Abstract
Electroporation (EP) is a method used to physically deliver therapeutic molecules such as plasmid DNA directly to tissues. It has been used safely and successfully in clinical studies and preclinical cancer models to deliver genes to a variety of tissues. In cancer research cytokine therapy is emerging as a promising tool that can be used to boost the host response to tumor antigens. The delivery of cytokines as recombinant proteins can result in toxicity and other adverse effects; however the delivery of cytokine genes using EP has been shown to be safe and effective. Interleukin 15 (IL-15) is a cytokine that promotes the innate as well as the adaptive immune response to cancer cells and bacterial pathogens. In this study we used EP to deliver a human IL-15 plasmid (phIL-15) directly to tumors to examine its anti-cancer effects. B16.F10 melanoma tumors were induced in C57BL/6J mice and phIL-15 was delivered three times over the course of a week. Expression of the transgene, tumor volume, long-term survival and resistance to challenge were monitored in these animals. Delivery of IL-15 plasmid by EP resulted in increased IL-15 expression within the tumor compared to the injection only control. This expression peaked at 12 to 18 hours after the first delivery and was sustained at lower levels after the second and third deliveries. The delivery of the phIL-15 resulted in tumor regression, long-term survival and greater protection against tumor recurrence when cancer cells were reintroduced compared to control plasmid. From these results we can conclude that the delivery of IL-15 plasmid to tumors using EP is a promising avenue to investigate for its anti-tumor effects, however more work needs to be done to increase the stability of the gene once it is delivered and to elucidate the anti-tumor mechanism.
Keywords: Electroporation, Interleukin-15, Melanoma
Introduction
Electroporation (EP) is a physical method for delivering molecules such as DNA into cells. It utilizes electric fields to alter the permeability of the cell membrane in order to allow molecules to enter the cell (1). Many studies evaluate the effectiveness of using EP to deliver therapeutic genes to a variety of tumor types to control tumor burden by inducing proteins that through downstream signaling cause anti-tumor effects (2 –5). The advantage to using this approach is delivering therapeutic molecules directly to the tumor site without affecting the surrounding normal tissue and generating unwanted side effects. The transfection efficiency of gene electrotransfer can be modulated by changing the EP parameters used such as the voltage, duration and the number of pulses (6, 7). EP for gene delivery has been extensively studied in a number of preclinical melanoma models (8) which lead to the first-in-man clinical trial that utilized EP to deliver inter leukin-12 (IL-12) directly to tumors (9). Studies have also shown that delivery of non-coding plasmid DNA using EP can stimulate inflammatory as well as anti-tumor responses (10, 11) and EP can recruit immune cells to the site of administration (12). This provides an added advantage when using this delivery method for cancer therapy.
Interleukin 15 (IL-15) is a 15 kDa cytokine that binds to IL-15 alpha receptor (IL-15Ra) chain and signals through the gamma and beta chains of the IL-2 receptor complex (13, 14). It is closely related to IL-2 and since it shares the same receptor, there is some overlap of their functions. In vivo IL-15 has been shown to regulate the homeostasis of both innate and adaptive immune cells (15). It induces the proliferation of B cells, the activation and proliferation of CD4+ and CD8 + lymphocytes, promotes the induction of cytolytic effector cells, maintains survival of memory CD8+ T cells, and acts as a potent T cell chemoattractant (16). It also stimulates the proliferation and activation of NK cells and acts as a costimulator with IL-12 to produce interferon gamma and tumor necrosis factor (17 –19). IL-15 has a high affinity for binding its IL-15Rα (20). This complex can then stimulate neighboring cells through transpresentation which is essential for mediating the biological effects of IL-15 (21). The innate immune system plays a crucial role in host defense against tumor cells and pathogens. IL-15 is an attractive anti-cancer therapeutic target because of its ability to not only stimulate the adaptive immune system but the innate immune system as well (22, 23).
Melanoma is an aggressive cancer that evades the host immune system by increasing the production of immunosuppressive genes, reducing antigen presentation and preventing the induction of effector cells (24). Within the tumor, there are alterations in cell-cell communication and adhesion which leads to disease progression (25). The increased expression of a therapeutic molecule such as IL-15 in combination with the inflammatory responses generated by electrically mediated transfer of plasmid DNA may allow for an increased presence of immune effector cells within the tumor and promote tumor regression. Specifically it would enhance non-specific killing of tumor cells by the innate immune system, enhance presentation of tumor antigens, recruit cells of the adaptive immune system and generate long-term memory against recurring tumor cells.
Intratumoral electrotransfer of various cytokines such as IL-21, and IFN-y in experimental cancer models have resulted in long-term tumor regression (26 –28). Delivery of IL-12 with EP to melanoma resulted in local and systemic expression of the gene, tumor regression, long-term survival and resistance to challenge (9, 29, 30). Delivery of IL-15 using EP to melanoma in vivo resulted in tumor regression, long-term survival and protection from re-introduced cancer cells (31). Previous studies in the mouse model have shown that a single delivery of IL-12 with EP was not sufficient to cause tumor regression, although two treatments were able to induce regression in 47% of the animals (30, 32). Similarly, two deliveries of IL-15 with EP were only able to induce tumor regression in 40% of the animals. Here we examine the therapeutic potential of three deliveries of IL-15 using EP. It is hypothesized that increasing the number of treatments will increase the rate of survival.
Materials and Methods
Tumor Cells
B16.F10 mouse melanoma cells (ATCC, Manassas, VA) were maintained in McCoy’s medium supplemented with 10% FBS and 1% gentamycin at 37°C and 5% CO2 humidified air. Cells were removed from flasks using trypsin without EDTA (Atlanta Biologicals, Lawrenceville, GA) and washed in sterile DPBS without calcium and magnesium (Mediatech, Cellgro, Manassas, VA). Cell viability was assessed by trypan blue exclusion dye method. Cells with a viability >90% were resuspended in sterile DPBS at a concentration of 2 × 107 per ml for injection.
Tumor Induction
Tumors were established in the shaved left flank of female 6-8 week-old C57BL/6J mice (Jackson Labs, Bar Harbor, ME) by subcutaneous injection of 0.05 ml (1 × 106) B16.F10 melanoma cells. Tumors were allowed to grow for 6-10 days to a volume of 30-60 mm3 before treatment. For challenge studies, tumors were established on the right flank by subcutaneous injection of 0.05 ml (5 × 105) B16.F10 melanoma cells.
Plasmids
phIL-15 was a generous gift from Dr. David B. Weiner (University of Pennsylvania, College of Medicine). The plasmid contains an optimized IL-15 sequence that was cloned into a pVAX1 cloning vector (Invitrogen, Carlsbad, CA) (33, 34). phIL-15 and control vector pVAX1 were commercially prepared to endotoxin levels of <100 EU/mg (Aldevron, Fargo, ND) and diluted in 0.9% sterile injectable saline to the appropriate concentration for each experiment.
Plasmid Delivery by Electroporation
Plasmid was delivered directly to tumors on days 0, 3, and 6 after the tumors grew to the appropriate volume (30-60 mm3). The number of times the plasmid was delivered to a given tumor varied depending on the experimental group. Mice were anesthetized by placing animals into an induction chamber infused with a mixture of 3% isoflurane and 97% oxygen for several minutes. They were then fitted with a standard rodent mask and exposed to 2-3% isoflurane in oxygen during all treatments. Mice received 50 pl of plasmid (1.0 mg/ml or 2.0 mg/ml of phIL-15 or pVAX1) by intratumoral injection. An applicator containing 6-needle penetrating electrodes in a circular array was placed around the tumor and pulses were applied using the ECM 830 Square Wave Pulse generator (BTX Harvard Apparatus, Holliston, MA). Mice were divided into three electroporation groups: one group received 6 pulses of field strength 1300 V/cm (voltage across distance (0.92 cm)) and pulse length 100 μs, the second group received 6 pulses of field strength 500V/cm and pulse length 20 ms, and the third group received 6 pulses of field strength 200V/cm and pulse length of 20 ms. Control groups received intratumoral injections of hIL-15 expressing plasmid only. Experimental groups used to monitor long-term survival had a starting n of 15. Some animals had tumors that were deep and embedded in the underlying muscle tissue or had developed a second tumor near the site of the primary tumor. These animals were dropped from the study.
Tumor Measurements
Tumors were measured every 3 to 4 days using a digital caliper. Tumor volume was determined using the standard formula V = πab2/6, where (a) is the longest diameter and (b) is the shortest diameter perpendicular to (a) (35). The mice were monitored for tumor growth for the duration of the experiment or until tumor volumes reached 1000mm3, at which point the animals were humanely euthanized with CO2.
ELISA
Tumors were collected from humanely euthanized mice, frozen immediately on dry ice and stored at -80°C. Blood, was collected from mice under mild anesthesia via retroorbital bleeding into serum separator tubes (BD). Serum was collected after centrifugation (5 minutes at 1000 rpm) at 4°C and stored at -20°C until analyzed. To measure cytokine levels within the tumor tissue, samples were thawed, weighed, and diluted in 500 pl-800 pl of PBS with protease inhibitor cocktail (Roche, Brandford, CT) dependent on sample size. Tissue samples were kept on ice and homogenized using a PowerGen 700 (Fisher Scientific, Pittsburg, PA). The homogenate was centrifuged at 1000 rpm for 5 minutes at 4°C. Expression levels of IL-15 were measured using a human IL-15 Quantikine ELISA kit (R&D Systems, Minneapolis, MN) per the manufacturer’s instructions. hIL-15 levels were calculated as pg/ml of tumor homogenate and normalized to tumor weight. The data is expressed as pg/0.1 g of tumor.
Statistical Analysis
The results were analyzed using Microsoft Excel and Statistical Package for the Social Sciences (SPSS-IBM, Chicago, IL) software to determine significance. A two-tailed Student’s t-test was utilized for the analysis of hIL-15 in the tumor lysate from treated groups compared to the injection only control at each time point. The Three Sigma Rule and Z score were used to identify and remove outliers greater than 2.5 standard deviations during analysis of significance. Differences were deemed significant when a p value was <0.05. At least three independent experiments were performed for these experiments.
Results and Discussion
Intratumoral Delivery of phIL-15 Using EP Results in IL-15 Expression
In order to determine the usefulness of delivering IL-15 using EP as an in vivo treatment for melanoma we first tested the expression of IL-15 in the tumor after delivery of phIL-15. Previous studies have characterized the expression kinetics and therapeutic effects of multiple intratumoral deliveries of pDNA using EP (30 –32). Studies have shown, using various tumor models and various methods of delivering the plasmids, that local expression of immune modulators is important for successful tumor regression (36 –39). 50 pl of plasmid DNA was injected into the tumor and a train of six pulses of various electric field strengths and pulse lengths were applied to the tumor using an applicator with six penetrating electrodes in a circular array. These pulse protocols include 1300 V/cm 100 μs pulses, 500 V/cm 20 ms pulses or 200 V/cm 20 ms pulses. Two concentrations of plasmid were tested, 1 mg/ml and 2 mg/ml and the level of expression in the tumor was determined. Tumor tissue was collected at 12, 18, 24 and 48 hours after a single delivery, 12 hours after two deliveries on days 0 and 3, and 12 hours after three deliveries on days 0, 3, and 6. The tissue was homogenized in PBS and analyzed by hIL-15 ELISA. The data are reported as pg/0.1 g of tumor over time as shown in Figure 1. IL-15 expression levels were also measured in serum (data not shown) and found to be less than 3 pg/ml for all groups.
Figure 1:
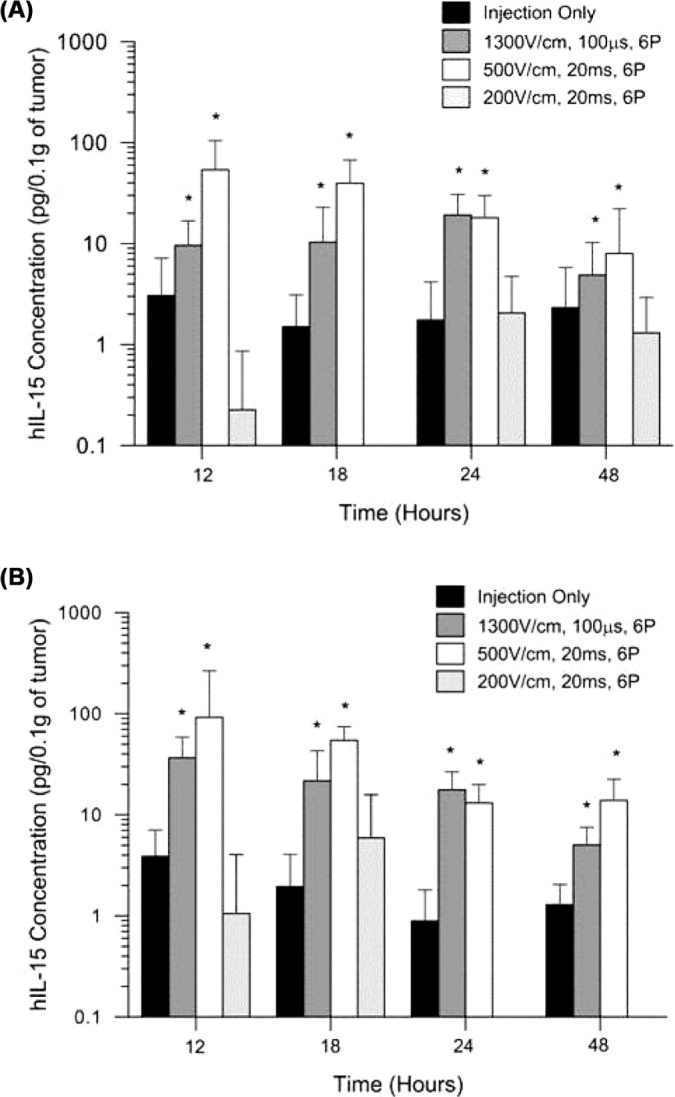
Analysis of tumor homogenate for hIL-15 expression after a single delivery of phIL-15. Expression of hIL-15 was measured by ELISA at 12, 18, 24, and 48 hours after a single intratumoral delivery of 50 |il of phIL-15 at a concentration of 1.0 mg/ml (A) or at a concentration of 2.0 mg/ml (B) using electroporation with various parameters delivered by a circular 6 penetrating electrode array. Data is represented as the mean hIL-15 concentration (pg/0.1g of tumor). Error bars represent standard deviation. Student f-test was performed to calculate significance for each group compared to injection only at that time point. The number of animals in each group tested were between 8 and 16. P pulses; *p value < 0.05. No samples were tested at 24 and 48 hours for mice that received phIL-15 with six 20 ms pulses at 200 V/cm.
Statistically significant, early, short-term increases in the levels of IL-15 protein were observed when phIL-15 was delivered using 1300 V/cm, 100 μs pulses and 500 V/cm, 20 ms pulses compared to injection alone. The expression levels of IL-15 decreased steadily among all groups over the 48 hours period. When phIL-15 was delivered using 200 V/cm 20 ms pulses, expression appeared later and the levels were lower than that of the other pulse protocols. These values were not statistically significant. Expression was not measured in tumors using the 200 V/cm 20 ms EP protocol to deliver phIL-15 (2 mg/ml) at 24 and 48 hours. The highest IL-15 expression levels were detected at 12 and 18 hours after a single delivery in all samples that were treated with 500 V/cm 20 ms pulses. Mouse tumors that received 1 mg/ml of phIL-15 and EP using this protocol showed a 17.6 and 26.4 fold increase over injection only control (p < 0.001) at 12 and 18 hours after delivery respectively. Expression decreased thereafter but remained significantly higher than the injection only control through 48 hours. Mice with tumors that received 2 mg/ml of phIL-15 and EP at this protocol showed a 10.5 and 28 fold increase over the injection only control (p < 0.001) at 12 and 18 hours after delivery respectively and maintained an elevated level of expression from 24 to 48 hours. When phIL-15 was delivered to tumors using 1300 V/cm 100 |is pulses as a single delivery the IL-15 expression was lower that of the groups that received 500 V/cm 20 ms pulses at 12, 18, and 48 hours but comparable at 24 hours. When phIL-15 (1 mg/ml) was delivered to tumors using 1300 V/cm 100 |is pulses expression appeared to peak after 24 hours and showed a 15.1 fold increase over the injection only control (p < 0.0001). When phIL-15 (2 mg/ml) was delivered by EP using this protocol, IL-15 expression peaked at 12 hours after the single delivery and remained through 24 hours before falling off at 48 hours.
When phIL-15 (2 mg/ml) was delivered using 500 V/cm, 20 ms or 1300 V/cm, 100 |is pulses, IL-15 expression remained elevated at similar levels above control at 12 hours after delivery. Using these parameters and this plasmid concentration we further investigated the effects on IL-15 expression after three deliveries of pIL-15 using EP on days 0, 3, and 6 (Figure 2). Tumors were collected 12 hours after the day 3 and day 6 deliveries in separate experiments. IL-15 expression was also measured in the serum by ELISA (data not shown) and found to be less than 2 mg/ml in all groups tested. When tumors were collected after the day 3 and 6 deliveries, both EP conditions used showed significantly elevated IL-15 expression in the tumor compared to the injection only control. These levels were not higher than the levels detected 48 hours after a single delivery. From these results it does not appear that multiple deliveries of plasmid using EP elevates IL-15 levels in the tumors to those seen 12 hours after a single delivery. However, it is clear that multiple deliveries can effectively sustain IL-15 expression at statistically significant levels above injection only control in tumors for more than six days. This indicates that the animals are either building a resistance to the foreign human protein or that the human IL-15 is unstable and is being regulated in some other way after it is secreted. Studies have shown that the IL-15Rα is important in stabilizing IL-15 in vivo and preventing degradation through the proteasome (40). Other studies show that formation of a complex between the IL-15 and the IL-15Rα is essential for trans-presentation and subsequent signaling on neighboring cells (41). Since the experiments in this report did not include co-delivery of an IL-15Rα plasmid this could be a contributing factor to the low levels of IL-15 seen in the tumor after plasmid delivery with EP and the low levels seen in the serum at corresponding time points (data not shown).
Figure 2:
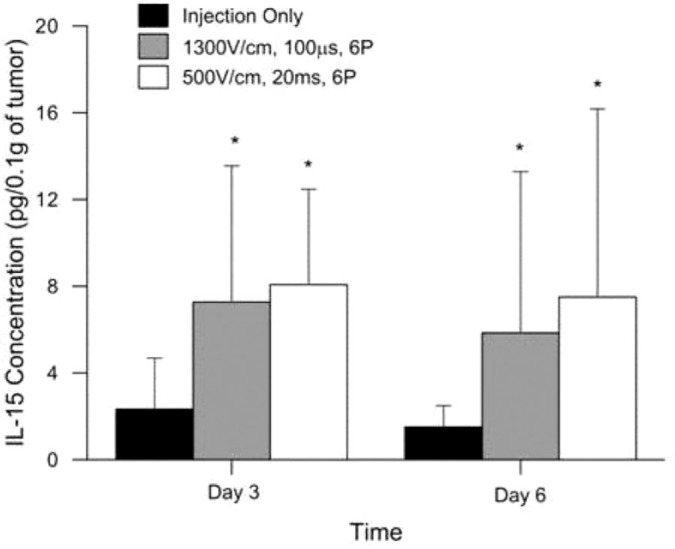
Analysis of tumor homogenate for hIL-15 expression after a multiple deliveries of phIL-15. Expression of phIL-15 was measured by ELISA 12 hours after two intratumoral deliveries of 50 |il of phIL-15 (2.0 mg/ml) on days 0 and 3 and 12 hours after three deliveries of 50 |il of phIL-15 (2.0 mg/ml) on days 0, 3, and 6 using electroporation with various parameters delivered by a circular 6 penetrating electrode array. Data is presented as the mean hIL-15 concentration (pg/0.1 g of tumor) over time. Error bars represent standard deviation. Student f-test was performed on each sample and significance calculated based on the injection only group at each time point. n = 12 for each group tested. P pulses; *p value < 0.05.
IL-15 is a tightly regulated cytokine and its expression is controlled at the levels of transcription, translation and intracellular trafficking (42). The plasmid used in this study encodes human IL-15 and was optimized in an attempt to overcome these impediments and produces a biologically active protein that expresses 80-fold higher than the native construct (33). Human IL-15 shares a 73% homology with the mouse gene. It enhances antigen specific CD8+ immune responses and elicits an anti-tumor response when delivered by EP to melanoma (31, 33). Human IL-15 binds to mouse IL-15Rα with a similar affinity as the mouse IL-15 but the specific activity of the human protein expressed in the mouse is much lower than the mouse protein (43). The human IL-15 was shown to be less effective at increasing the number and activity of spleen NK and CD8+ cells in the mouse than the mouse IL-15 protein. It was also suggested that IL-15 can be physiologically active in some systems without the involvement of the IL-15Ra. This provides evidence that the levels of human IL-15 generated in the tumors bind to the endogenous mouse IL-15Rα and are most likely physiologically active.
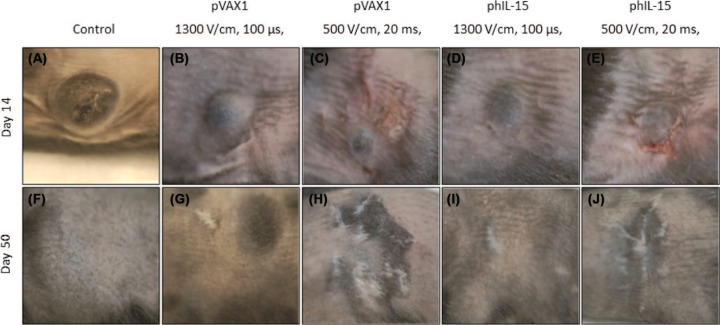
Electroporation has been shown to be an effective means of delivering plasmid DNA to tissues. The selection of electrodes and pulse parameters such as pulse number, length and amplitude are important to the efficiency of permeabilization of cells in a given tissue (7, 44). The objective is to achieve tissue permeabilization without causing cell death. It has been reported that various EP protocols can result in gene transfer (45). Protocols using millisecond pulses at relatively low voltage usually result in higher, prolonged gene expression compared to high voltage millisecond pulses. In this study we evaluated three EP conditions: six 200 V/cm, 20 ms pulses; six 500 V/cm, 20 ms pulses; and six 1300 V/cm, 100 μs pulses. All pulses were delivered using an applicator with a circular array of six penetrating electrodes placed around the tumor. We found that the 200 V/cm, 20 ms EP pulses were not sufficient to generate high levels of IL-15 after the plasmid was delivered. The most efficient delivery of the plasmid as evidenced by gene expression was shown by the 500 V/cm, 20 ms pulses followed by the 1300 V/cm, 100 μs pulses. Though the 500 V/cm pulses generate higher gene expression, they generate visible tissue damage (Figure 3) that resulted in scarring of the skin and tissue.
Figure 3:
Effect of delivery of plasmid by electroporation on animal skin. All animals except controls received three deliveries of plasmid using EP on days 0, 3, and 6. Images were captured on day 14 (A-E) and day 50 (F-J). Images depict untreated (A) and treated tumors (B-E and G-J) and (F) naive mouse.
Delivery of pIL-15 Using EP Results in Tumor Regression and Long-term Survival
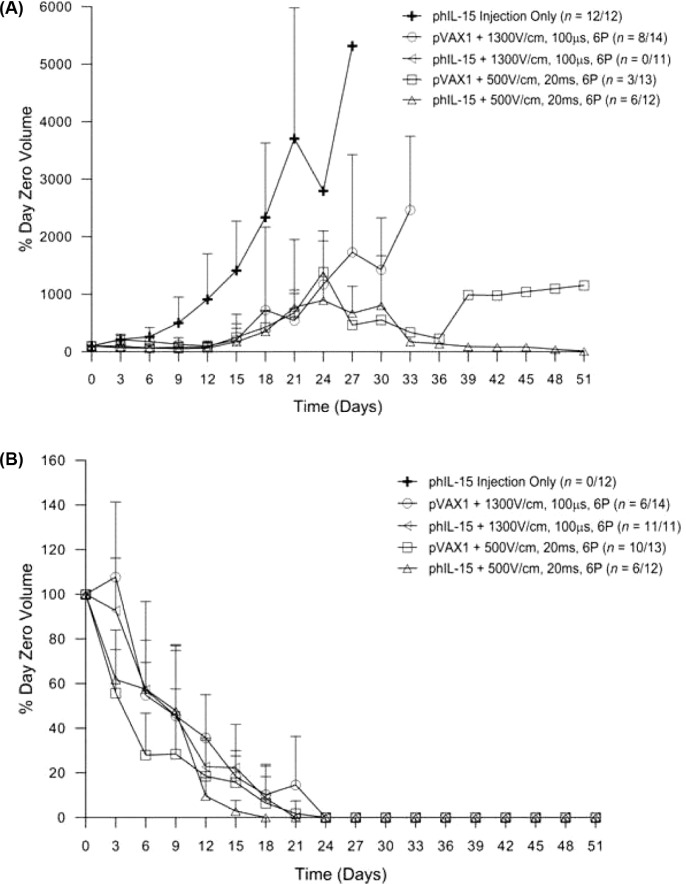
To determine the potential of delivering phIL-15 to tumors using EP as an antitumor therapy, we delivered 50 pg of phIL-15 (2 mg/ml) in sterile saline into established tumors on C57BL/6J mice followed by EP on days 0, 3, and 6. Previous studies have shown the efficacy of a three treatment EP mediated delivery of plasmid DNA for tumor regression (32, 46). An applicator consisting of six penetrating electrodes in a circular array was used to deliver six 1300 V/cm, 100 μs pulses or 500 V/cm, 20 ms pulses to the tumor. The mice were monitored over the course of 50 days and the tumor volume recorded (Figure 4). The data are expressed as the percent of the tumor volume on day zero (% day zero volume) and reported for non-responders (Figure 4A) as well as responders (Figure 4B). Percent survival is reported in Figure 5.
Figure 4:
Tumor regression after multiple deliveries of phIL-15 using electroporation. Tumor volume (mm3) monitored over 50 days for non-responders (A) and responders (B) following intratumoral delivery of 50 pl of phIL-15 (2.0 mg/ml) on days 0, 3, and 6 using either six 100 μs pulses at 1300 V/cm or six 20 ms pulses at 500 V/cm delivered by penetrating electrode array. The group that received phIL-15 using six 20 ms pulses at 500 V/cm had one mouse represented on the non-responder graph (A), with a tumor that increased in volume up to day 30 and then regressed. On day 51 there was a measureable mass. Data is represented as the average percentage of the volume of the tumor at day zero. Error bars represent standard deviation. The number of responders or nonresponders is indicated on the graph as a fraction of the total number of animals in each group. P pulses; pVAX1 control plasmid; phIL-15 plasmid encoding the hIL-15 gene.
Figure 5:
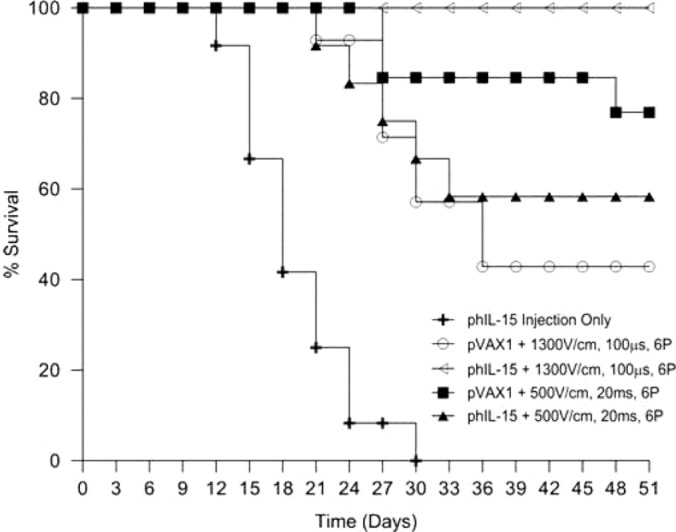
Survival after multiple deliveries of phIL-15 using electroporation. The percentage survival of mice represented in Figure 4 is presented as a survival curve. Survival was monitored after the delivery of 50 pl of phIL-15 (2.0 mg/ml) on days 0, 3, and 6 using EP with the indicated conditions.
Control mice that received only injection of phIL-15 (no EP) showed no response to treatment, their tumors increased in size and the animals were humanely euthanized about two weeks after the last delivery. Plasmid DNA (phIL-15 as well as control plasmid pVAX1) delivered to tumors using EP induced tumor regression and long-term survival in a percentage of the animals in each group (Figure 4B). Tumor volume steadily decreased over the first three weeks and the animals were all tumor free by day 24. These animals remained tumor free for the duration of the experiment and were challenged on day 56. No additional treatments were administered. One animal in the group that was treated with control plasmid and 20 ms pulses at 500 V/cm showed a partial response until day 39, after which the tumor volume began increasing (Figure 4A). In the group that was treated with phIL-15 using this pulse protocol, one animal showed a partial response as well. In this case the tumor volume slowly increased over the first four weeks, remained constant for a few days and then decreased thereafter (Figure 4A). Melanoma is an aggressive cancer with metastatic properties so it is important to note that in some cases cells may have already migrated from the primary site before the plasmid was delivered and therefore would not be treated. This would result in the animal being classified as non-responsive to the phIL-15 therapy due to the growth of a distant untreated tumor.
Mice that received phIL-15 delivered with 1300 V/cm 100 μs pulses showed 100% survival compared to a 43% survival for mice given control plasmid using the same EP conditions. phIL-15 delivered using 500 V/cm 20 ms pulses showed 58% survival compared to a 77% survival for mice given the control vector (pVAX1). Pulses delivered under this EP condition using this penetrating electrode array caused visible damage to the skin of the animal and potentially to the underlying tissues (Figure 3). Pulses delivered at 1300 V/cm for 100 μs did not cause any visible damage to the skin of the animals. The interesting finding in this study is the tumor regression, long-term survival and the generation of a tumorspecific memory response by the mice that received control plasmid. Studies have shown that EP can act as an adjuvant in recruiting immune cells to the site of administration (11, 12). This effect of the EP pulses combined with the tissue damaging nature of the 20 ms pulses at 500 V/cm could lead to an increased presence of immune cells in the tumor and account for the increase in tumor regression and long-term survival seen in mice receiving the control plasmid with EP. The lack of visible damage to the tissue when 1300 V/cm pulses were delivered might lead to fewer immune cells recruited to the tumor, resulting in the lower survival rates observed for mice receiving the control plasmid. Since the EP adjuvant effect appears to be muted under these conditions the higher survival rate of mice receiving phIL-15 with EP at 1300 V/cm could then be attributed to the physiological function of IL-15 in the tumor.
Plasmid DNA that does not encode a therapeutic gene delivered by EP has anti-tumor effects (11). Heller et al. showed that the delivery of a plasmid encoding luciferase using ten 5 ms pulses at 800 V/cm with caliper electrodes induced greater tumor regression and long-term survival than plasmid delivered using 1300 V/cm pulses. Delivery of pUC18, a plasmid that contains no mammalian sequences, using 5 ms pulses at 800 V/cm resulted in tumor regression and long-term survival in 70% of the mice. These findings are similar to what was observed for pVAX1 delivered by EP in this report where vector control plasmid delivered by 20 ms pulses at 500 V/cm resulted in greater survival than vector plasmid delivered by 100 μs pulses at 1300 V/cm. These antitumor effects could be attributed to the CpG motifs that are present on the plasmid DNA as these sequences can cause tumor-specific immune responses (47 –50). Plasmid delivery by EP alters endogenous mRNA and protein expression of cytokines and chemokines in melanoma based on the pulse parameters used (10). EP itself has also been shown to activate proinflammatory chemokine and stress genes and cause an influx of inflammatory cells to the site of administration (51, 52). The generation of these cytokines could play a role in the tumor-specific responses demonstrated in this report in groups of mice that received control plasmid with EP.
From this study we have shown that three deliveries of phIL-15 can increase the percentage survival of the animals treated when compared to a previous study with two administrations of phIL-15 (31). Using three deliveries the group treated with six 100 μs pulses at 1300 V/cm and showed 100% survival at day 51, an improvement over the 40% survival shown by mice that received phIL-15 delivered using a similar electroporation protocol, six 100 μs pulses at 1500 V/cm. The three treatment protocol using the 1300 V/cm pulses also increased the survival of mice that were given the vector control plasmid when compared to the two treatment protocol using the 1500 V/cm pulses.
Delivery of phIL-15 Using EP Confers Resistance to Challenge
Mice with tumors that completely regressed with long-term, tumor-free survival were challenged by subcutaneous injection on the opposite flank of 50 pl B16.F10 melanoma cells (0.5 × 106 cells). No additional treatments with phIL-15 or EP were administered. The mice were monitored for an additional 50 days and tumor growth, if any was quantified. The outcome of the challenge was represented as percentage overall survival in Table I. Mice were called “resistant” if they remained tumor free for the duration and “non-resistant” if tumors grew at the secondary inoculation site. Of the mice given phIL-15 delivered with 1300 V/cm, 100 μs pulses 5 out of 11 (45% of the original n) were resistant compared to the control plasmid group in which 3 out of the 6 challenged (21% of the original n) were resistant. The mice that received phIL-15 using 500 V/cm, 20 ms pulses had 5 out of 7 mice challenged (42% of the original n) were resistant to challenge compared to 3 out of 9 (23% of the original n) being resistant in the control plasmid group using the same EP condition. This is a noteworthy observation, however the underlying molecular mechanism of how delivery of control plasmid leads to long-term survival and resistance to challenge in a small percentage of animals is unclear at this time. These results suggest that EP plays a large role in the tumor regression and resistance to challenge that were observed. They also imply the generation of a memory immune response by the animals to melanoma tumor antigens resulting from the treatment of the initial subcutaneous tumor on the left flank. This study has demonstrated the usefulness of using EP to deliver phIL-15 as an anti-melanoma therapy. We have demonstrated that the therapeutic protein IL-15 can be expressed in the tumor at levels sufficient to cause tumor regression and generate a specific memory response that protects the animal from developing tumors when challenged. We cannot rule out that the delivery of the empty vector using EP may play a role in the overall anti-tumor effect. However, it is clear from these data that the delivery of IL-15 expressing plasmid by EP results in a higher percentage of animals resistant to challenge. Additional studies need to be carried out to enhance the stability of theIL-15 protein once it is produced to improve the efficacy of the therapy. Another plasmid backbone could be used in future experiments to express the therapeutic gene in order to further study the effect of delivering non-coding DNA by electroporation.
Table I.
Delivery of phIL-15 by electroporation results in long-term survival and resistance to challenge. Mice that remained tumor free after the primary inoculation of B16.F10 melanoma cells and delivery of pDNA using electroporation were challenged on the opposite flank with a subcutaneous injection of B16.F10 melanoma cells. They were monitored for an additional 50 days. Data represents animals that were tumor free 50 days post challenge.
| Challenge | ||||
|---|---|---|---|---|
| Total animals (n) | Day 0 (n) | Day 50 (n) | Overall survival (%) | |
| phIL-15 injection | 12 | 0 | 0 | - |
| pVAX1 + 1300 V/cm | 14 | 6 | 3 | 21 |
| phIL-15+1300 V/cm | 11 | 11 | 5 | 45 |
| pVAX1+500 V/cm | 13 | 9+ | 3 | 23 |
| phIL-15+500 V/cm | 12 | 7* | 5 | 42 |
+Of the 10 surviving animals in this group one was found dead on day 48.
*There was one mouse in this group that had a small tumor on day 51 that completely regressed by day 0 of challenge and was included in the challenge.
Acknowledgements
The funding sources were provided by NIH National Cancer Institute Fellowship Grant 5F31CA119950-01A2 and National Cancer Institute R01 CA122518 Grant. Our appreciation is extended to David Weiner (University of Pennsylvania Philadelphia, PA) for supplying the optimized phIL-15 and Mark Jaroszeski (University of South Florida Tampa, FL) for engineering the 6-needle electrode.
Abbreviations:
- EP:
Electroporation
- phIL-15:
Human Interleukin 15 Plasmid
Footnotes
Conflict of Interest: There is no conflict of interest associated with this manuscript.
References
- 1.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J 1, 841–845, (1982). DOI: 0261-4189/82/0107-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardlik R, Palffy R, Hodosy J, Lukacs J, Turna J, Celec P. Vectors and delivery systems in gene therapy. Med Sci Monit 11, RA110–121 (2005). DOI: 6257 [pii]. [PubMed] [Google Scholar]
- 3.Rochlitz CF. Gene therapy of cancer. Swiss Med Wkly 131, 4–9, (2001). DOI: 2001/01/smw-09649. [DOI] [PubMed] [Google Scholar]
- 4.Heller LC, Heller R. In vivo electroporation for gene therapy. Hum Gene Ther 17, 890–897, (2006). DOI: 10.1089/hum.2006.17.890. [DOI] [PubMed] [Google Scholar]
- 5.Kirkwood JM, Moschos S, Wang W. Strategies for the development of more effective adjuvant therapy of melanoma: current and future explorations of antibodies cytokines vaccines and combinations. Clin Cancer Res 12, 2331s–2336s (2006). DOI: 10.1158/1078-0432.CCR-05-2538. [DOI] [PubMed] [Google Scholar]
- 6.Andre F, Mir LM. DNA electrotransfer: its principles and an updated review of its therapeutic applications. Gene Ther 11 (Suppl. 1), S33–42 (2004). DOI: 10.1038/sj.gt.3302367. [DOI] [PubMed] [Google Scholar]
- 7.Miklavcic D, Beravs K, Semrov D, Cemazar M, Demsar F, Sersa G. The importance of electric field distribution for effective in vivo electroporation of tissues. Biophys J 74, 2152–2158, (1998). DOI: 10.1016/S0006-3495(98)77924-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heller LC, Heller R. Electroporation gene therapy preclinical and clinical trials for melanoma. Curr Gene Ther 10, 312–317, (2010). DOI: ABS-39. [DOI] [PubMed] [Google Scholar]
- 9.Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, Munster PN, Sullivan DM, Ugen KE, Messina JL, Heller R. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol 26, 5896–5903, (2008). DOI: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heller LC, Cruz YL, Ferraro B, Yang H, Heller R. Plasmid injection and application of electric pulses alter endogenous mRNA and protein expression in B16.F10 mouse melanomas. Cancer Gene Ther 17, 864–871, (2010). DOI: 10.1038/cgt.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heller LC, Coppola D. Electrically mediated delivery of vector plasmid DNA elicits an antitumor effect. Gene Ther 9, 1321–1325, (2002). DOI: 10.1038/sj.gt.3301802. [DOI] [PubMed] [Google Scholar]
- 12.Dayball K, Millar J, Miller M, Wan YH, Bramson J. Electroporation enables plasmid vaccines to elicit CD8+ T cell responses in the absence of CD4+ T cells. J Immunol 171, 3379–3384, (2003). DOI: 0022-1767/03. [DOI] [PubMed] [Google Scholar]
- 13.Tagaya Y, Bamford RN, DeFilippis AP, Waldmann TA. IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity 4, 329–336, (1996). DOI: 10.1016/S1074-7613(00)80246-0. [DOI] [PubMed] [Google Scholar]
- 14.Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, Namen A, Park LS, Cosman D, Anderson D. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J 13, 2822–2830, (1994). DOI: 8026467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodolce JP, Burkett PR, Koka RM, Boone DL, Ma A. Regulation of lymphoid homeostasis by interleukin-15. Cytokine Growth Factor Rev 13, 429–439, (2002). DOI: 10.1016/S1359-6101(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 16.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science 264, 965–968, (1994). DOI: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 17.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med 180, 1395–1403, (1994). DOI: 0022-1007/94/10/1395/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev 17, 259–280, (2006). DOI: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol 86, 209–239, (2005). DOI: 10.1016/S0065-2776(04)86006-1. [DOI] [PubMed] [Google Scholar]
- 20.Lorenzen I, Dingley AJ, Jacques Y, Grotzinger J. The structure of the interleukin-15 alpha receptor and its implications for ligand binding. J Biol Chem 281, 6642–6647, (2006). DOI: 10.1074/jbc.M513118200. [DOI] [PubMed] [Google Scholar]
- 21.Stonier SW, Schluns KS. Trans-presentation: a novel mechanism regulating IL-15 delivery and responses. Immunol Lett 127, 85–92, (2010). DOI: 10.1016/j.imlet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies E, Reid S, Medina MF, Lichty B, Ashkar AA. IL-15 has innate anti-tumor activity independent of NK and CD8 T cells. J Leukoc Biol 88, 529–536, (2010). DOI:10.1189/jlb.0909648. [DOI] [PubMed] [Google Scholar]
- 23.Ohteki T. Critical role for IL-15 in innate immunity. Curr Mol Med 2, 371–380, (2002). DOI: 1012174/1566524023362519. [DOI] [PubMed] [Google Scholar]
- 24.Real LM, Jimenez P, Kirkin A, Serrano A, Garcia A, Canton J, Zeuthen J, Garrido F, Ruiz-Cabello F. Multiple mechanisms of immune evasion can coexist in melanoma tumor cell lines derived from the same patient. Cancer Immunol Immunother 49, 621–628, (2001). DOI: 10.1007/s002620000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haass NK, Smalley KS, Li L, Herlyn M. Adhesion migration and communication in melanocytes and melanoma. Pigment Cell Res 18, 150–159, (2005). DOI: 10.1111/j.1600-0749.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 26.Li S, Zhang X, Xia X, Zhou L, Breau R, Suen J, Hanna E. Intramuscular electroporation delivery of IFN-alpha gene therapy for inhibition of tumor growth located at a distant site. Gene Ther 8, 400–407, (2001). DOI: 10.1038/sj.gt.3301418. [DOI] [PubMed] [Google Scholar]
- 27.Heller LC, Ingram SF, Lucas ML, Gilbert RA, Heller R. Effect of electrically mediated intratumor and intramuscular delivery of a plasmid encoding IFN alpha on visible B16 mouse melanomas. Technol Cancer Res Treat 1, 205–209, (2002). DOI: 53007&c54058&p510979&do5detail [pii]. [DOI] [PubMed] [Google Scholar]
- 28.Hanari N, Matsubara H, Hoshino I, Akutsu Y, Nishimori T, Murakami K, Sakata H, Miyazawa Y, Ochiai T. Combinatory gene therapy with electrotransfer of midkine promoter-HSV-TK and interleukin-21. Anticancer Res 27, 2305–2310, (2007). DOI: 0250-7005/2007. [PubMed] [Google Scholar]
- 29.Heller R, Jaroszeski MJ, Reintgen DS, Puleo CA, DeConti RC, Gilbert RA, Glass LF. Treatment of cutaneous and subcutaneous tumors with electrochemotherapy using intralesional bleomycin. Cancer 83, 148–157, (1998). DOI: 10.1002/(SICI)1097-0142(19980701)83:1<148::AID-CNCR20>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 30.Lucas ML, Heller L, Coppola D, Heller R. IL-12 plasmid delivery by in vivo electroporation for the successful treatment of established subcutaneous B16.F10 melanoma. Molecular Therapy 5, 668–675, (2002). DOI: 10.1006/mthe.2002.0601. [DOI] [PubMed] [Google Scholar]
- 31.Ugen KE, Kutzler MA, Marrero B, Westover J, Coppola D, Weiner DB, Heller R. Regression of subcutaneous B16 melanoma tumors after intratumoral delivery of an IL-15-expressing plasmid followed by in vivo electroporation. Cancer Gene Ther 13, 969–974, (2006). DOI: 10.1038/sj.cgt.7700973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucas ML, Heller R. IL-12 gene therapy using an electrically mediated nonviral approach reduces metastatic growth of melanoma. DNA Cell Biol 22, 755–763, (2003). DOI: 10.1089/104454903322624966. [DOI] [PubMed] [Google Scholar]
- 33.Kutzler MA, Robinson TM, Chattergoon MA, Choo DK, Choo AY, Choe PY, Ramanathan MP, Parkinson R, Kudchodkar S, Tamura Y, Sidhu M, Roopchand V, Kim JJ, Pavlakis GN, Felber BK, Waldmann TA, Boyer JD, Weiner DB. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J Immunol 175, 112–123, (2005). DOI: 175/1/112. [DOI] [PubMed] [Google Scholar]
- 34.Jalah R, Rosati M, Kulkarni V, Patel V, Bergamaschi C, Valentin A, Zhang GM, Sidhu MK, Eldridge JH, Weiner DB, Pavlakis GN, Felber BK. Efficient systemic expression of bioactive IL-15 in mice upon delivery of optimized DNA expression plasmids. DNA Cell Biol 26, 827–840, (2007). DOI: 10.1089/dna.2007.0645. [DOI] [PubMed] [Google Scholar]
- 35.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol 24, 148–154, (1989). DOI: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 36.Yu WG, Ogawa M, Mu J, Umehara K, Tsujimura T, Fujiwara H, Hamaoka T. IL-12-induced tumor regression correlates with in situ activity of IFN-gamma produced by tumor-infiltrating cells and its secondary induction of anti-tumor pathways. J Leukoc Biol 62, 450–457, (1997). DOI: 9335314. [DOI] [PubMed] [Google Scholar]
- 37.Colombo MP, Vagliani M, Spreafico F, Parenza M, Chiodoni C, Melani C, Stoppacciaro A. Amount of interleukin 12 available at the tumor site is critical for tumor regression. Cancer Res 56, 2531–2534, (1996). DOI: 8653692. [PubMed] [Google Scholar]
- 38.Wang YS, Li D, Shi HS, Wen YJ, Yang L, Xu N, Chen XC, Chen X, Chen P, Li J, Deng HX, Wang CT, Xie G, Huang S, Mao YQ, Chen LJ, Zhao X, Wei YQ. Intratumoral expression of mature human neutrophil peptide-1 mediates antitumor immunity in mice. Clin Cancer Res 15, 6901–6911, (2009). DOI: 10.1158/1078-0432.CCR-09-048439. [DOI] [PubMed] [Google Scholar]
- 39.Chu Y, Yang X, Xu W, Wang Y, Guo Q, Xiong S. In situ expression of IFN-gamma-inducible T cell alpha chemoattractant in breast cancer mounts an enhanced specific anti-tumor immunity which leads to tumor regression. Cancer Immunol Immunother 56, 1539–1549, (2007). DOI: 10.1007/s00262-007-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergamaschi C, Jalah R, Kulkarni V, Rosati M, Zhang GM, Alicea C, Zolotukhin AS, Felber BK, Pavlakis GN. Secretion and biological activity of short signal peptide IL-15 is chaperoned by IL-15 receptor alpha in vivo. J Immunol 183, 3064–3072, (2009). DOI: 10.4049/jimmunol.0900693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castillo EF, Schluns KS. Regulating the immune system via IL-15 transpresentation. Cytokine 59, 479–490, (2012). DOI: 10.1016/j.cyto.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol 17, 19–49, (1999). DOI: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 43.Eisenman J, Ahdieh M, Beers C, Brasel K, Kennedy MK, Le T, Bonnert TP, Paxton RJ, Park LS. Interleukin-15 interactions with interleukin-15 receptor complexes: characterization and species specificity. Cytokine 20, 121–129, (2002). DOI: 10.1006/cyto.2002.1989. [DOI] [PubMed] [Google Scholar]
- 44.Miklavcic D. Electrical engineering aspects of electroporation drug and gene delivery. Medicon 2001: Proceedings of the International Federation for Medical & Biological Engineering Pts 1 and 2, 15–18, (2001). DOI: 953-184-023-7. [Google Scholar]
- 45.Lucas ML, Heller R. Immunomodulation by electrically enhanced delivery of plasmid DNA encoding IL-12 to murine skeletal muscle. Mol Ther 3, 47–53, (2001). DOI: 0.1006/mthe.2000.0233. [DOI] [PubMed] [Google Scholar]
- 46.Heller L, Merkler K, Westover J, Cruz Y, Coppola D, Benson K, Daud A, Heller R. Evaluation of toxicity following electrically mediated interleukin-12 gene delivery in a B16 mouse melanoma model. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research 12, 3177–3183 (2006). DOI: 10.1158/1078-0432.CCR-05-2727. [DOI] [PubMed] [Google Scholar]
- 47.Weiner GJ, Liu HM, Wooldridge JE, Dahle CE, Krieg AM. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci U S A 94, 10833–10837, (1997). DOI: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu HM, Newbrough SE, Bhatia SK, Dahle CE, Krieg AM, Weiner GJ. Immunostimulatory CpG oligodeoxynucleotides enhance the immune response to vaccine strategies involving granulocyte-macrophage colony-stimulating factor. Blood 92, 3730–3736, (1998). DOI: 006-4971/98/9210-0023. [PubMed] [Google Scholar]
- 49.Shen W, Waldschmidt M, Zhao X, Ratliff T, Krieg AM. Antitumor mechanisms of oligodeoxynucleotides with CpG and polyG motifs in murine prostate cancer cells: decrease of NF-kappaB and AP-1 binding activities and induction of apoptosis. Antisense Nucleic Acid Drug Dev 12, 155–164, (2002). DOI: 10.1089/108729002760220752. [DOI] [PubMed] [Google Scholar]
- 50.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation Nat Rev Drug Discov 5, 471–484, (2006). DOI: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 51.Peng B, Zhao Y, Xu L, Xu Y. Electric pulses applied prior to intramuscular DNA vaccination greatly improve the vaccine immunogenicity. Vaccine 25, 2064–2073, (2007). DOI: 10.1016/j.vaccine.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 52.Liu J, Kjeken R, Mathiesen I, Barouch DH. Recruitment of antigen-presenting cells to the site of inoculation and augmentation of human immunodeficiency virus type 1 DNA vaccine immunogenicity by in vivo electroporation. J Virol 82, 5643–5649, (2008). DOI: 10.1128/JVI.02564-07. [DOI] [PMC free article] [PubMed] [Google Scholar]