Abstract
Current means of measuring RT-induced fibrosis are subjective. We evaluated the DermaLab suction cup system to measure objectively skin deflection as a surrogate for fibrosis. Sixty-nine patients with E-STS were treated with limb-sparing surgery and 50-66 Grays (Gy) of RT. Using a “scleroderma” DermaLab Suction Cup, the skin stiffness was measured by two clinicians. The National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) scale, the Musculoskeletal Tumor Rating Scale (MSTS) and Toronto Extremity Salvage Score (TESS) questionnaires were completed for each patient. Levels of agreement between measurers were estimated using the Kappa (κ) coefficient and the concordance correlation coefficient (CCC). All sixty-nine patients were included. The level of agreement between measurers for NCI-CTCAE grading was moderate (range κ = 0.41-0.59). The CCC for the elasticity measurements were higher, with CCC = 0.82 for fibrotic skin and CCC = 0.84 for normal skin. The elasticity measurements were significantly higher when MSTS scores were <30 and or TESS scores were <90. Suction Cup measurement of skin elasticity is more reproducible than CTCAE grading and shows promise in generating reproducible measurements for radiation-induced skin fibrosis. Furthermore, it correlates well with the MSTS and TESS.
Keywords: Radiation, Fibrosis, Sarcoma, Elasticity
Introduction
Current protocols for treatment of extremity soft tissue sarcomas (E-STS) include radiation (RT) and limb-sparing surgery (1, 2). Multiple trials have demonstrated the advantages of limb-sparing therapy compared to amputation or surgical resection of entire limb muscle compartments, including better function with comparable disease-free and overall survival (3 -6). However, with the use of external beam RT (EBRT), up to 50% of patients develop clinically detectable fibrosis after receiving 60-65 Grays (Gy) delivered in 2-3 Gy fractions (7, 8), making it one of the most common late effects in this patient population.
This is comparable to many other sites treated with RT. As an example, in patients with head and neck cancers treated with curative doses of RT, up to 45% will experience trismus (9). This manifestation of fibrosis may have adverse effects on chewing, swallowing, maintenance of oral hygiene and pulmonary function. In a more common malignancy, women treated with breast-conserving surgery and a median dose of 55 Gy of RT had 43% risk of fibrosis, 77% of retraction or atrophy of the skin and 54% of pain (10).
Subcutaneous fibrosis (11, 12) is manifested by a loss of pliability and flexibility of the soft tissues down to the muscle layers. The underlying cellular processes of radiation-induced fibrosis are not well understood. However, it is known that normal tissues are replaced by mesenchymal cells and that these cells overproduce extracellular matrix. Transforming growth factor-β has a role in the development of fibrosis via the induction and deposition of extracellular matrix, stimulation of fibroblast growth, collagen deposition, and angiogenic effects on vascular endothelial cells. Radiation has been reported to induce a premature differentiation of fibroblasts into mature collagen-forming fibrocytes.
Symptoms associated with fibrosis vary by anatomic site but may include pain, neuropathy, loss of joint range of motion, and distal lymphedema (13 -16). These symptoms and impairments may, in turn, limit functionality and activity, including difficulty with activities of daily living, mobility and self-care (9, 10).
Current means of measuring and documenting radiation-induced fibrosis are subjective. A common scale is the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) (17) for grading fibrosis in the deep connective tissue. Functional scales include the revised Musculoskeletal Tumor Society Rating Scale (MSTS 1993) (18, 19) and the Toronto Extremity Salvage Score (TESS) (20 -22). Davis et al. (23) noted that existing fibrosis rating scales have limited inter-rater reliability and do not discriminate severity. Consequently, quantitative measures have been developed, using compression or impedance. Although more precise in measuring tissue fibrosis these devices require skilled technicians for application and interpretation of the data, thus limiting their implementation.
Skin elasticity, a surrogate for fibrosis, can be measured through vacuum suction devices. The DermaLab® skin elasticity module (Cortex Technology, Hadsund, Denmark) uses a suction chamber to measure objectively the visco-elastic properties of the skin. This study was designed to analyze the utility of this suction cup device as a means to evaluate radiation related fibrosis in E-STS. The results using the device were also compared to the NCI-CTCAE, MSTS and TESS scores for all patients.
Materials and Methods
The prospective study was reviewed and approved by the local research ethics committee. The study was carried out in the multidisciplinary sarcoma clinic. No funding or material support was provided by the manufacturer of the device.
Skin fibrosis in patients with E-STS undergoing limb-sparing therapy was measured with the suction cup device, the NCICTCAE, the MSTS and the TESS scores. Measurements from the suction cup device were compared to scoring using established subjective scales.
Patients
Inclusion criteria were defined as:
Patients aged 18 years or older having been treated limbsparing surgery with curative intent
Patients having received pre- or post-operative radiation therapy
Patients having received a dose ≥50 Gy, delivered in 2 Gy fractions, at least 90 days prior to registration into the study
Exclusion criteria were defined as:
Patients with active or previous chemotherapy/biotherapy within 30 days of recruitment
Prior brachytherapy
Connective tissue disease known to affect skin elasticity or unhealed wounds
Local tumor recurrence
Recruitment
Patients diagnosed with E-STS between 1998 and 2008 were identified at our multidisciplinary clinic from 2008 to 2010. Patients provided written informed consent and relevant patient, tumour and treatment information were extracted from the medical chart.
Fibrosis Measurements
Skin fibrosis was assessed during a single routine follow-up appointment for each patient, using four different methods: NCI-CTCAE, MSTS, TESS and the suction cup device. Suction cup measurements were performed using a scleroderma probe since this probe allows for use with less elastic tissues as the detectors are placed closer to the skin surface. Measurements were performed with the patients lying down for the lower extremity or sitting with the arm supported for the upper extremity. Two measurements were taken independently by two health care workers, to assess for reproducibility of the process. One measurement was taken on a cleaned area of the skin deemed by the observer to display the most fibrosis. The second measure was taken at a corresponding area on the untreated contralateral extremity. Each investigator also graded connective tissue toxicity along the CTCAE scales. For each patient, MSTS and TESS questionnaires were also completed.
NCI-CTCAE
The NCI-CTCAE v4.03 is a descriptive terminology for adverse events reporting with a grading (severity) scale for each adverse events. The grading system for radiationinduced fibrosis is explained in Table I.
Table I.
NCI-CTCAE v4.03 grading for radiation-induced fibrosis.
| Fibrosis grade | Appearance |
|---|---|
| 0 | Normal skin |
| 1 | Mild induration, able to move skin parallel to plane (sliding) and perpendicular to skin (pinching up) |
| 2 | Moderate induration, able to slide skin, unable to pinch skin; limiting instrumental ADL (including preparing meals, shopping for groceries or clothes, using thetelephone, etc.) |
| 3 | Severe induration, unable to slide or pinch skin; limiting joint movement or orifice (e.g., mouth, anus); limiting self care ADL (bathing, dressing and undressing, feeding self, using the toilet, taking medications, and not bedridden) |
| 4 | Generalized; associated with signs or symptoms of impaired breathing or feeding |
MSTS
The MSTS is a clinician-rated scale that evaluates seven items including pain, joint range of motion, strength, joint stability, joint deformity, overall function, and general acceptance of the treatment. The score ranges from 0 to 35, with the lowest score indicating worse symptoms.
TESS
The TESS is a measure of physical disability developed specifically for patients with extremity sarcoma; the score ranges from 0 to 100. Patients rate the difficulty they experience performing routine daily activities. The lowest score indicates greater difficulty in performing these activities.
DermaLab Suction Cup (24)
The DermaLab elasticity module (Figure 1) consists of a light plastic probe that forms a closed chamber when attached to the skin surface using double-sided sticky tape. Within the probe chamber, two narrow beams of light run at different heights parallel to the skin surface that serve as elevation detectors. A computer-controlled vacuum pump is used to progressively increase the suction within the chamber over 30 to 60 seconds. The amount of suction in kilopascals (kPa) required to lift the skin is recorded electronically at the time where each of the light beams is blocked and when the negative pressure within the chamber is maintained in equilibrium with the capacity of the pump. The software then calculates the mechanical properties of the skin based on Hooke’s law, “the strain of any material is proportional to the load applied to it”. With certain basic assumptions, the stiffness of the skin or its Young’s modulus (E) is expressed in mm/kPa and can be calculated from this stress-strain relationship as follows:
| 1 |
where p = pressure in kPa, x = distance in mm, ψ = elasticity constant for a measured object estimated from civil engineering tables (0.5), r = radius of surface measured in mm (defined by the chamber geometry as 5 mm), s = thickness of the skin in mm (set to a standard of 1.0 mm by the manufacturer). The skin that is firm and taught will thus have a much higher stiffness index. The upper and lower elevation detectors of a normal probe are farther from the skin surface, when compared to a scleroderma probe. Many times, the stiff irradiated skin cannot be stretched enough to reach the level of its detectors. As a result, measurements were not registered consistently using a normal probe. Therefore, a scleroderma probe was used, as its upper and lower elevation detectors are closer to the skin surface, allowing measuring tight skin without excessive stretching.
Figure 1:

The DermaLab Suction Cup (Cortex, Hadsund, Denmark).
Statistical Analysis
Levels of agreement between clinicians were estimated using the Kappa coefficient (κ) for ordinal variables. The Pearson correlation coefficient (PCC) and concordance correlation coefficient (CCC) were used for continuous variables. The PCC estimates is a measure of association between the two variables whereas the CCC measures how close the two variables are to the line of unity. Association between ordinal (i.e., NCI-CTCAE grade) and continuous outcomes were assessed using Spearman correlation coefficients (SCC).
All correlation coefficients are estimated values of association, which range from −1 to 1. A value of −1 indicates perfect, negative association, +1 indicates perfect, positive association and 0 indicates no association. Correlation coefficients between −0.3 and 0.3 are considered to be weak or no association, between 0.3 and 0.6 or −0.3 and −0.6 are considered moderately associated, and values >0.6 or <−0.6 are considered strongly associated. Two-sided confidence intervals were constructed at the 95% level for selected outcomes.
Results
Patient Population
From 2008 to 2010, 69 patients with E-STS treated with limb-sparing surgery and RT met inclusion criteria and were included in the final analysis.
Patient and tumour characteristics are reported in Table II. Fifty-five patients (79.7%) had a tumour in the lower extremity (LE) and 14 (20.3%), in the upper extremity (UE). Thirtyseven (53.6%) participants were male with a mean age of 54.9 years (ranging from 23 to 85 years). The mean interval from radiation was 56.1 months, ranging from 13 to 130 months. All patients received a total radiation dose of 50-66 Gy at 2 Gy per fraction.
Table II.
Characteristics of patients and tumours.
| Variable | No. |
|---|---|
| n | 69 |
| Age | |
| Mean, yrs | 54.9 |
| Range, yrs | 23-85 |
| Gender, no. (%) | |
| Female | 32 (47.4) |
| Male | 37 (53.6) |
| Anatomic site, no. (%) | |
| Upper extremity | 14 (20.3) |
| Lower extremity | 55 (79.7) |
| Interval from RT | |
| Mean, months | 56.1 |
| Range, months | 13-130 |
| Radiation dose, no. (%) | |
| 50 Gy | 28 (41.6) |
| 66 Gy | 41 (59.4) |
Measurement Analysis
For the LE and UE groups, the mean MSTS total score was 31.2 (ranging from 5 to 35) and 32.9 (ranging from 21 to 35) respectively, while the mean score for TESS was 90.6 (ranging from 33 to 100) and 91.0 (ranging from 45 to 100) respectively. The health care workers had a tendency to score most patients as having RT-related toxicities (e.g., Grade 1 or higher) and almost 90% of patients having subcutaneous fibrosis and approximately 45% of patients having edema. These results are summarized in Table III.
Table III.
Subjective scales grading, including the NCI-CTCAE, MSTS and TESS scores.
| Grading scale | No. (%) |
|---|---|
| NCI-CTCAE Edema Grade-A | |
| 0 | 43 (62.3) |
| 1 | 15 (21.7) |
| 2 | 8 (11.6) |
| 3 | 3 (4.4) |
| NCI-CTCAE Edema Grade-B | |
| 0 | 34 (49.3) |
| 1 | 20 (29.0) |
| 2 | 9 (13.0) |
| 3 | 6 (8.7) |
| NCI-CTCAE Fibrosis Grade-A | |
| 0 | 7 (10.1) |
| 1 | 26 (37.7) |
| 2 | 23 (33.3) |
| 3 | 13 (18.8) |
| NCI-CTCAE Fibrosis Grade-B | |
| 0 | 6 (8.7) |
| 1 | 35 (50.7) |
| 2 | 11 (15.9) |
| 3 | 17 (24.6) |
| MSTS Score (SD) | |
| Mean | 31.2 (5.5) |
| N (%) ≥30 | 51 (73.9) |
| TESS Score (SD) | |
| Mean | 90.7 (12.2) |
| N (%) ≥90 | 44 (63.8) |
A: Measured by health care worker A.
B: Measured by health care worker B.
SD: Standard deviation.
The level of agreement between clinicians in terms of NCICTCAE grading was moderate and ranged from κ = 0.41 for edema to κ = 0.59 subcutaneous tissue-related late radiation morbidity. The concordance correlation coefficients (CCC) for the elasticity measurements were much higher, 0.82 for fibrotic skin and 0.84 for normal skin, suggesting a strong correlation between the two measurements. This relationship was illustrated in Figures 2 and 3. In addition, the mean difference in the measure of elasticity between clinician A and clinician B was 0.2 and −0.2 for both the treated and contralateral limb, indicating that the difference (noise) appears to be random.
Figure 2:

Concordance correlation coefficient (CCC) for elasticity measurements on the treated limb. CCC = 0.82 (95% CI = 0.72-0.88). Each point represents a patient for which elasticity was measured by both observers.
Figure 3:
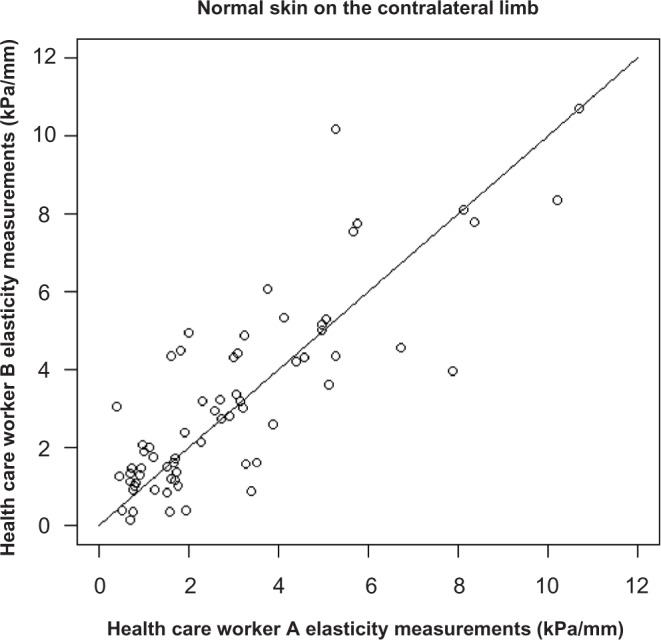
Concordance correlation coefficient (CCC) for elasticity measurements on normal skin on the contralateral limb. CCC = 0.84 (95% CI 0.75-0.90). Each point represents a patient for which elasticity was measured by both observers.
Statistically significant associations between the elasticity measurements and the MSTS were found, with an elasticity of 7.9 kPa/mm vs. 5.7 kPa/mm for MSTS <30 and ≥30 respectively (p = 0.002). The elasticity also correlated with the TESS, with an elasticity of 6.9 kPa/mm and 5.9 kPa/mm for the TESS score of <90 and ≥90 respectively (p = 0.020).
Discussion
Our results suggest that the DermaLab suction cup device generates reproducible elasticity measurements that correlate well with subjective scores such as the MSTS and TESS scales.
It has been well established that fibrosis is associated with impaired extremity function (25). Therapeutic intervention with various agents such as pentoxyfyllin and vitamin E (26, 27), copper/zinc superoxide dismutase (28, 29), Mn SOD (30 -32)), hyperbaric oxygen or even physiotherapy as done with head and neck patients, have been used in patients with measurable fibrosis. The efficacy of these sporadically used interventions has been difficult to measure.
Furthermore, it is pertinent to investigate new approaches to delivering radiation with reduced morbidity. The RTOG trial 0630 is a contemporary approach where the treated volume was reduced through the use of image-guided RT.
Evaluating these techniques and therapeutic interventions requires sensitive, reliable and valid measurements of fibrosis. Existing studies using clinician or patient-rated measurements of fibrosis suggest that these scales have poor reliability, and hence validity, that may limit their value as outcome measures (23). Objective measurements potentially offer greater accuracy and sensitivity in quantifying fibrosis. As well, a continuous rather than discrete scale may be more sensitive to change.
In this study, we utilized the DermaLab Suction Cup to evaluate the degree of fibrosis objectively. This device has been investigated in multiple clinical settings such as active burn scars (33), age-related connective tissue weakness (e.g., cellulite) response following treatments (34), vaginal prolapse (35), and mapping of normal breast elasticity (36).
To assess the reproducibility of this apparatus, two independent clinicians measured skin elasticity. The mean difference in the measure of elasticity between clinician A and B appeared to be random. Furthermore, the DermaLab Suction Cup’s elasticity measurement yielded a CCC suggesting a strong level of concordance between the two measurers, for both fibrotic and normal skin. These results thus suggest that the DermaLab generates reproducible results.
Other devices have previously been studied as an objective measurement of E-STS fibrosis with various limitations. Leung et al. first reported using an ultrasound probe and computer algorithm in 2002 (37). The deformation of the soft tissue due to a measured applied force was measured. Huang et al. (38) tested the same system, using a minimized version of the tissue ultrasound palpation system used by Leung et al. (37). Thirty-eight patients who received RT to the neck for nasopharyngeal carcinoma were studied. The intraclass correlation coefficient (ICC) values for intra- and inter-rater measurement were generally larger than 0.80, which are comparable to the DermaLab Suction Cup results. It was noted however, that this device’s use had some limitations. Stringent measurement site consistency was required to maintain inter- and intrapatient consistency. It was also estimated that several hours of training were required for an ultrasonography technician to learn the technique. In contrast, the DermaLab Suction cup does not require precise delineation of the skin site and any healthcare worker may use the device with minimal training.
Other objective methods of measurement such as the tissue compliance meter (TCM) used by Marinus et al. (39) showed significantly superior inter-observer reliability compared to that of the palpation method. Most importantly, its ICC was slightly higher than the CCC in our study for the DermaLab Suction Cup (ICC 0.91 vs. CCC 0.82 for radiated skin). However, the use of the TCM also had restrictions. The plastic disc, with its diameter of 8 cm, was rather large, therewith limiting an even positioning of the device and thus accurate measurement of tissue compliance. For instance, areas such as the axilla, the skin folds and other areas with convex or concave surfaces were not assessable. The DermaLab Suction cup’s 2 cm diameter, in contrast, allowed more versatility with less anatomical limitations. Therefore, implementation in clinical settings will be easier, especially for patients with E-STS where the limb surface is often smaller than 8 cm.
Davis et al. (40) studied the usefulness of the BTC-2000 in evaluating the biomechanical tissue parameters of laxity and energy absorption in 41 patients treated for E-STS by surgery alone and surgery with preoperative RT (50 Gy), and postoperative RT (66 Gy). This method of measuring post-radiation fibrosis was the most similar to the DermaLab Suction Cup, having a laser beam system measuring the skin deflection by subatmospheric pressure through a chamber aperture. Promising quantitative measures of soft-tissue fibrosis were generated; discriminating patients treated with surgery alone, pre-operative EBRT and post-operative EBRT. However, BTC-2000’s reliability and validity was not evaluated.
The CCC of the DermaLab Suction Cup in this study was also compared to the kappa for the NCI-CTCAE edema and fibrosis score. Our results showed a stronger correlation between measurements from the DermaLab Suction Cup compared to the NCI-CTCAE scale (κ= 0.41 for edema to κ= 0.59 subcutaneous tissue-related late radiation morbidity compared to CCC of 0.82 for fibrotic skin and 0.84 for normal skin). This is in concordance with all the aforementioned studies (23, 33, 34, 41), which also demonstrated that objective measurements have better reproducibility than clinical scales.
Skin and subcutaneous fibrosis is a known progressive process (42). Data from Jung et al. indicate an apparent lifelong risk of developing late complications, without a plateau, suggesting that different kinetic mechanisms are in play (43). Interestingly, it appears that after four to six years, more than 50% of patients who received similar treatment protocols to our population will have experienced RT-related fibrosis (44). Although our measurements were taken at different interval from RT, the mean interval from RT was of 56.1 months (13-130 months). Thus, we believe we would have measured the majority of patients who would have been affected by RT-related fibrosis.
Finally, correlating our results with the MSTS and the TESS showed statistically significant association between elasticity and the MSTS with a higher elasticity measurement for MSTS < 30 compared to > = 30. The elasticity also correlated with the TESS with a higher elasticity with TESS score of <90 compared to > = 90. This suggests that the higher the elasticity measurement value, the more the patient’s daily activities are affected by radiation-induced fibrosis. This is consistent with many studies that have demonstrated that increased fibrosis results in greater impairment in functionality (14, 45 -48). The measurements of the suction cup device thus correlate with both patient and physician-reported measures of functional outcome. This has not been demonstrated with other devices.
Conclusion
Sensitive, reproducible and clinically relevant measurements of radiation-induced fibrosis are necessary if new radiation approaches are to be shown to reduce this common late effect. The same is required for the study of therapy for preexisting fibrosis.
With its simplicity and ease of use, a suction cup measurement of skin elasticity shows promise in generating simple and reproducible measurements of radiation-induced skin fibrosis. It is more reliable than CTCAE grading and correlates well with validated patient and physician-reported scores — in the case of E-STS, MSTS and TESS questionnaires.
Acknowledgement
Nil.
Abbreviations
- NCI-CTCAE:
National Cancer Institute Common Terminology Criteria for Adverse Events
- MSTS:
Musculoskeletal Tumor Rating Scale
- TESS:
Toronto Extremity Salvage Score
- κ:
Kappa; CCC: Concordance Correlation Coefficient
- E-STS:
Extremity Soft Tissue Sarcomas
- RT:
Radiation
- EBRT:
External Beam Radiation
- Gy:
Grays
- PCC:
Pearson Correlation Coefficient
- SCC:
Spearman Correlation Coefficients
- LE:
Lower Extremity
- UE:
Upper Extremity
- RTOG:
Radiation Therapy Oncology Group
- ICC:
Intraclass Correlation Coefficient
- TCM:
Tissue Compliance Meter
Footnotes
Conflict of Interest: The authors state no conflict of interest.
References
- 1.Robinson MH, Keus RB, Shasha D, Harrison LB. Is pre-operative radiotherapy superior to postoperative radiotherapy in the treatment of soft tissue sarcoma? Eur J Cancer 34, 1309-1316 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Bell RS, O’Sullivan B, Davis A, Langer F, Cummings B, Fornasier VL. Functional outcome in patients treated with surgery and irradiation for soft tissue tumours. Journal of Surgical Oncology 48, 224-231 (1991). [DOI] [PubMed] [Google Scholar]
- 3.Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, DeLaney T, Glatstein E, Steinberg SM, Merino MJ, Rosenberg SA. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. Journal of Clinical Oncology 16, 197-203 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Tepper J, Glatstein E, Costa J, Baker A, Brennan M, DeMoss EV, Seipp C, Sindelar WF, Sugarbaker P, Wesley R. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Annals of Surgery 196, 305-315 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muhic A, Hovgaard D, Petersen M Mork, Daugaard S, Bech B Hojlund, Roed H, Kjaer-Kristoffersen F, Engelholm S Aage. Local control and survival in patients with soft tissue sarcomas treated with limb sparing surgery in combination with interstitial brachytherapy and external radiation. Radiotherapy and Oncology 88, 382-387 (2008). DOI: 10.1016/j.radonc.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Koshy M, Rich SE, Mohiuddin MM. Improved survival with radiation therapy in high-grade soft tissue sarcomas of the extremities: a SEER analysis. International Journal of Radiation Oncology, Biology, Physics 77, 203-209 (2010). DOI: 10.1016/j.ijrobp.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Kogel A. Basic clinical radiobiology, 3rd Edition, In: Stell GG. (Ed.), New York: Oxford University Press, 31-41 (2002). [Google Scholar]
- 8.Davis AM, O’Sullivan B, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Hammond A, Benk V, Kandel R, Goddard K, Freeman C, Sadura A, Zee B, Day A, Tu D, Pater J. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiotherapy and Oncology 75, 48-53 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Kent M Louise, Brennan MT, Noll JL, Fox PC, Burri SH, Hunter JC, Lockhart PB. Radiation-induced trismus in head and neck cancer patients. Supportive Care in Cancer 16, 305-309 (2008). DOI: 10.1007/s00520-007-0345-5. [DOI] [PubMed] [Google Scholar]
- 10.Hoeller U, Tribius S, Kuhlmey A, Grader K, Fehlauer F, Alberti W. Increasing the rate of late toxicity by changing the score? A comparison of RTOG/EORTC and LENT/SOMA scores. International Journal of Radiation Oncology, Biology, Physics 55, 1013-1018 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Ryan JL. Ionizing radiation: the good, the bad, and the ugly. The Journal of Investigative Dermatology 132, 985-993 (2012). DOI: 10.1038/jid.2011.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JH, Kolozsvary AJ, Jenrow KA, Brown SL. Mechanisms of radiation-induced skin injury and implications for future clinical trials. International Journal of Radiation Biology (Epub January 10, 2013). DOI: 10.3109/09553002.2013.765055. [DOI] [PubMed] [Google Scholar]
- 13.Johansson S, Svensson H, Denekamp J. Dose response and latency for radiation-induced fibrosis, edema, and neuropathy in breast cancer patients. International Journal of Radiation Oncology, Biology, Physics 52, 1207-1219 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Davis AM, O’Sullivan B, Bell RS, Turcotte R, Catton CN, Wunder JS, Chabot P, Hammond A, Benk V, Isler M, Freeman C, Goddard K, Bezjak A, Kandel RA, Sadura A, Day A, James K, Tu D, Pater J, Zee B. Function and health status outcomes in a randomized trial comparing preoperative and postoperative radiotherapy in extremity soft tissue sarcoma. Journal of Clinical Oncology 20, 4472-4477 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Davis AM. Functional outcome in extremity soft tissue sarcoma. Seminars in Radiation Oncology 9, 360-368 (1999). DOI: 10.1053/ SRAO00900360. [DOI] [PubMed] [Google Scholar]
- 16.Bentzen SM, Dische S. Morbidity related to axillary irradiation in the treatment of breast cancer. Acta Oncol 39, 337-347 (2000). [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute. Common Terminology Criteria for Adverse Events v.3.0 and v.4.0 (CTCAE). <http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm>.
- 18.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clinical Orthopaedics and Related Research 286, 241-246 (1993). [PubMed] [Google Scholar]
- 19.Enneking WF. In: Limb Salvage in Musculoskeletal Oncology 626-639 (Churchill Livingston, 1987). [Google Scholar]
- 20.Davis AM, Wright JG, Williams JI, Bombardier C, Griffin A, Bell RS. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Quality of Life Research 5, 508-516 (1996). [DOI] [PubMed] [Google Scholar]
- 21.Davis AM, Bell RS, Badley EM, Yoshida K, Williams JI. Evaluating functional outcome in patients with lower extremity sarcoma. Clinical Orthopaedics and Related Research 358, 90-100 (1999). [PubMed] [Google Scholar]
- 22.Clayer M, Doyle S, Sangha N, Grimer R. The toronto extremity salvage score in unoperated controls: an age, gender, and country comparison. Sarcoma 2012, 717213 (2012). DOI: 10.1155/2012/717213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis AM, Dische S, Gerber L, Saunders M, Leung SF, O’Sullivan B. Measuring postirradiation subcutaneous soft-tissue fibrosis: state-of-the-art and future directions. Seminars in Radiation Oncology 13, 203-213 (2003). DOI: 10.1016/S1053-4296(03)00022-5. [DOI] [PubMed] [Google Scholar]
- 24.Serup J, Jemec B, Grove G. In: Handbook of Non-Ivasive Methods and the Skin, 2nd Edition, Chapter 68, 593-599 (2006). [Google Scholar]
- 25.Estrella E, Wang E, Caro L, Castillo V. Functional outcomes of reconstruction for soft tissue sarcomas of the foot and ankle. The Foot and Ankle Online Journal 2 (2009). DOI: 10.3827/faoj.2009.0203.0002. [Google Scholar]
- 26.Jacobson G, Bhatia S, Smith BJ, Button AM, Bodeker K, Buatti J. Randomized trial of pentoxifylline and vitamin e vs standard follow-up after breast irradiation to prevent breast fibrosis, evaluated by tissue compliance meter. International Journal of Radiation Oncology, Biology, Physics 85, 604-608 (2012). DOI: 10.1016/j.ijrobp.2012.06.042. [DOI] [PubMed] [Google Scholar]
- 27.Gothard L, Cornes P, Earl J, Hall E, MacLaren J, Mortimer P, Peacock J, Peckitt C, Woods M, Yarnold J. Double-blind placebo-controlled randomised trial of vitamin E and pentoxifylline in patients with chronic arm lymphoedema and fibrosis after surgery and radiotherapy for breast cancer. Radiotherapy and Oncology 73, 133-139 (2004). DOI: 10.1016/j.radonc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Okunieff P, Augustine E, Hicks JE, Cornelison TL, Altemus RM, Naydich BG, Ding I, Huser AK, Abraham EH, Smith JJ, Coleman N, Gerber LH. Pentoxifylline in the treatment of radiation-induced fibrosis. Journal of Clinical Oncology 22, 2207-2213 (2004). DOI: 10.1200/JCO.2004.09.101. [DOI] [PubMed] [Google Scholar]
- 29.Campana F, Zervoudis S, Perdereau B, Gez E, Fourquet A, Badiu C, Tsakiris G, Koulaloglou S. Topical superoxide dismutase reduces post-irradiation breast cancer fibrosis. Journal of Cellular and Molecular Medicine 8, 109-116 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lefaix JL, Delanian S, Leplat JJ, Tricaud Y, Martin M, Nimrod A, Baillet F, Daburon F. Successful treatment of radiation-induced fibrosis using Cu/Zn-SOD and Mn-SOD: an experimental study. International Journal of Radiation Oncology, Biology, Physics 35, 305-312 (1996). [DOI] [PubMed] [Google Scholar]
- 31.Delanian S, Lefaix JL. Current management for late normal tissue injury: radiation-induced fibrosis and necrosis. Seminars in Radiation Oncology 17, 99-107 (2007). DOI: 10.1016/j.semradonc.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Delanian S, Baillet F, Huart J, Lefaix JL, Maulard C, Housset M. Successful treatment of radiation-induced fibrosis using liposomal Cu/Zn superoxide dismutase: clinical trial. Radiotherapy and Oncology 32, 12-20 (1994). [DOI] [PubMed] [Google Scholar]
- 33.Anthonissen M, Daly D, Fieuws S, Massage P, Van Brussel M, Vranckx J, Van den Kerckhove E. Measurement of elasticity and transepidermal water loss rate of burn scars with the Dermalab(R). Burns pii, S0305-4179 (2012). DOI: 10.1016/j.burns.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 34.Christ C, Brenke R, Sattler G, Siems W, Novak P, Daser A. Improvement in skin elasticity in the treatment of cellulite and connective tissue weakness by means of extracorporeal pulse activation therapy. Aesthetic Surgery Journal 28, 538-544 (2008). DOI: 10.1016/j.asj.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Epstein LB, Graham CA, Heit MH. Systemic and vaginal biomechanical properties of women with normal vaginal support and pelvic organ prolapse. American Journal of Obstetrics and Gynecology 197, 165 e161-166 (2007). DOI: 10.1016/j.ajog.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 36.Sutradhar A, Miller MJ. In vivo measurement of breast skin elasticity and breast skin thickness. Skin Res Technol 19, e191-199 (2013). DOI: 10.1111/j.1600-0846.2012.00627.x. [DOI] [PubMed] [Google Scholar]
- 37.Leung SF, Zheng Y, Choi CY, Mak SS, Chiu SK, Zee B, Mak AF. Quantitative measurement of post-irradiation neck fibrosis based on the young modulus: description of a new method and clinical results. Cancer 95, 656-662 (2002). DOI: 10.1002/cncr.10700. [DOI] [PubMed] [Google Scholar]
- 38.Huang YP, Zheng YP, Leung SF, Choi AP. High frequency ultrasound assessment of skin fibrosis: clinical results. Ultrasound in Medicine & Biology 33, 1191-1198 (2007). DOI: 10.1016/j.ultrasmedbio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Marinus J, Niel CG, de Bie RA, Wiggenraad RG, Schoppink EM, Beukema LH. Measuring radiation fibrosis: the interobserver reliability of two methods of determining the degree of radiation fibrosis. International Journal of Radiation Oncology, Biology, Physics 47, 1209-1217 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Davis AM, Gerrand C, Griffin A, O’Sullivan B, Hill RP, Wunder JS, Abudu A, Bell RS. Evaluation of clinical utility of BTC-2000 for measuring soft tissue fibrosis. International Journal of Radiation Oncology, Biology, Physics 60, 286-294 (2004). DOI: 10.1016/j.ijrobp.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 41.Wang D, Zhang Q, Kirsch D, Okuno S, Kane J, Li X, Roberge D, Finkelstein S, DeLaney T, Eisenberg B. In: ASTRO’s 53rd Annual Meeting.
- 42.Turesson I, Nyman J, Holmberg E, Oden A. Prognostic factors for acute and late skin reactions in radiotherapy patients. International Journal of Radiation Oncology, Biology, Physics 36, 1065-1075 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Jung H, Beck-Bornholdt HP, Svoboda V, Alberti W, Herrmann T. Quantification of late complications after radiation therapy. Radiotherapy and Oncology 61, 233-246 (2001). [DOI] [PubMed] [Google Scholar]
- 44.O’Sullivan B, Levin W. Late radiation-related fibrosis: pathogenesis, manifestations, and current management. Seminars in Radiation Oncology 13, 274-289 (2003). DOI: 10.1016/S1053-4296(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 45.Schreiber D, Bell RS, Wunder JS, O’Sullivan B, Turcotte R, Masri BA, Davis AM. Evaluating function and health related quality of life in patients treated for extremity soft tissue sarcoma. Quality of Life Research 15, 1439-1446 (2006). DOI: 10.1007/s11136-006-0001-4. [DOI] [PubMed] [Google Scholar]
- 46.Johansson S, Svensson H, Denekamp J. Timescale of evolution of late radiation injury after postoperative radiotherapy of breast cancer patients. International Journal of Radiation Oncology, Biology, Physics 48, 745-750 (2000). [DOI] [PubMed] [Google Scholar]
- 47.Gerrand CH, Wunder JS, Kandel RA, O’Sullivan B, Catton CN, Bell RS, Griffin AM, Davis AM. The influence of anatomic location on functional outcome in lower-extremity soft-tissue sarcoma. Annals of Surgical Oncology 11, 476-482 (2004). DOI: 10.1245/ASO.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 48.Alektiar KM, Brennan MF, Singer S. Influence of site on the therapeutic ratio of adjuvant radiotherapy in soft-tissue sarcoma of the extremity. International Journal of Radiation Oncology, Biology, Physics 63, 202-208 (2005). DOI: 10.1016/j.ijrobp.2005.01.011. [DOI] [PubMed] [Google Scholar]


