Equivalent (CGE) using the accepted relative biologic effectiveness multiplier of 1.1. Four optimized plans were generated for each patient: 1) photons (x-rays) to treat the high-risk CTV (PET-positive gross disease only) to 74 Gy (XG); 2) photons (x-rays) to treat the high-risk CTV (PET-positive gross disease) to 74 Gy and the standard-risk CTV (high-risk nodes) to 44 Gy (XNG); 3) protons to treat the high-risk CTV (PET-positive gross disease only) to 74 CGE (PG); and 4) protons to treat the high-risk CTV (PET-positive gross disease) to 74 CGE and the standard-risk CTV (high-risk nodes) to 44 CGE (PNG). For all plans, the maximum spinal cord dose (to 0.1cc) was limited to 50.50 Gy/CGE. The normal lung (total lung - GTV) V20 Gy/CGE was limited to 37%. During planning we also attempted to limit the mean esophageal dose to 34Gy/CGE, the esophageal V60 Gy/CGE to 40%, the cardiac V60 Gy/CGE to 30%, and the cardiac V40 Gy/CGE to 50%. For each CTV target, 100% of the target received at least 95% of the prescribed dose, and at least 95% of each CTV target received 100% of the prescribed dose.
Results
The median (range) pulmonary V10 Gy/CGE, V20 Gy/CGE, and mean lung doses (MLD) are shown in Table II for each group’s treatment plans.
Table II.
Median (range) of normal lung exposures for each of the 4 groups of treatment plans.
| V10 Gy (CGE) | V20 Gy (CGE) | Mean lung dose | |
|---|---|---|---|
| XG | 31% | 26% | 18 Gy |
| (range, 20%-40%) | (range, 17%-31%) | (range, 13-20 Gy) | |
| XNG | 37% | 30% | 20 Gy |
| (range, 31%-41%) | (range, 23%-32%) | (range, 16-21 Gy) | |
| PG | 21% | 17% | 9 CGE |
| (range, 13%-25%) | (range, 11%-21%) | (range, 6-12 CGE) | |
| PNG | 25% | 22% | 13 CGE |
| (range, 21%-32%) | (range, 17%-24%) | (range, 10-14 CGE) |
XG = photons (x-rays) for positron emission tomography (PET)-positive gross disease only to 74 Gy; XNG = photons (x-rays) to treat high-risk nodes to 44 Gy and PET-positive gross disease to 74 Gy; PG = protons to treat PET-positive gross disease only to 74 CGE; PNG = protons to treat high-risk nodes to 44 CGE and PET-positive gross disease to 74 CGE.
The median (range) spinal cord (0.1 cm3) doses, median (range) mean esophageal doses, median (range) esophageal V60 Gy/CGE, median (range) cardiac V40 Gy/CGE, and median (range) cardiac V60 Gy/CGE are shown in Table III for each group’s treatment plans.
Table III.
Spinal Cord, esophageal, and cardiac normal tissue exposures for each of the 4 groups of treatment plans.
| XG | XNG | PG | PNG | |
|---|---|---|---|---|
| Spinal Cord Dose to 0.1 cm3 – | 18.26 Gy | 38.90 Gy | 10.52 CGE | 21.12 CGE |
| Median (Range) | (2.25 to 31.30 Gy) | (15.00 to 45.82 Gy) | (0 to 38.28 CGE) | (0.93 to 35.21 CGE) |
| Mean Esophageal Dose – | 10.05 Gy | 27.25 Gy | 6.76 CGE | 17.55 CGE |
| Median (Range) | (1.70 to 40.77 Gy) | (13.71 to 44.36 Gy) | (0.09 to 28.42 CGE) | (7.56 to 34.45 CGE) |
| Esophageal V60 Gy/CGE – | 2.9% | 6.7% | 0% | 0% |
| Median (Range) | (0 to 42.9%) | (0 to 47.2%) | (0 to 27.4%) | (0 to 32.9%) |
| Cardiac V40 Gy/CGE – | 2.6% | 9.2% | 1.2% | 4.8% |
| Median (Range) | (0 to 17.5%) | (0 to 17.1%) | (0 to 8.6%) | (0 to 10.7%) |
| Cardiac V60 Gy/CGE – | 1.6% | 2.7% | 0.6% | 1.6% |
| Median (Range) | (0 to 13.2%) | (0 to 11.5%) | (0 to 38.28 CGE) | (0 to 3.8%) |
XG = photons (x-rays) to positron emission tomography (PET)-positive gross disease only to 74 Gy; XNG = photons (x-rays) to treat high-risk nodes to 44 Gy and PET-positive gross disease to 74 Gy; PG = protons to treat PET-positive gross disease only to 74 CGE; PNG = protons to treat high-risk nodes to 44 CGE and PET-positive gross disease to 74 CGE.
In each of the 5 patients, V10 Gy/CGE, V20 Gy/CGE, and MLD parameters were superior in the PG plans compared to the XG plans. These parameters were also superior in the PNG plans compared to the XNG plans.
In spite of the larger volume irradiated, the median V10 Gy/CGE, V20 Gy/CGE, and MLD were lower for the proton PNG plans (which included high-risk nodes) compared with the photon (x-ray) XG plans which covered only gross PET positive disease.
The highest V20 CGE in any of the PNG plans was only 24%. The highest MLD in any of the PNG plans was only 14 CGE.
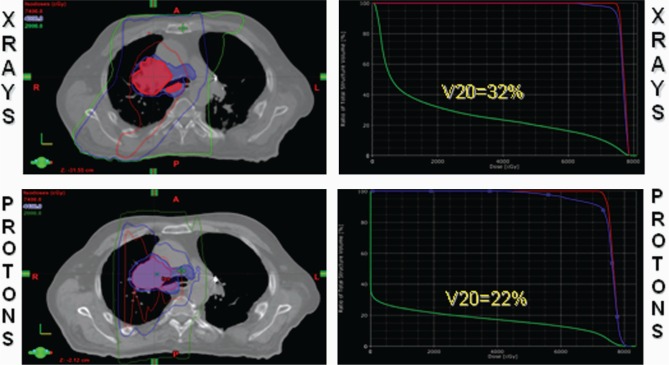
While the proton plans offered superior lung sparing compared to the isovolumetric photon (x-ray) plans for all 5 patients, protons offered the greatest lung-sparing benefit in the patient with gross mediastinal disease that involved the subcarinal lymph nodes (Figure 1). Specifically, by manipulating the spread-out Bragg peak, protons allowed for treatment of the subcarinal lymph nodes (as well as other midline and contralateral mediastinal nodes) without having to resort to deep tangential fields, which inevitably irradiate large swaths of ipsilateral and contralateral normal lung parenchyma. In this setting, the majority of the dose with protons could be delivered with anterior and posterior fields.
Figure 1:
A patient with AJCC stage T3N2 non-small-cell carcinoma of the right middle lobe with gross subcarinal involvement. Avoiding deep tangential fields with protons results in a significant improvement in the normal lung V20 Gy/CGE from 32% to 22%.
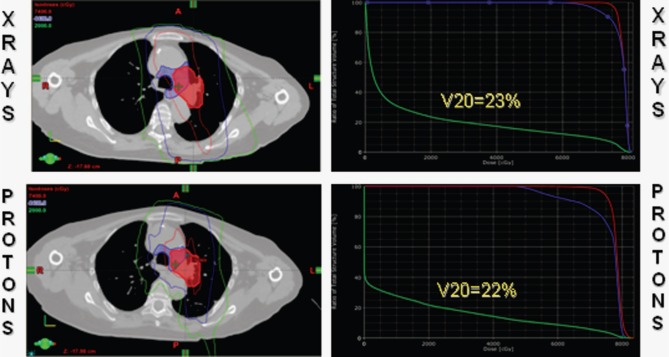
The benefit of protons was less dramatic in the patient whose gross disease was well lateralized (Figure 2) since the majority of the dose with photons (x-rays) or protons, could be delivered without the use of deep tangential fields. Even in this case, however, while the normal-lung V20 Gy/CGE was only reduced from 23% to 22%, the MLD was decreased from 16 Gy to 13 CGE.
Figure 2:
A patient with a well lateralized T2N2 non-small-cell carcinoma of the left upper lobe with direct extension into the aorto-pulmonary window. Protons reduced the mean lung dose from 16 Gy to 13 CGE, although the pulmonary V20 Gy/CGE was virtually unchanged.
The proton plans’ reduction of normal lung exposure was not achieved by transferring dose to other critical intrathoracic structures such as the spinal cord, esophagus or heart. In fact, the median spinal cord, esophageal, and cardiac exposures were lower for the isovolumetric proton plans compared to the photon (x-ray) plans.
Discussion
While the potential benefits of dose escalation in lung cancer are accepted, the value of elective nodal irradiation (ENI) in the setting of locally advanced non-small-cell lung cancer remains one of the more intensely debated controversies within the field of radiation oncology. The arguments supporting ENI are articulated in an editorial by Kelsey et al. (7). While acknowledging the studies by Schild (1), Rosenzweig (2), and Senan (3) that demonstrate a low rate of isolated clinical failure in elective nodal regions when only gross disease is targeted, Kelsey et al point out that surgical series in the PET era demonstrate a 13% (8) to 16% (9) rate of occult nodal dissemination for patients undergoing thoracotomy for early lung cancers. They also highlight the surgical data from Cleveland Clinic which indicates a 39% rate of nodal understaging in patients with documented N2 disease (10). Kelsey et al. emphasize that elimination of even the 9% isolated nodal failure rate appreciated in the Memorial Sloan-Kettering Cancer Center series could potentially result in a significant relative improvement in survival for a group of patients who only have a 15% to 20% expectation of cure. Finally, it is relevant that, when treating with 3DCRT or IMRT, even when the elective nodes are not specifically targeted, these nodal stations often receive radiation doses which may be adequate to control subclinical disease (2,11,12). As such, it is reasonable to believe that the low reported incidence of clinical failure in these nodal stations may be partly due to incidental irradiation.
The most persuasive argument against elective nodal irradiation is that its use increases the volume of normal lung irradiated to the extent that it may produce unacceptable toxicity, particularly in conjunction with dose escalation to the primary target and concurrent chemotherapy. Given the dosimetric limitations of photons (x-rays), it is understandable that a rational clinician might choose a scenario that omits ENI.
Randomized clinical data, which is frequently cited to support the argument in favor of GTV dose escalation over ENI, is provided by Yuan (4). In this study, patients with unresectable stage III non-small cell lung cancer were randomized to receive either: 1) involved-field RT to doses between 68 Gy and 74 Gy or 2) ENI with a GTV boost to a total dose ranging from 60 Gy to 64 Gy. Patients in both arms received concomitant cisplatin-based chemotherapy. Two-year survival was statistically superior in the involved-field arm (39.4% vs. 25.6%; p = 0.048). The pneumonitis rate was also lower in the involved-field arm (17% vs. 29%; p = 0.044). Although this study is often cited to demonstrate the superiority of limiting treatment to the involved field, we would argue that this trial simply demonstrates improved outcomes with higher GTV doses. We do not believe that the study by Yuan should be used to conclude the irrelevance or inadvisability of ENI. We believe that our data suggests that protons have enough of a potential to reduce normal-tissue exposure so that the choice between GTV dose escalation and ENI may be unnecessary.
Conclusion
While our dosimetric findings will need to be validated in clinical practice, it is possible that protons may save us from having to make the difficult choice between dose escalation to gross disease and treatment of high-risk nodal stations. Our current phase II protocol at the University of Florida Proton Therapy Institute (UFPTI LU-02) delivers 80 CGE to PET-positive foci of gross disease as well as 40 CGE to high-risk nodal stations with concomitant weekly chemotherapy.
Abbreviations:
- PET:
Positron Emission Tomography
- ENI:
Elective Nodal Irradiation
- 3DCRT:
Three-dimensional Conformal Radiotherapy
- IMRT:
Intensity-modulated Radiotherapy
- RT:
Radiotherapy.
Footnotes
Conflicts of Interest Statement: The authors have no conflicts of interest to report.
References
- 1. Schild S. E. Elective Nodal Irradiation (ENI) doesn’t appear to provide a clear benefit for patients with unresectable non-small-cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 72, 311–312 (2008). [DOI] [PubMed] [Google Scholar]
- 2. Rosenzweig K. E., Sura S., Jackson A., Yorke E. Involved-field radiation therapy for inoperable non small-cell lung cancer. J Clin Oncol 25, 5557–5561 (2007). [DOI] [PubMed] [Google Scholar]
- 3. Senan S., Burgers S., Samson M. J., van Klaveren R. J., Oei S. S., van Sornsen D. K., Voet P. W., Lagerwaard F. J., Maarten V. H., Aerts J. G., van Meerbeeck J. P. Can elective nodal irradiation be omitted in stage III non-small-cell lung cancer? Analysis of recurrences in a phase II study of induction chemotherapy and involvedfield radiotherapy. Int J Radiat Oncol Biol Phys 54, 999–1006 (2002). [DOI] [PubMed] [Google Scholar]
- 4. Yuan S., Sun X., Li M., Yu J., Ren R., Yu Y., Li J, Liu X., Wang R., Li B., Kong L., Yin Y. A randomized study of involved-field irradiation versus elective nodal irradiation in combination with concurrent chemotherapy for inoperable stage III nonsmall cell lung cancer. Am J Clin Oncol 30, 239–244 (2007). [DOI] [PubMed] [Google Scholar]
- 5. Sulman E. P., Komaki R., Klopp A. H., Cox J. D., Chang J. Y. Exclusion of elective nodal irradiation is associated with minimal elective nodal failure in non-small cell lung cancer. Radiat Oncol 4, 5 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chapet O., Kong F. M., Quint L. E., Chang A. C., Ten Haken R. K., Eisbruch A., Hayman J. A. CT-based definition of thoracic lymph node stations: an atlas from the University of Michigan. Int J Radiat Oncol Biol Phys 63, 170–178 (2005). [DOI] [PubMed] [Google Scholar]
- 7. Kelsey C. R., Marks L. B., Glatstein E. Elective nodal irradiation for locally advanced non-small-cell lung cancer: it’s called cancer for a reason. Int J Radiat Oncol Biol Phys 73, 1291–1292 (2009). [DOI] [PubMed] [Google Scholar]
- 8. Kim B. T., Lee K. S., Shim S. S., Choi J. Y., Kwon O. J., Kim H., Shim Y. M., Kim J., Kim S. Stage T1 non-small cell lung cancer: preoperative mediastinal nodal staging with integrated FDG PET/CT-a prospective study. Radiology 241, 501–509 (2006). [DOI] [PubMed] [Google Scholar]
- 9. Al Sarraf N., Aziz R., Gately K., Lucey J., Wilson L., McGovern E., Young V. Pattern and predictors of occult mediastinal lymph node involvement in non-small cell lung cancer patients with negative mediastinal uptake on positron emission tomography. Eur J Cardiothorac Surg 33, 104–109 (2008). [DOI] [PubMed] [Google Scholar]
- 10. Videtic G. M., Rice T. W., Murthy S., Suh J. H., Saxton J. P., Adelstein D. J., Mekhail T. M. Utility of positron emission tomography compared with mediastinoscopy for delineating involved lymph nodes in stage III lung cancer: insights for radiotherapy planning from a surgical cohort. Int J Radiat Oncol Biol Phys 72, 702–706 (2008). [DOI] [PubMed] [Google Scholar]
- 11. Kepka L., Maciejewski B., Withers R. H. Does incidental irradiation with doses below 50 gy effectively reduce isolated nodal failures in non-small-cell lung cancer: dose-response relationship. Int J Radiat Oncol Biol Phys 73, 1391–1396 (2009). [DOI] [PubMed] [Google Scholar]
- 12. Kimura T., Togami T., Nishiyama Y., Ohkawa M., Takashima H. Impact of Incidental Irradiation on Clinically Uninvolved Nodal Regions in Patients with Advanced Non-Small-Cell Lung Cancer Treated with Involved-Field Radiation Therapy: Does Incidental Irradiation Contribute to the Low Incidence of Elective Nodal Failure? Int J Radiat Oncol Biol Phys (2009). [DOI] [PubMed]