Abstract
The increasing efficacy of pediatric cancer therapy over the past four decades has produced many long-term survivors that now struggle with serious treatment related morbidities affecting their quality of life. Radiation therapy is responsible for a significant proportion of these late effects, but a relatively new and emerging modality, proton radiotherapy hold great promise to drastically reduce these treatment related late effects in long term survivors by sparing dose to normal tissues. Dosimetric studies of proton radiotherapy compared with best available photon based treatment show significant dose sparing to developing normal tissues. Furthermore, clinical data are now emerging that begin to quantify the benefit in decreased late treatment effects while maintaining excellent cancer control rates.
Keywords: Protons, Radiation therapy, Pediatric cancer
Fundamentals of Pediatric Cancer Treatment
Approximately 10,400 children under the age of 15 are diagnosed with cancer annually and 1500 will die of their disease (1). While the incidence of childhood malignancy has increased slightly over the last three decades, five year survival rates have improved markedly from 58.1 percent in 1975-77 to 79.6 percent in 1996-2003 (1), owed in large part to multidisciplinary treatment strategies incorporating improved surgical and radiation delivery techniques and advances in chemotherapy and supportive care. At present, there are estimated to be greater than 250,000 survivors of childhood cancer in the United States (2).
The substantial improvement in treatment outcomes, and the resultant increase in long-term survivors of pediatric malignancy, has placed an increased emphasis on treatment toxicity. The goal of both current and future therapies focuses on both improvements in treatment efficacy as well as decreasing the unwanted acute and long-term side effects of curative therapy. In the multidisciplinary setting, this may include less invasive surgical procedures or development of improved chemotherapeutics or novel biologic agents. Historically, radiation therapy (RT) has been associated with unwanted cosmesis, significant long-term treatment morbidity within multiple organ systems and has been definitively shown to increase the risk of second malignancy (3).
There are several avenues by which to decrease radiation-associated morbidity. First, radiation treatment can be avoided altogether if other methods of treatment prove equally effective. Historically, craniospinal irradiation (CSI) to 24Gy was shown to be extremely effective at preventing central nervous system relapses in childhood acute lymphoblastic leukemia (4), but was associated with significant neurocognitive deficits (5, 6). The introduction of intrathecal methotrexate has provided equivalent efficacy leading to the elimination of CSI in this setting. Similarly, full surgical resection of low-grade glioma eliminates the need for postoperative RT (7). Second, due to the increased toxicity of radiation in the very young (age <3 years) (8), radiation therapy can be delayed with the judicious use of chemotherapy in select disease sites until the child reaches a more appropriate age for treatment (9). Third, the radiation dose delivered can be decreased if supplemented with effective surgery or chemotherapy. For example, while CSI remains an integral component of medulloblastoma treatment, the addition of cisplatin-based chemotherapy has lead to a decrease in dose from 36 Gy to 23.4 Gy with equivalent disease related outcomes in average risk patients (10). Similarly, a gross total resection of intermediate risk rhabdomyosarcoma allows for a decrease in dose from 50.4 Gy to 36 Gy. Lastly, the radiation dose distribution can be improved to decrease the dose to normal tissues in close proximity to the target. This reduction in dose to normal tissues is better achieved by proton radiotherapy compared with standard external beam radiation techniques.
Improvements in RT Target Localization and Delivery
The ability to both identify the area to be treated (target volume) and to direct the radiation beam to conform around the target volume has evolved substantially over the last three decades. Historically, radiation was delivered based on twodimensional anatomy under fluoroscopic or X-ray imaging. The last three decades have seen drastic improvements in the ability to localize the gross tumor, surrounding sites of microscopic tumor spread, and adjacent normal tissue structures in three dimensions (3-D) using computed tomography (CT) based imaging. Magnetic resonance imaging (MRI) and positron emission tomography (PET) have also been incorporated into the planning process further improving target delineation. More recent advances have included 4-dimensional scanning and real time tumor localization during treatment administration for moving targets. Additionally, improved immobilization devices have lessened variation in daily treatment positioning allowing practitioners to decrease the error margins around the treatment target (11–13).
Improvement in the radiological localization of tumor and surrounding normal tissues has coincided with marked improvements in treatment delivery to these three dimensional target volumes. These techniques have led to decreased treatment morbidity at traditionally accepted dose levels and may allow for dose intensification in settings where additional dose could not previously be administered safely. 3-D conformal radiation uses multiple static beams at various angles and beam energies to optimize the radiation coverage to the tumor while minimizing dose to normal structures.
The separate beams can be shaped with blocks and wedges to match patient contours and beams can be weighted so that the majority of radiation can be given via a preferred angle. Intensity modulated radiation therapy (IMRT) is a variant of 3-D conformal radiation where blocks move in and out of the active treatment beam to vary the intensity at specific points within the beam profile. This variant fluence leads to a heterogeneous dose distribution within each beam and allows for a further increase in treatment conformality, further sparing normal tissue. A substantial drawback of IMRT, especially in the pediatric population, is the increased volume of relatively low dose radiation delivered to tissue by this technique. This effect can be mitigated by careful arrangement of beam angles, but is increased with the use of newer techniques including helical tomotherapy and volumetric modulated arc therapy (14). While the longterm morbidity of increased low dose radiation is not well studied in the pediatric population, there is concern that it may have a deleterious effect on normal tissue development and increase the probability of second malignancies (15).
3D conformal RT and IMRT are techniques that have improved the delivery of photon radiation. Interest in proton radiation stems from the physical properties of these charged particles and their inherent advantages in dose distribution when delivered via the above techniques.
History of Proton Radiotherapy
Proton therapy deployed as large field fractionated treatment was first used in clinical practice in the United States at the Harvard Cyclotron Laboratory in Cambridge, Massachusetts beginning in 1974. To date greater than 73,800 patients have been treated with proton radiotherapy worldwide. Internationally there are now 38 active proton facilities (http://ptcog.web.psi.ch/ptcentres.html), including ten within the United States, namely the Francis H. Burr Proton Therapy Center (Boston, MA), the MD Anderson Cancer Center (Houston, TX), the University of Pennsylvania (Philadelphia, PA), Loma Linda University Medical Center (Loma Linda, CA), the Florida Proton Therapy Institute (Jacksonville, FL), the University of California–Davis (Davis, CA), the Midwest Proton Therapy Institute (Bloomington, IN), the Hampton University Proton Therapy Institute (Hampton, VA) and two ProCure Proton Therapy Centers (Oklahoma City, OK and Warrenville (Chicago area), IL). Several additional centers are presently under development throughout the United States. The capital costs of constructing a multi-gantry proton center is well over 100 million dollars, although newer treatment designs hope to modestly decrease this cost, and smaller one gantry facilities are being developed and offered in the range 20-30 million dollars. The high cost of building a proton center means that proton radiation will remain a limited resource.
The biologic effect of proton radiation is thought to be essentially identical to that of photons where ionization events lead to free radical generation and subsequent DNA damage. However, the relative biological effectiveness (RBE) of protons is estimated to be 10% greater than photons (16). As such, a proton dose is described as a cobalt Gray equivalent (CGE), which translates to an equivalent photon dose measured in Gray (Gy).
The advantage of proton radiation over photon radiation lies in its physical properties only. Photon radiation consists of high-energy electromagnetic waves that penetrate tissue and deposit dose along the beam path. Dose delivery is maximal just below the skin surface and continues to be exponentially attenuated along the entire treatment path until exiting the body. In order to treat a tumor at depth to an effective dose, photon radiation is by necessity delivered in substantial amounts to tissue both proximal and distal to the tumor along the path of the treatment beam (Figure 1). Conversely, proton radiation consists of charged particles with mass, which deposit a small amount of their energy along the particle path, until reaching a maximum penetration depth where the remaining energy is lost rapidly over a short distance, a phenomenon termed a Bragg Peak (Figure 1). The maximum penetration depth of the proton beam, which is determined by beam energy, can be manipulated to place the Bragg peak within a tumor at a specified depth in the body (17). Because the Bragg peak of a monoenergetic proton beam is narrow (approximately 1 cm), several beams with closely spaced penetration depths are used to treat the entirety of the tumor. This area of uniform dose over the entirety of the tumor is termed a Spread Out Bragg peak (SOBP) (Figure 1). While the SOBP does increase dose deposition proximal to the tumor, the entrance dose usually remains substantially lower than that of photon radiotherapy. Most importantly, proton radiation deposits no dose distal to the tumor along the particle path. The absence of radiation distal to the target is the principal advantage of proton radiotherapy, allowing for substantial tissue sparing at certain anatomic sites. A visual representation of the considerable normal tissue sparing provided by proton radiotherapy in the treatment of the craniospinal axis is provided in Figure 2.
Figure 1:
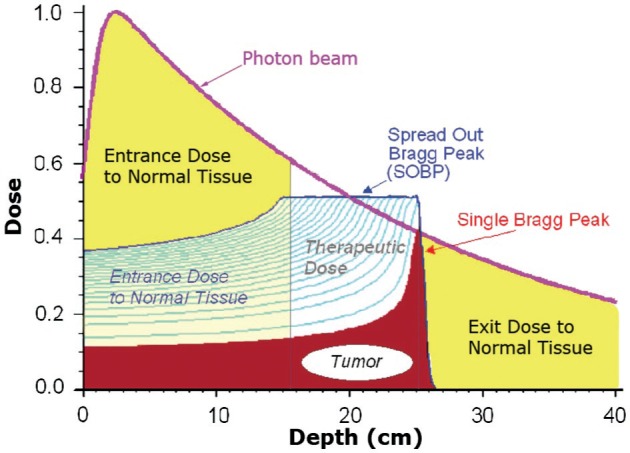
Radiation Dose Profiles: Photons vs. Protons. Photon radiation enters the body and deposits dose along the entirety of the beam path. Dose delivery is maximal just below the skin surface and continues until exiting the body. Proton radiation delivers the majority of its dose at the end of its range, a phenomenon termed a Bragg peak. Passively scattered proton radiation requires a spread out Bragg peak to cover the entire target volume, increasing dose at the skin surface. Notable in this figure is the relative decrease in entry dose compared to photons and the lack of any dose distal to the tumor with proton treatment. Dose as a ratio of maximum dose in represented on the y-axis. Depth of penetration into the patient is represented in centimeters on the x-axis. A tumor is depicted from 17 to 24 centimeters.
Figure 2:
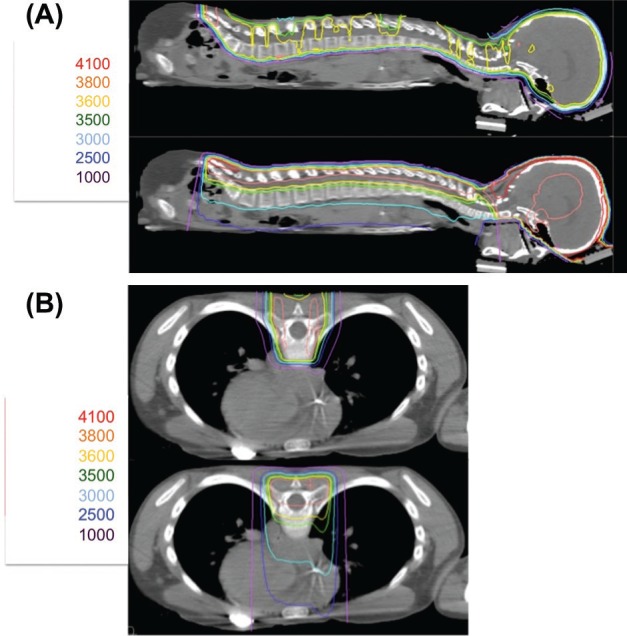
Dosimetric Comparison of Proton Radiation and Intensity Modulated Radiation Therapy (IMRT) with Photons: Sagittal (A) and Axial (B) images of proton radiation (top) and IMRT (bottom) in a pediatric patient treated to the craniospinal axis to a prescribed dose of 3600 cGy for high risk medulloblastoma. Doses in centiGray (cGy) are listed to the left of each panel. Protons provide equivalent target coverage while limiting dose distal to the treatment area. Increased dose is noted in Panel A to the entire abdominal viscera with IMRT and increased dose to the heart is noted in Panel B with IMRT.
Methods of Proton Delivery
There are presently two principal methods of proton radiotherapy delivery.
Passively scattered proton radiotherapy is by far the most common treatment technique currently employed (18). This method consists of 3D-conformal treatments with multiple static beams. Protons are spread laterally and shaped via brass apertures that are placed in the gantry head. Beam depth is manipulated via a modulation wheel, which produces the varying energies needed to treat the entire target under the SOBP. The beams are further shaped to conform to the distal edge of the tumor with Lucite compensators that account for both tissue inhomogeneity and tumor shape.
Pencil beam scanning, also known as active scanning, is a more recent technological advance employed at a handful of centers worldwide. In this technique, magnets steer a small pencil beam of protons to specific positions within a tumor target without the need for brass apertures or compensators (19). The depth of the beam is varied in the accelerator itself, in a process termed active modulation. Pencil beam technology has two main advantages. First, it allows for shaping of both the proximal and distal edges of the treatment field, decreasing entry dose while maintaining a lack of exit dose. Second, there is no neutron scatter associated with active scanning due to the lack of shielding and blocks in the gantry head, an advantage that will be particularly important for the pediatric patient.
The fact that the biologic effect of protons and photons on target tissue is essentially identical makes the rate of tumor control or cure unlikely to differ between these modalities. The physical properties of protons should however allow for dose reduction to surrounding normal tissues. Consequently, an important endpoint in pediatric proton radiotherapy trials is a measurable reduction in treatment-associated morbidity.
Protons in Clinical Practice
CNS Malignancies
Central nervous system tumors are the most common solid malignancies in childhood. Some of the most common tumors include gliomas, medulloblastoma and other primitive neuroectodermal tumors, germ cell tumors and ependymoma. Radiation plays a critical role in the management of most of these tumors.
As survival rates have improved, there has been an increasing focus on treatment related side effects, which are determined by the treatment dose and location of the tumor. Within the CNS of the developing child, these treatment effects can be numerous. Radiation to the brain has been associated with significant neurocognitive deficits. Intellectual functioning, as measured by the Weschler Intelligence Scales for Children, has been noted to decline over time in children receiving cranial irradiation (20). This decline correlates with age, with children less than seven years old being the most profoundly affected (21). The amount of neurocognitive dysfunction is further correlated to the mean dose of radiation received by the brain (22) and deficits relative to peers are commonly seen in working memory, sustained attention and processing speed (23, 24). It is important to note however, that baseline neurocognitive functioning can be compromised prior to the use of radiation therapy and is evident in patients who never receive radiotherapy. In these cases, neurocognitive decline is related to the tumor itself, surgical morbidity, chemotherapy and/or hydrocephalus.
Neuro-endocrine function can be perturbed as a result of tumor involvement of the hypothalamic-pituitary axis but can also be negatively affected by radiation to these structures. Growth hormone and thyroid hormone are the most commonly affected by radiation, followed by effects on sex hormone levels and adrenocorticotropic hormone (ACTH) secretion (25, 26).
Radiation therapy can have detrimental effects on other tissues in a dose dependent manner. Radiation to the cochlea can lead to hearing loss at doses greater than 35-45 Gy in the absence of chemotherapy (27, 28). The risk of ototoxicity is markedly increased in children who receive ototoxic platinum based chemotherapy regimens (29–31). Craniospinal irradiation, most commonly used as part of medulloblastoma treatment, can further lead to primary thyroid dysfunction and damage to the lung, heart and intestinal tract (32, 33). Additionally, radiation can be carcinogenic and patients irradiated at a young age are at an increased risk of developing a radiation induced second tumor compared to their adult counterparts.
Medulloblastoma
Medulloblastoma is the most common malignant CNS tumor in the pediatric population. Treatment regimens are determined by inclusion in one of two risk categories, based on patient age, extent of surgical resection, the presence or absence of CNS dissemination, and histologic characteristics. Survival for standard risk patients is 80-85% (34) while greater than 50% of patients with high-risk disease are cured with aggressive therapy (35). Standard therapy in both risk groups is a maximal safe surgical resection followed by craniospinal irradiation and platinum based chemotherapy. Children under the age of three are treated with additional chemotherapy in an effort to delay or avoid CSI, due to the increased toxicity of CSI in the very young. Classification as standard risk allows for a reduced CSI dose of 23.4 Gy, compared to 36 Gy for those in the high-risk group. In both groups, the posterior fossa or tumor bed receives additional radiation to a total dose of 54-55.8 Gy.
There is a relatively large experience with proton therapy for medulloblastoma, given the large treatment fields and the young age of these patients. However, late effects data comparing proton radiation to photon treatment has not yet matured. One practical difference in the delivery of proton RT is that the entire vertebral body needs to be treated in developing children due to the sharp dose fall off inherent to proton RT. In these cases, asymmetric radiation of the vertebral body may increase the risk of scoliosis or kyphosis. Children who have completed bone growth are treated to the thecal sac alone, allowing for more vertebral body sparing. In these patients, radiation induced fatty replacement of marrow is noted as a sharp demarcation on T1 images, providing in vivo evidence of the sharp dose fall off provided by proton therapy (36, 37). Dosimetric studies have been undertaken which suggest that proton RT in medulloblastoma should lead to decreased long-term toxicity. In an example case of CSI followed by a posterior fossa boost in a 3.5 year old boy, proton radiotherapy markedly decreased dose to the cochlea, pituitary gland, hypothalamus, temporomandibular joints, parotid glands and heart compared to conventional X-Ray and IMRT plans (38). Similar results were reported by Loma Linda on three children between three and four years of age who received proton CSI (39). A separate study comparing proton to photon RT in ten medulloblastoma patients revealed significant dose savings to the cochlea and the supratentorial brain with proton radiation (40). This reduction in brain dose, using a dose-cognitive effects model, was predicted to result in significantly higher IQ scores in children treated with protons. A further study comparing 3D-conformal RT, IMRT, and protons in two medulloblastoma cases revealed significant dose savings with protons to the cochlea, hypothalamicpituitary axis, the optic chiasm, eyes, mandible, thyroid, lung, kidneys, liver, and heart (41). Protons provide the additional advantage of significantly decreasing dose to the lens of the eye when treatment beams are angled 15°-20° to the posterior, an effect most noticeable in children under 10 years of age (42).
Low Grade Glioma
Low-grade gliomas can occur anywhere in the brain and are effectively managed with a gross total resection when feasible (7, 43). Gliomas in locations where surgical resection can lead to unacceptable morbidity, including the optic nerves or chiasm, brainstem, diencephalon and cervical-medullary junction, are often treated with chemotherapy in young patients in the hopes of delaying radiotherapy (44). Radiation to a dose of 54 Gy is often reserved for unresectable, progressive lesions (45). The goal of proton radiotherapy in these patients is a reduction in late-effects, not improved survival, since the total dose to the target tumor is the same as with photons. Loma Linda University Medical Center has reported on the use of protons in the treatment of low-grade gliomas (46). As expected, three quarters of the patients receiving RT had tumors located in the brainstem or diencephalon. Among the 27 patients treated, six experienced a local failure. Acute side effects were minimal and Moyamoya syndrome developed in one patient. At a median follow up of three years, all children with local control maintained their performance status. A separate dosimetric comparison of protons to photons for seven optic pathway gliomas treated at Loma Linda revealed a marked decrease in dose to the contralateral optic nerve and temporal lobes and a more modest reduction in dose to the pituitary gland and optic chiasm with the use of protons (47). An additional study comparing proton to photon RT in ten patients with optic pathway glioma revealed significant dose savings to the cochlea and supratentorial brain (40). This decreased brain dose was expected to correlate with improved cognitive outcomes based on a dosecognitive effects model.
Craniopharyngioma
Craniopharyngiomas are benign, often cystic lesions, which occur most commonly in children in the late first and second decades of life. The vast majority of craniopharyngiomas occur in the suprasellar regions and present with neuroendocrine abnormalities, visual field deficits or hydrocephalus (48). Historically, treatment consisted of a curative gross total resection with radiation reserved for residual or recurrent disease (49). Due to the substantial morbidity associated with gross total resections, current treatment often consists of a biopsy and/or partial debulking, followed by planned adjuvant RT. Due to the cystic nature of these tumors, routine surveillance is warranted during the radiation treatment course, as a change in cyst size may necessitate a replanning of the treatment volume (50).
The Massachusetts General Hospital has reported on 15 patients, five of whom were children, treated with combined photon/proton radiation or proton radiation alone with a median follow-up of 15.5 years (51). All five pediatric patients achieved local control without evidence of radiation related long-term deficits in endocrine or cognitive function. Additionally, Loma Linda has reported on the use of proton RT in 16 craniopharyngioma patients treated to doses of 50.4-59.4 cobalt Gray equivalent (CGE) with 5 year follow-up (52). Local control was achieved in 14 of the 15 patients with follow-up data. Three patients died, one of recurrent disease, one of sepsis and one of a right middle cerebral artery stroke. Among survivors, one developed panhypopitutarism 36 months after two debulking surgeries and RT, a second had a cerebrovascular accident 34 months after combined primary treatment, and a third developed a meningioma 59 months after initial photon RT, followed by salvage resection and proton radiation. A dosimetric study comparing proton to photon RT in ten craniopharyngioma patients revealed significant dose savings to the cochlea, infratentorial and supratentorial brain with protons (40). This decreased brain dose was expected to result in significantly higher IQ scores in children treated with protons based on a dose-cognitive effects model.
Ependymoma
Ependymomas arise from the ependymal lining of the ventricular system. Two thirds of these tumors are infratentorial, arising from the lining of fourth ventricle. One third of children are diagnosed under the age of three with the majority diagnosed by age six (53). The standard of care is a maximal surgical resection followed by adjuvant radiation therapy, with a gross total resection being the most important predictor of outcome (54, 55). Radiation therapy is delivered to the post-operative tumor bed and/or residual disease to doses of 54-60 Gy and has been shown to improve outcomes compared to surgery alone (55, 56). Chemotherapy can be used to delay post-operative RT in the very young in an effort to reduce radiation toxicities, but this approach is associated with an increased rate of failure (57). Currently, children as young as 1 year of age are treated with focal field irradiation.
The Massachusetts General Hospital has reported on the use of protons in seventeen children with ependymoma with a median follow-up of 26 months (58). Radiation doses ranged from 52.2 to 59.4 cobalt Gray equivalent (CGE). Local control, progression-free survival, and overall survival rates were 86%, 80%, and 89%, respectively. Both local recurrences were seen in patients who underwent subtotal resections. Longer follow-up is necessary to comment on late effects although no deleterious acute effects were noted. In the same study, the authors generated two IMRT plans in order to measure the dosimetric advantages provided by protons for treatment of ependymoma in infratentorial and supratentorial locations, respectively. In both locations, proton radiation provided for a significant decrease in dose to the whole brain, as well as the temporal lobes specifically. Proton RT better spared the pituitary gland, hypothalamus, cochlea and optic chiasm while providing equivalent target coverage of the resection cavity as compared with IMRT. A separate dosimetric study comparing proton to photon RT in ten infratentorial ependymoma patients similarly revealed marked dose savings to the cochlea, hypothalamus, pituitary, optic chiasm, and temporal lobes (40).
Intracranial Germ Cell Tumors
Germ cell tumors (GCT) typically arise within the suprasellar region or the pineal region but can occur in other areas of the brain (59). GCTs are more likely to occur in adolescents than other childhood brain tumors and are subdivided into two highly prognostic histologic subgroups: the more common and more favorable germinomas, and non-germinomatous germ cell tumors (NGGCTs). Germinomas are very sensitive to radiation and historically were treated with radiation alone consisting of CSI and a tumor bed boost to 45-50 Gy, with cure rates of >90% (60). Due to the significant morbidity of CSI, whole ventricular radiation therapy (WVRT) followed by an involved field boost has become the standard of treatment in localized disease. Furthermore, the addition of chemotherapy to the treatment regimen has allowed for a further reduction in radiation dose, while maintaining high control rates provided WVRT is still combined with a tumor bed boost (61). CSI does remain the standard of care for disseminated germinoma, although chemotherapy may allow for reduced doses in this setting as well. NGGCTs are less radiosensitive and require aggressive multi-modality therapy (62, 63). Radiation volumes for NGGCTs remain controversial and may include CSI with an involved field boost, WVRT with an involved field boost, or involved field alone.
The Massachusetts General Hospital recently reported on the use of protons in the treatment of germ cell tumors (64). Among the 22 patients treated, 13 had germinoma and nine had NGGCTs. 21 patients were treated with CSI, WVRT, or whole brain radiation followed by an involved field boost, while one patient received involved field alone. Radiation doses ranged from 30.6 to 57.6 CGE and all NGGCT patients received chemotherapy prior to RT. At a median follow-up of 28 months there were no CNS recurrences and no deaths. Following radiation two patients developed growth hormone deficiency and two developed central hypothyroidism. Longer follow-up will be necessary to define neurocognitive effects. In the same study, the authors compared dosimetric outcomes of photons and protons for a representative WVRT and involved field boost treatment. As expected, proton radiotherapy provided substantial sparing to the whole brain and temporal lobes. Dose savings were also noted for the optic nerves.
Pediatric Tumors Outside the CNS
Radiation therapy plays a role in the management of several solid tumors including retinoblastoma, rhabdomyosarcoma, Ewing’s Sarcoma, Wilms’ tumor, Hodgkin’s Lymphoma, and neuroblastoma. While the utility of proton radiation is well established within the CNS, its utility in extracranial tumors depends primarily on the tumor location and the treatment fields necessary for effective management. It stands to reason that curable tumors located adjacent to or within critical normal structures may benefit substantially from the dosimetric advantages of proton radiotherapy.
Retinoblastoma
While not a CNS tumor, the proximity of the orbit to the brain affords protons similar advantages to those seen in CNS malignancies. The median age for the development of sporadic lesions is 2 years, with the vast majority being unilateral. Conversely, the one-third of retinoblastoma patients harboring germline mutations in the Rb gene, standardly present with bilateral involvement, often during the first year of life (65). For small tumors, several curative options exist including thermotherapy, photocoagulation, cryotherapy and plaque brachytherapy, where radioactive sources are placed within the orbit adjacent to the tumor itself. In cases where vision cannot be preserved, enucleation is considered the standard of care. Induction chemotherapy followed by external beam radiation is employed in the setting of bilateral disease or in large unilateral tumors where vision may be salvaged.
The Massachusetts General Hospital has treated pediatric retinoblastoma patients with protons since 1986. This group recently undertook a dosimetric analysis of proton radiotherapy for lesions located at nasal, central and temporal locations within the eye (66). In no instance was appreciable radiation dose deposited in the brain parenchyma, and dose to the orbital bones was minimized. A separate analysis of three patients treated at the MD Anderson Cancer Center demonstrated superior target coverage and normal tissue sparing, most notably a decrease in dose to the orbital bones, with protons compared to electrons, 3D conformal RT and IMRT (41). It is reasonable to expect that long term follow up will provide evidence that proton radiation decreases the incidence of dry eye, cataract, retinal injury, orbital hypoplasia and neuroendocrine dysfunction seen with conventional photon treatment (67, 68). Reducing the integral dose in these children is of great importance since the risk of secondary tumors is approximately 1% per year in the genetic form (69).
Rhabdomyosarcoma
Rhabdomyosarcoma (RMS) is a malignant tumor of mesenchymal origin and is the most common pediatric soft tissue sarcoma (70). There is a bimodal distribution in incidence with two thirds of cases diagnosed before age six followed by an additional peak in adolescence. The head and neck is the most common site of presentation with the majority of these tumors in a parameningeal location, often abutting or invading the base of skull. Rhabdomyosarcomas also occur in the genitourinary system, extremity, trunk and orbit. RMS patients are stratified into low, intermediate and high-risk groups based on site of origin, size, histology, lymph node involvement, metastatic spread and extent of surgical resection (71). Patients with low risk disease who undergo a negative margin resection do not need radiation. Low risk patients with a gross total resection but positive margins receive 36 Gy, those with positive nodes 41.4 Gy and those with gross residual disease 50.4 Gy. Intermediate risk patients receive RT regardless of surgical margin status and those children with orbital primaries are treated to 45 Gy after biopsy alone. All patients receive chemotherapy.
The utility of proton radiation is dependent on the site of RMS origin. The Massachusetts General Hospital has recently published a dosimetric comparison of proton and intensity-modulated photon radiotherapy for ten pediatric parameningeal rhabdomyosarcoma patients, eight of whom had radiographic evidence of intracranial extension (72). Each patient was treated with protons, and the proton plan used for treatment was compared to an IMRT plan generated for the study. Proton treatment afforded equivalent target coverage while significantly decreasing dose to the optic structures (globe, lens, optic nerves, optic chiasm, and retina), the hypothalamic-pituitary axis, the brainstem, temporal lobes, cochlea, lacrimal glands and parotids. An additional study reports the clinical outcomes and late effects for seventeen patients treated with proton RT for parameningeal rhabdomyosarcoma at the MGH with a median follow-up of 5 years (73). 10/17 (59%) of patients were without tumor recurrence at study completion. Among these patients, late effects of multimodality treatment included mild facial hypoplasia (n = 7), lack of permanent tooth eruption (n = 3), decreased height velocity (n = 3), endocrinopathies (n = 2) and chronic sinus congestion (n = 2), all of which compare favorably to traditional photon based treatment.
The MGH has also reported their proton experience for seven children treated for orbital RMS to a median dose of 46.6 CGE with a median follow-up of 6.3 years (74). Six of the seven patients were without evidence of disease and the remaining child was salvaged with enucleation and stereotactic radiosurgery after local recurrence. Late effects of treatment were minimal and included mild to moderate orbital bony asymmetry or enophthalmous in all patients. No children had evidence of corneal pathology or dry eye syndrome. All patients with intact orbits had good vision in the treated eye and all were without neuroendocrine deficits. In the same publication, the authors compared the proton plans used for treatment to IMRT plans generated for the study. Proton radiation markedly decreased dose to the brain, temporal lobes, hypothalamic-pituitary axis, and both the ipsilateral and contralateral orbital structures.
The MGH has further reported their proton experience for seven children treated for bladder/prostate RMS to 36 to 50.4 CGE with a median follow-up of 27 months (75). Five of seven patients were without evidence of disease with intact bladders at study completion. One patient had a local recurrence in the treatment field, while a second had a local and a distant recurrence. Two of the five patients with intact bladders (40%) had bladder dysfunction, both of which were related to surgical procedures. No skeletal or gastrointestinal effects were noted, and all patients were too young to assess sexual function. The authors compared the proton plans used for treatment to IMRT plans generated for the study. Proton radiotherapy markedly decreased mean organ dose to the bladder, testes, femoral heads, pelvic growth plates and pelvic bones compared to IMRT. A separate dosimetric analysis of two female and one male patient treated at the MD Anderson Cancer Center for pelvic sarcoma demonstrated a marked reduction in ovarian dose with protons compared to both 3D conformal RT and IMRT (41). Protons provided the least dose to the pelvic bones, while IMRT afforded the best bladder sparing.
Ewing’s Sarcoma
Ewing’s Sarcoma is the second most common bone tumor of childhood with the majority of patients presenting early in the second decade of life with disease in the long bones of the appendicular skeleton or the pelvis (76). Standard treatment for localized disease consists of induction chemotherapy followed by local treatment and additional chemotherapy (77). Local therapy can consist of surgery, radiation, or both (78). Radiation is delivered to a dose of 55.8 Gy for gross disease, and 50.4 Gy for microscopic disease, respectively.
The Massachusetts General Hospital has reported the clinical outcomes for 30 patients treated with proton radiotherapy for Ewing’s sarcoma with a median follow-up of 38.4 months (79). The median RT dose was 54 CGE, and all patients received chemotherapy. Three year local control was 86% and few adverse advents were noted after multimodality therapy. The MGH has separately reported on the use of proton RT in two patients with Ewing’s Sarcoma of the paranasal sinuses, both of who obtained local control with multi-modality therapy (80).
Other Pediatric Non-CNS Malignancies
Radiation therapy plays an important role in the management of Hodgkin’s Lymphoma (81, 82), neuroblastoma (83, 84) and Wilms tumor (85). Proton radiotherapy is starting to be explored in these malignancies. Specifically, the University of Florida performed a dosimetric study comparing proton radiotherapy to 3D-conformal RT and IMRT in nine adult patients with Stage II Hodgkin’s Lymphoma of the neck and/or mediastinum treated with involved field RT to 30 Gy (86). Proton radiotherapy afforded statistically significant decreases in total body dose, dose to the lungs, and dose to the breasts compared to 3D conformal RT and IMRT. Furthermore, the University of Florida recently published their initial clinical experience in Hodgkin’s Lymphoma, describing the use of proton radiotherapy in a 41 year old woman with a mediastinal relapse receiving consolidative radiotherapy to 30.6 CGE (87). The authors comment on the significant dose savings to the heart protons afforded in this setting.
The Loma Linda group has reported on the administration of proton radiotherapy in a 4-year boy with neuroblastoma of the right adrenal gland, treated to 34.2 CGE after chemotherapy and delayed surgical resection (88). A separate dosimetric study of proton radiotherapy compared to 2-field photon abdominal irradiation or IMRT for advanced Wilms’ tumor and neuroblastoma, revealed decreased integral total body dose and decreased liver dose with proton radiotherapy (89).
New Directions/Controversies
Carbon Ion Therapy
Carbon ion therapy holds additional promise as a particle therapy that may provide improved dose distributions. Carbon ions are similar to protons as no dose is deposited distal to the maximum depth of penetration along the particle path. Interestingly, carbon ions have a similar RBE to protons along the particle path but have a markedly increased RBE (estimated at 3-4) at their maximum depth of penetration (90). As such, the deleterious effects on normal tissues proximal to the tumor are expected to be similar to proton radiotherapy, while tumor killing is enhanced at maximum depth. This may be especially important in hypoxic tumors as higher RBE modalities are less dependent on oxygen and are therefore expected to be more effective (91).
Carbon ion therapy has been used safely for numerous tumors in the adult population, including, but not limited to, prostate, lung, head and neck and base of skull (92). The pediatric experience with carbon therapy is extremely limited and includes 17 patients treated for skull base tumors at a median age of 18 years (range 5-21) at Gesellschaft für Schwerionenforschung (GSI) in Darmstadt, Germany with a median follow-up of 49 months (93). Local control was excellent at 94% and acute toxicities were minimal. Follow-up was too short to effectively analyze long term treatment risks, including second malignancies, which is of significant concern considering the high RBE of carbon therapy. At present, there are no operational or planned carbon centers in the United States, although centers in Germany and Japan are actively treating patients.
Second Malignancy Risks
The risk of a treatment induced secondary malignancy is especially important in the pediatric population where survivorship is measured in decades and whose developing tissues are especially susceptible to the carcinogenic effects of radiation. The cumulative incidence of a second malignancy 30 years after a childhood cancer diagnosis is 7.9% (excluding nonmelanoma skin cancer) and 3.1% for meningioma (94). Furthermore, among these patients, the relative risk of second malignancy is 2.7 fold higher in those who received radiation as part of a curative regimen (94). Miralbell et al. modeled 3D conformal RT, IMRT, and proton dose distributions to non-target organs in order to calculate the expected risk of a second malignancy in a model parameningeal rhabdomyosarcoma and medulloblastoma case, respectively. Based on these calculations, proton RT decreased the expected rate of a radiation-induced second malignancy two-fold in the rhabdomyosarcoma case and 8-15 fold in the medulloblastoma case (95). Notably this study did not take into account the role of neutron scatter, which can be significant in passive scatter proton radiation due to the production of neutrons in the head of the gantry as the beams are shaped. It has been suggested that neutron scatter can contribute substantially to malignancy risk (96). Notably, active scanning proton RT shapes the beam with magnets alone markedly decreasing the risks from neutron scatter. A more recent study of a passive scatter proton craniospinal RT plan for medulloblastoma did account for neutron production and estimated the lifetime risk of a fatal radiation induced secondary malignancy to be low at 3.4% (97).
There is limited long-term clinical data to date on second malignancy rates in children treated with proton radiotherapy. A retrospective case control study of adult and pediatric patients treated at the Harvard Cyclotron with proton radiotherapy matched to comparative photon treated patients from the SEER database revealed the risk of second malignancy to be reduced by greater than half in the proton cohort, with 6.8 year median follow-up after treatment (98).
Cost Effectiveness
Proton radiotherapy is significantly more expensive than standard photon treatment (99, 100). In order to be considered cost effective, proton therapy needs to either provide a survival benefit over standard treatment, or needs to reduce long-term treatment morbidity without sacrificing efficacy. As noted above, proton radiation provides significant dose savings to normal structures that are expected to translate into decreased long-term morbidity, although limited data showing decreased morbidity exists to date. Consequently, all cost effectiveness studies must make assumptions as to the degree of benefit proton therapy will provide. In a recent study of childhood medulloblastoma treatment, proton radiation was considered to be a cost-effective option, assuming improvement in IQ and endocrine function compared to standard photon treatment (101). Long-term outcomes studies of proton RT patients will be necessary to determine whether these assumptions are in fact correct. Additionally, the reduction in dose to normal tissues that proton radiotherapy provides may allow for safe and effective hypofractionation. By treating to an equivalent dose in fewer fractions, patients can be treated more efficiently, thereby decreasing health care costs and increasing the number of patients who could make use of this limited resource.
Conclusion
Proton radiotherapy holds significant promise in the treatment of pediatric malignancies and the number of children being treated with proton therapy for solid tumors is increasing rapidly throughout the world. Numerous clinical studies show protons to be as effective as standard photon treatment in disease control and dosimetric studies show significant dose sparing to developing normal tissues. Early clinical data is beginning to suggest that the dosimetric advantages of proton radiation equate to a true clinical advantage with decreased late effects. As proton centers continue to proliferate and the number of children treated with proton RT further increases, it will be of paramount importance to document the long term morbidity of proton treatments, both by objective clinical criteria (bone growth, endocrine function, etc.) and patient centered quality of life metrics.
Acknowledgements
We would like to thank Dr. Jay Loeffler, MD for helpful discussion of the manuscript and Judith Adams, CMD for assistance with the generation of figures.
Abbreviations
- (CGE)
Cobalt Gray Equivalent
- (CSI)
Craniospinal Irradiation
- (Gy)
Gray
- (IMRT)
Intensity Modulated Radiation Therapy
- (RT)
Radiation Therapy
- (RBE)
Relative Biological Effectiveness
- (SOBP)
Spread Out Bragg Peak
- (WVRT)
Whole Ventricular Radiation Therapy
Footnotes
Conflicts of Interest: There are no actual or potential conflicts of interest associated with the publication of this manuscript.
References
- 1. NCI Fact Sheet, 1/October/2008; http://www.cancer.gov/cancertopics/factsheet/Sites-Types/childhood.
- 2. Hewitt M., Weiner S. L., Simone J. V. Editors. Childhood Cancer Survivorship: Improving Care and Quality of Life (National Academies Press, 2003). [PubMed] [Google Scholar]
- 3. Schwartz C. L. Long-term survivors of childhood cancer: the late effects of therapy. The Oncologist 4, 45–54 (1999). [PubMed] [Google Scholar]
- 4. Hustu H. O., Aur R. J. Extramedullary leukaemia. Clinics in Haematology 7, 313–337 (1978). [DOI] [PubMed] [Google Scholar]
- 5. Hertzberg H., Huk W. J., Ueberall M. A., Langer T., Meier W., Dopfer R., Skalej M., Lackner H., Bode U., Janssen G., Zintl F., Beck J. D. CNS late effects after ALL therapy in childhood. Part I: Neuroradiological findings in long-term survivors of childhood ALL–an evaluation of the interferences between morphology and neuropsychological performance. The German Late Effects Working Group. Medical and Pediatric Oncology 28, 387–400 (1997). [DOI] [PubMed] [Google Scholar]
- 6. Paolucci G., Vecchi V., Favre C., Miniero R., Madon E., Pession A., Rondelli R., De Rossi G., Lo Nigro L., Porta F., Santoro N., Indolfi P., Basso G., Conter V., Arico M. Treatment of childhood acute lymphoblastic leukemia. Long-term results of the AIEOP-ALL 87 study. Haematologica 86, 478–484 (2001). [PubMed] [Google Scholar]
- 7. Pollack I. F., Claassen D., al-Shboul Q., Janosky J. E., Deutsch M. Low-grade gliomas of the cerebral hemispheres in children: an analysis of 71 cases. Journal of Neurosurgery 82, 536–547 (1995). [DOI] [PubMed] [Google Scholar]
- 8. Kiltie A. E., Lashford L. S., Gattamaneni H. R. Survival and late effects in medulloblastoma patients treated with craniospinal irradiation under three years old. Medical and Pediatric Oncology 28, 348–354 (1997). [DOI] [PubMed] [Google Scholar]
- 9. Reddy A. T., Packer R. J. Chemotherapy for low-grade gliomas. Childs Nerv Syst 15, 506–513 (1999). [DOI] [PubMed] [Google Scholar]
- 10. Packer R. J., Goldwein J., Nicholson H. S., Vezina L. G., Allen J. C., Ris M. D., Muraszko K., Rorke L. B., Wara W. M., Cohen B. H., Boyett J. M. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children’s Cancer Group Study. J Clin Oncol 17, 2127–2136 (1999). [DOI] [PubMed] [Google Scholar]
- 11. Hurkmans C. W., Remeijer P., Lebesque J. V., Mijnheer B. J. Set-up verification using portal imaging; review of current clinical practice. Radiotherapy & Oncology 58, 105–120 (2001). [DOI] [PubMed] [Google Scholar]
- 12. Kooy H. M., Dunbar S. F., Tarbell N. J., Mannarino E., Ferarro N., Shusterman S., Bellerive M., Finn L., McDonough C. V., Loeffler J. S. Adaptation and verification of the relocatable Gill-Thomas-Cosman frame in stereotactic radiotherapy. International Journal of Radiation Oncology, Biology, Physics 30, 685–691 (1994). [DOI] [PubMed] [Google Scholar]
- 13. Nevinny-Stickel M., Sweeney R. A., Bale R. J., Posch A., Auberger T., Lukas P. Reproducibility of patient positioning for fractionated extracranial stereotactic radiotherapy using a double-vacuum technique. Strahlentherapie und Onkologie 180, 117–122 (2004). [DOI] [PubMed] [Google Scholar]
- 14. Lee Y. K., Brooks C. J., Bedford J. L., Warrington A. P., Saran F. H. Development and Evaluation of Multiple Isocentric Volumetric Modulated Arc Therapy Technique for Craniospinal Axis Radiotherapy Planning. International journal of Radiation Oncology, Biology, Physics 82, 1006–1012 (2012). [DOI] [PubMed] [Google Scholar]
- 15. Hall E. J., Wuu C. S. I. H. E. J. Radiation-induced second cancers: the impact of 3D-CRT and IMRT.[see comment]. International Journal of Radiation Oncology, Biology, Physics 56, 83–88 (2003). [DOI] [PubMed] [Google Scholar]
- 16. Paganetti H. Interpretation of proton relative biological effectiveness using lesion induction, lesion repair, and cellular dose distribution. Med Phys 32, 2548–2556 (2005). [DOI] [PubMed] [Google Scholar]
- 17. Gerweck L., Paganetti H. in Proton and Charged Particle Radiotherapy (Ed. Kooy H. M., Delaney T. F.) 8–18 (Lipincott Williams and Wilkins, 2008). [Google Scholar]
- 18. Gottschalk B. in Proton and Charged Particle Radiotherapy (Ed. Kooy H. M., Delaney T. F.) 33–49 (Lippincott Williams and Wilkins, 2008). [Google Scholar]
- 19. Lomax A. J., Bohringer T., Bolsi A., Coray D., Emert F., Goitein G., Jermann M., Lin S., Pedroni E., Rutz H., Stadelmann O., Timmermann B., Verwey J., Weber D. C. Treatment planning and verification of proton therapy using spot scanning: initial experiences. Med Phys 31, 3150–3157 (2004). [DOI] [PubMed] [Google Scholar]
- 20. Spiegler B. J., Bouffet E., Greenberg M. L., Rutka J. T., Mabbott D. J. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol 22, 706–713 (2004). [DOI] [PubMed] [Google Scholar]
- 21. Ris M. D., Packer R., Goldwein J., Jones-Wallace D., Boyett J. M. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children’s Cancer Group study. J Clin Oncol 19, 3470–3476 (2001). [DOI] [PubMed] [Google Scholar]
- 22. Merchant T. E., Kiehna E. N., Li C., Shukla H., Sengupta S., Xiong X., Gajjar A., Mulhern R. K. Modeling radiation dosimetry to predict cognitive outcomes in pediatric patients with CNS embryonal tumors including medulloblastoma. Int J Radiat Oncol Biol Phys 65, 210–221 (2006). [DOI] [PubMed] [Google Scholar]
- 23. Butler R. W., Copeland D. R. Attentional processes and their remediation in children treated for cancer: a literature review and the development of a therapeutic approach. J Int Neuropsychol Soc 8, 115–124 (2002). [PubMed] [Google Scholar]
- 24. Schatz J., Kramer J. H., Ablin A., Matthay K. K. Processing speed, working memory, and IQ: a developmental model of cognitive deficits following cranial radiation therapy. Neuropsychology 14, 189–200 (2000). [DOI] [PubMed] [Google Scholar]
- 25. Heikens J., Michiels E. M., Behrendt H., Endert E., Bakker P. J., Fliers E. Long-term neuro-endocrine sequelae after treatment for childhood medulloblastoma. Eur J Cancer 34, 1592–1597 (1998). [DOI] [PubMed] [Google Scholar]
- 26. Merchant T. E., Conklin H. M., Wu S., Lustig R. H., Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol 27, 3691–3697 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hua C., Bass J. K., Khan R., Kun L. E., Merchant T. E. Hearing loss after radiotherapy for pediatric brain tumors: effect of cochlear dose. Int J Radiat Oncol Biol Phys 72, 892–899 (2008). [DOI] [PubMed] [Google Scholar]
- 28. Merchant T. E., Gould C. J., Xiong X., Robbins N., Zhu J., Pritchard D. L., Khan R., Heideman R. L., Krasin M. J., Kun L. E. Early neuro-otologic effects of three-dimensional irradiation in children with primary brain tumors. International Journal of Radiation Oncology, Biology, Physics. 58, 1194–1207 (2004). [DOI] [PubMed] [Google Scholar]
- 29. Miettinen S., Laurikainen E., Johansson R., Minn H., Laurell G., Salmi T. T. Radiotherapy enhanced ototoxicity of cisplatin in children. Acta Oto-laryngologica 529, 90–94 (1997). [DOI] [PubMed] [Google Scholar]
- 30. Schell M. J., McHaney V. A., Green A. A., Kun L. E., Hayes F. A., Horowitz M., Meyer W. H. Hearing loss in children and young adults receiving cisplatin with or without prior cranial irradiation. Journal of Clinical Oncology: Official Journal of The American Society of Clinical Oncology 7, 754–760 (1989). [DOI] [PubMed] [Google Scholar]
- 31. Walker D. A., Pillow J., Waters K. D., Keir E. Enhanced cis-platinum ototoxicity in children with brain tumours who have received simultaneous or prior cranial irradiation. Medical and Pediatric Oncology 17, 48–52 (1989). [DOI] [PubMed] [Google Scholar]
- 32. Jakacki R. I., Goldwein J. W., Larsen R. L., Barber G., Silber J. H. Cardiac dysfunction following spinal irradiation during childhood. J Clin Oncol 11, 1033–1038 (1993). [DOI] [PubMed] [Google Scholar]
- 33. Jakacki R. I., Schramm C. M., Donahue B. R., Haas F., Allen J. C. Restrictive lung disease following treatment for malignant brain tumors: a potential late effect of craniospinal irradiation. J Clin Oncol 13, 1478–1485 (1995). [DOI] [PubMed] [Google Scholar]
- 34. Packer R. J., Sutton L. N., Elterman R., Lange B., Goldwein J., Nicholson H. S., Mulne L., Boyett J., D’Angio G., Wechsler-Jentzsch, et al. , Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. Journal of Neurosurgery 81, 690–698 (1994). [DOI] [PubMed] [Google Scholar]
- 35. McLean T. W. Medulloblastomas and central nervous system primitive neuroectodermal tumors. Current Treatment Options in Oncology 4, 499–508 (2003). [DOI] [PubMed] [Google Scholar]
- 36. Krejcarek S. C., Grant P. E., Henson J. W., Tarbell N. J., Yock T. I. Physiologic and radiographic evidence of the distal edge of the proton beam in craniospinal irradiation. Int J Radiat Oncol Biol Phys 68, 646–649 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gensheimer M. F., Yock T. I., Liebsch N. J., Sharp G. C., Paganetti H., Madan N., Grant P. E., Bortfeld T. In vivo proton beam range verification using spine MRI changes. Int J Radiat Oncol Biol Phys 78, 268–275 (2010). [DOI] [PubMed] [Google Scholar]
- 38. St Clair W. H., Adams J. A., Bues M., Fullerton B. C., La Shell S., Kooy H. M., Loeffler J. S., Tarbell N. J. Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. International Journal of Radiation Oncology, Biology, Physics. 58, 727–734 (2004). [DOI] [PubMed] [Google Scholar]
- 39. Yuh G. E., Loredo L. N., Yonemoto L. T., Bush D. A., Shahnazi K., Preston W., Slater J. M., Slater J. D. Reducing toxicity from craniospinal irradiation: using proton beams to treat medulloblastoma in young children. Cancer Journal 10, 386–390 (2004). [DOI] [PubMed] [Google Scholar]
- 40. Merchant T. E., Hua C. H., Shukla H., Ying X., Nill S., Oelfke U. Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatric Blood & Cancer 51, 110–117 (2008). [DOI] [PubMed] [Google Scholar]
- 41. Lee C. T., Bilton S. D., Famiglietti R. M., Riley B. A., Mahajan A., Chang E. L., Maor M. H., Woo S. Y., Cox J. D., Smith A. R. Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: how do protons compare with other conformal techniques? Int J Radiat Oncol Biol Phys 63, 362–372 (2005). [DOI] [PubMed] [Google Scholar]
- 42. Cochran D. M., Yock T. I., Adams J. A., Tarbell N. J. Radiation dose to the lens during craniospinal irradiation-an improvement in proton radiotherapy technique. Int J Radiat Oncol Biol Phys 70, 1336–1342 (2008). [DOI] [PubMed] [Google Scholar]
- 43. Forsyth P. A., Shaw E. G., Scheithauer B. W., O’Fallon J. R., Layton D. D., Jr., Katzmann J. A. Supratentorial pilocytic astrocytomas. A clinicopathologic, prognostic, and flow cytometric study of 51 patients. Cancer 72, 1335–1342 (1993). [DOI] [PubMed] [Google Scholar]
- 44. Silva M. M., Goldman S., Keating G., Marymont M. A., Kalapurakal J., Tomita T. Optic pathway hypothalamic gliomas in children under three years of age: the role of chemotherapy. Pediatric neurosurgery 33, 151–158 (2000). [DOI] [PubMed] [Google Scholar]
- 45. Merchant T. E., Kun L. E., Wu S., Xiong X., Sanford R. A., Boop F. A. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol 27, 3598–3604 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hug E. B., Muenter M. W., Archambeau J. O., DeVries A., Liwnicz B., Loredo L. N., Grove R. I., Slater J. D. Conformal proton radiation therapy for pediatric low-grade astrocytomas. Strahlenther Onkol 178, 10–17 (2002). [DOI] [PubMed] [Google Scholar]
- 47. Fuss M., Hug E. B., Schaefer R. A., Nevinny-Stickel M., Miller D. W., Slater J. M., Slater J. D. Proton radiation therapy (PRT) for pediatric optic pathway gliomas: comparison with 3D planned conventional photons and a standard photon technique. Int J Radiat Oncol Biol Phys 45, 1117–1126 (1999). [DOI] [PubMed] [Google Scholar]
- 48. Ohmori K., Collins J., Fukushima T. Craniopharyngiomas in children. Pediatric neurosurgery 43, 265–278 (2007). [DOI] [PubMed] [Google Scholar]
- 49. Kiehna E. N., Merchant T. E. Radiation therapy for pediatric craniopharyngioma. Neurosurgical Focus 28, E10. [DOI] [PubMed] [Google Scholar]
- 50. Winkfield K. M., Linsenmeier C., Yock T. I., Grant P. E., Yeap B. Y., Butler W. E., Tarbell N. J. Surveillance of craniopharyngioma cyst growth in children treated with proton radiotherapy. Int J Radiat Oncol Biol Phys 73, 716–721 (2009). [DOI] [PubMed] [Google Scholar]
- 51. Fitzek M. M., Linggood R. M., Adams J., Munzenrider J. E. Combined proton and photon irradiation for craniopharyngioma: long-term results of the early cohort of patients treated at Harvard Cyclotron Laboratory and Massachusetts General Hospital. Int J Radiat Oncol Biol Phys 64, 1348–1354 (2006). [DOI] [PubMed] [Google Scholar]
- 52. Luu Q. T., Loredo L. N., Archambeau J. O., Yonemoto L. T., Slater J. M., Slater J. D. Fractionated proton radiation treatment for pediatric craniopharyngioma: preliminary report. Cancer Journal (Sudbury, Mass 12, 155–159 (2006). [PubMed] [Google Scholar]
- 53. Merchant T. E. Current management of childhood ependymoma. Oncology (Huntington). 16, 629–642, 644; discussion 645–626, 648 (2002). [PubMed] [Google Scholar]
- 54. Merchant T. E., Li C., Xiong X., Kun L. E., Boop F. A., Sanford R. A. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. The lancet Oncology 10, 258–266 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rousseau P., Habrand J. L., Sarrazin D., Kalifa C., Terrier-Lacombe M. J., Rekacewicz C., Rey A. Treatment of intracranial ependymomas of children: review of a 15-year experience. Int J Radiat Oncol Biol Phys 28, 381–386 (1994). [DOI] [PubMed] [Google Scholar]
- 56. Rogers L., Pueschel J., Spetzler R., Shapiro W., Coons S., Thomas T., Speiser B. Is gross-total resection sufficient treatment for posterior fossa ependymomas? Journal of Neurosurgery 102, 629–636 (2005). [DOI] [PubMed] [Google Scholar]
- 57. Duffner P. K., Horowitz M. E., Krischer J. P., Burger P. C., Cohen M. E., Sanford R. A., Friedman H. S., Kun L. E. The treatment of malignant brain tumors in infants and very young children: an update of the Pediatric Oncology Group experience. Neuro-Oncology 1, 152–161 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. MacDonald S. M., Safai S., Trofimov A., Wolfgang J., Fullerton B., Yeap B. Y., Bortfeld T., Tarbell N. J., Yock T. Proton radiotherapy for childhood ependymoma: initial clinical outcomes and dose comparisons. Int J Radiat Oncol Biol Phys 71, 979–986 (2008). [DOI] [PubMed] [Google Scholar]
- 59. Jennings M. T., Gelman R., Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. Journal of Neurosurgery 63, 155–167 (1985). [DOI] [PubMed] [Google Scholar]
- 60. Maity A., Shu H. K., Janss A., Belasco J. B., Rorke L., Phillips P. C., Sutton L. N., Goldwein J. W. Craniospinal radiation in the treatment of biopsy-proven intracranial germinomas: twenty-five years’ experience in a single center. Int J Radiat Oncol Biol Phys 58, 1165–1170 (2004). [DOI] [PubMed] [Google Scholar]
- 61. Allen J. C., DaRosso R. C., Donahue B., Nirenberg A. A phase II trial of preirradiation carboplatin in newly diagnosed germinoma of the central nervous system. Cancer 74, 940–944 (1994). [DOI] [PubMed] [Google Scholar]
- 62. Dearnaley D. P., A’Hern R. P., Whittaker S., Bloom H. J. Pineal and CNS germ cell tumors: Royal Marsden Hospital experience 1962–1987. Int J Radiat Oncol Biol Phys 18, 773–781 (1990). [DOI] [PubMed] [Google Scholar]
- 63. Robertson P. L., DaRosso R. C., Allen J. C. Improved prognosis of intracranial non-germinoma germ cell tumors with multimodality therapy. Journal of Neuro-oncology 32, 71–80 (1997). [DOI] [PubMed] [Google Scholar]
- 64. MacDonald S. M., Trofimov A., Safai S., Adams J., Fullerton B., Ebb D., Tarbell N. J., Yock T. I. Proton radiotherapy for pediatric central nervous system germ cell tumors: early clinical outcomes. Int J Radiat Oncol Biol Phys 79, 121–129 (2011). [DOI] [PubMed] [Google Scholar]
- 65. MacCarthy A., Birch J. M., Draper G. J., Hungerford J. L., Kingston J. E., Kroll M. E., Onadim Z., Stiller C. A., Vincent T. J., Murphy M. F. Retinoblastoma in Great Britain 1963–2002. The British Journal of Ophthalmology 93, 33–37 (2009). [DOI] [PubMed] [Google Scholar]
- 66. Krengli M., Hug E. B., Adams J. A., Smith A. R., Tarbell N. J., Munzenrider J. E. Proton radiation therapy for retinoblastoma: comparison of various intraocular tumor locations and beam arrangements. Int J Radiat Oncol Biol Phys 61, 583–593 (2005). [DOI] [PubMed] [Google Scholar]
- 67. Kaste S. C., Chen G., Fontanesi J., Crom D. B., Pratt C. B. Orbital development in long-term survivors of retinoblastoma. J Clin Oncol 15, 1183–1189 (1997). [DOI] [PubMed] [Google Scholar]
- 68. Schipper J., Tan K. E., van Peperzeel H. A. Treatment of retinoblastoma by precision megavoltage radiation therapy. Radiother Oncol 3, 117–132 (1985). [DOI] [PubMed] [Google Scholar]
- 69. Wong F. L., Boice J. D., Jr., Abramson D. H., Tarone R. E., Kleinerman R. A., Stovall M., Goldman M. B., Seddon J. M., Tarbell N., Fraumeni J. F., Jr., Li F. P. Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk. JAMA: the journal of the American Medical Association 278, 1262–1267 (1997). [DOI] [PubMed] [Google Scholar]
- 70. Punyko J. A., Mertens A. C., Baker K. S., Ness K. K., Robison L. L., Gurney J. G. Long-term survival probabilities for childhood rhabdomyosarcoma. A population-based evaluation. Cancer 103, 1475–1483 (2005). [DOI] [PubMed] [Google Scholar]
- 71. Raney R. B., Anderson J. R., Barr F. G., Donaldson S. S., Pappo A. S., Qualman S. J., Wiener E. S., Maurer H. M., Crist W. M. Rhabdomyosarcoma and undifferentiated sarcoma in the first two decades of life: a selective review of intergroup rhabdomyosarcoma study group experience and rationale for Intergroup Rhabdomyosarcoma Study V. J Pediatr Hematol Oncol 23, 215–220 (2001). [DOI] [PubMed] [Google Scholar]
- 72. Kozak K. R., Adams J., Krejcarek S. J., Tarbell N. J., Yock T. I. A dosimetric comparison of proton and intensity-modulated photon radiotherapy for pediatric parameningeal rhabdomyosarcomas. Int J Radiat Oncol Biol Phys 74, 179–186 (2009). [DOI] [PubMed] [Google Scholar]
- 73. Childs S. K., Kozak K. R., Friedmann A. M., Yeap B. Y., Adams J., Macdonald S. M., Liebsch N. J., Tarbell N. J., Yock T. I. Proton Radiotherapy for Parameningeal Rhabdomyosarcoma: Clinical Outcomes and Late Effects. International Journal of Radiation Oncology, Biology, Physics (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yock T., Schneider R., Friedmann A., Adams J., Fullerton B., Tarbell N. Proton radiotherapy for orbital rhabdomyosarcoma: clinical outcome and a dosimetric comparison with photons. Int J Radiat Oncol Biol Phys 63, 1161–1168 (2005). [DOI] [PubMed] [Google Scholar]
- 75. Cotter S. E., Herrup D. A., Friedmann A., Macdonald S. M., Pieretti R. V., Robinson G., Adams J., Tarbell N. J., Yock T. I. Proton radiotherapy for pediatric bladder/prostate rhabdomyosarcoma: clinical outcomes and dosimetry compared to intensity-modulated radiation therapy. International journal of radiation oncology, biology, physics 81, 1367–1373 (2011). [DOI] [PubMed] [Google Scholar]
- 76. Denny C. T. Ewing’s sarcoma–a clinical enigma coming into focus. J Pediatr Hematol Oncol 20, 421–425 (1998). [DOI] [PubMed] [Google Scholar]
- 77. Gibbs I. C., Tuamokumo N., Yock T. I. Role of radiation therapy in pediatric cancer. Hematol Oncol Clin North Am 20, 455–470 (2006). [DOI] [PubMed] [Google Scholar]
- 78. Krasin M. J., Rodriguez-Galindo C., Davidoff A. M., Billups C. A., Fuller C. E., Neel M. D., Kun L. E., Merchant T. E. Efficacy of combined surgery and irradiation for localized Ewings sarcoma family of tumors. Pediatric Blood & Cancer 43, 229–236 (2004). [DOI] [PubMed] [Google Scholar]
- 79. Rombi B., Delaney T. F., Macdonald S. M., Huang M. S., Ebb D. H., Liebsch N. J., Raskin K. A., Yeap B. Y., Marcus K. J., Tarbell N. J., Yock T. I. Proton Radiotherapy for Pediatric Ewing’s Sarcoma: Initial Clinical Outcomes. International Journal of Radiation Oncology, Biology, Physics (2011). [DOI] [PubMed] [Google Scholar]
- 80. Gray S. T., Chen Y. L., Lin D. T. Efficacy of Proton Beam Therapy in the Treatment of Ewing’s Sarcoma of the Paranasal Sinuses and Anterior Skull Base. Skull Base 19, 409–416 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hutchinson R. J., Fryer C. J., Davis P. C., Nachman J., Krailo M. D., O’Brien R. T., Collins R. D., Whalen T., Reardon D., Trigg M. E., Gilchrist G. S. MOPP or radiation in addition to ABVD in the treatment of pathologically staged advanced Hodgkin’s disease in children: results of the Children’s Cancer Group Phase III Trial. J Clin Oncol 16, 897–906 (1998). [DOI] [PubMed] [Google Scholar]
- 82. Nachman J. B., Sposto R., Herzog P., Gilchrist G. S., Wolden S. L., Thomson J., Kadin M. E., Pattengale P., Davis P. C., Hutchinson R. J., White K. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin’s disease who achieve a complete response to chemotherapy. J Clin Oncol 20, 3765–3771 (2002). [DOI] [PubMed] [Google Scholar]
- 83. Haas-Kogan D. A., Swift P. S., Selch M., Haase G. M., Seeger R. C., Gerbing R. B., Stram D. O., Matthay K. K. Impact of radiotherapy for high-risk neuroblastoma: a Children’s Cancer Group study. Int J Radiat Oncol Biol Phys 56, 28–39 (2003). [DOI] [PubMed] [Google Scholar]
- 84. Marcus K. J., Shamberger R., Litman H., von Allmen D., Grupp S. A., Nancarrow C. M., Goldwein J., Grier H. E., Diller L. Primary tumor control in patients with stage 3/4 unfavorable neuroblastoma treated with tandem double autologous stem cell transplants. J Pediatr Hematol Oncol 25, 934–940 (2003). [DOI] [PubMed] [Google Scholar]
- 85. Kalapurakal J. A., Dome J. S., Perlman E. J., Malogolowkin M., Haase G. M., Grundy P., Coppes M. J. Management of Wilms’ tumour: current practice and future goals. The Lancet Oncology 5, 37–46 (2004). [DOI] [PubMed] [Google Scholar]
- 86. Chera B. S., Rodriguez C., Morris C. G., Louis D., Yeung D., Li Z., Mendenhall N. P. Dosimetric comparison of three different involved nodal irradiation techniques for stage II Hodgkin’s lymphoma patients: conventional radiotherapy, intensity-modulated radiotherapy, and three-dimensional proton radiotherapy. Int J Radiat Oncol Biol Phys 75, 1173–1180 (2009). [DOI] [PubMed] [Google Scholar]
- 87. Hoppe B. S., Flampouri S., Li Z., Mendenhall N. P. Cardiac sparing with proton therapy in consolidative radiation therapy for Hodgkin lymphoma. Leukemia & Lymphoma 51, 1559–1562. [DOI] [PubMed] [Google Scholar]
- 88. Hug E. B., Nevinny-Stickel M., Fuss M., Miller D. W., Schaefer R. A., Slater J. D. Conformal proton radiation treatment for retroperitoneal neuroblastoma: introduction of a novel technique. Medical and pediatric oncology 37, 36–41 (2001). [DOI] [PubMed] [Google Scholar]
- 89. Hillbrand M., Georg D., Gadner H., Potter R., Dieckmann K. Abdominal cancer during early childhood: a dosimetric comparison of proton beams to standard and advanced photon radiotherapy. Radiother Oncol 89, 141–149 (2008). [DOI] [PubMed] [Google Scholar]
- 90. Kanai T., Matsufuji N., Miyamoto T., Mizoe J., Kamada T., Tsuji H., Kato H., Baba M., Tsujii H. Examination of GyE system for HIMAC carbon therapy. Int J Radiat Oncol Biol Phys 64, 650–656 (2006). [DOI] [PubMed] [Google Scholar]
- 91. Tobias C. A., Blakely E. A., Alpen E. L., Castro J. R., Ainsworth E. J., Curtis S. B., Ngo F. Q., Rodriguez A., Roots R. J., Tenforde T., Yang T. C. Molecular and cellular radiobiology of heavy ions. Int J Radiat Oncol Biol Phys 8, 2109–2120 (1982). [DOI] [PubMed] [Google Scholar]
- 92. Schulz-Ertner D., Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol 25, 953–964 (2007). [DOI] [PubMed] [Google Scholar]
- 93. Combs S. E., Nikoghosyan A., Jaekel O., Karger C. P., Haberer T., Munter M. W., Huber P. E., Debus J., Schulz-Ertner D. Carbon ion radiotherapy for pediatric patients and young adults treated for tumors of the skull base. Cancer 115, 1348–1355 (2009). [DOI] [PubMed] [Google Scholar]
- 94. Friedman D. L., Whitton J., Leisenring W., Mertens A. C., Hammond S., Stovall M., Donaldson S. S., Meadows A. T., Robison L. L., Neglia J. P. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. Journal of the National Cancer Institute 102, 1083–1095 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Miralbell R., Lomax A., Cella L., Schneider U. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int J Radiat Oncol Biol Phys 54, 824–829 (2002). [DOI] [PubMed] [Google Scholar]
- 96. Hall E. J. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys 65, 1–7 (2006). [DOI] [PubMed] [Google Scholar]
- 97. Taddei P. J., Mirkovic D., Fontenot J. D., Giebeler A., Zheng Y., Kornguth D., Mohan R., Newhauser W. D. Stray radiation dose and second cancer risk for a pediatric patient receiving craniospinal irradiation with proton beams. Physics in Medicine and Biology 54, 2259–2275 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chung C. S., Keating T., Yock T. I., Tarbell N. Comparative Analysis of Second Malignancy Risk in Patients Treated with Proton Therapy versus Conventional Photon Therapy. International Journal of Radiation Oncology*Biology*Physics 72, S8 (2008). [Google Scholar]
- 99. Goitein M., Jermann M. The relative costs of proton and X-ray radiation therapy. Clin Oncol (R Coll Radiol) 15, S37–50 (2003). [DOI] [PubMed] [Google Scholar]
- 100. Lundkvist J., Ekman M., Ericsson S. R., Jonsson B., Glimelius B. Proton therapy of cancer: potential clinical advantages and cost-effectiveness. Acta Oncol 44, 850–861 (2005). [DOI] [PubMed] [Google Scholar]
- 101. Lundkvist J., Ekman M., Ericsson S. R., Jonsson B., Glimelius B. Cost-effectiveness of proton radiation in the treatment of childhood medulloblastoma. Cancer 103, 793–801 (2005). [DOI] [PubMed] [Google Scholar]


