Abstract
This planning study was performed to compare stereotactic linac based radiosurgery of Arteriovenous Malformations (AVM) with current Helical Tomotherapy (HT) and future HT techniques. For 10 patients with AVM, dose distributions and treatment times of “regular” HT delivery (Reg 2.5/1/0.6 cm field width), Running-Start-Stop Treatment (RSS 5/2.5 cm), Axial Mode (Axial 5 cm) and Dynamic Jaw/Dynamic Couch delivery with a maximum field width of 5 cm (DJDC 5) were analysed and compared to linac-based stereotactic radiosurgery. Axial produced the fastest treatment (Axial 4:47 min vs. Linac 32:42 min) at the cost of large brain exposure (V10% 289 ml). Except for Reg 0.6, all other HT techniques achieved significantly shorter treatment times than linac-based treatment (e.g. Reg 1, 19:42 min, DJDC 6:30 min). However, high-dose brain exposure (V60%) was higher in all HT plans (e.g. Reg 0.6, 10 ml, Linac 9 ml), and only Reg 0.6 showed better low-dose exposure (V10% of 167 ml vs. 199 ml, not significant). Neither current nor future HT modes in their current version outperformed linac-based stereotactic radiosurgery. However, AVM with special geometry might still benefit from HT.
Keywords: Helical tomotherapy, Dynamic jaws/Dynamic couch, Arteriovenous malformation, Radiosurgery
Introduction
Arteriovenous malformations (AVM) are aberrant fistulous connections between arteries and veins bypassing the normal capillary bed. They occur in 0.2-0.8% of the normal population (1). Untreated cerebral AVMs pose a constant risk of hemorrhage and subsequent neurological impairments. Annual rates of major hemorrhages reported in literature vary from 2 to 17% (2). Of these, up to 29% take a lethal course (3).
AVM are amenable to various treatment options, including surgery, embolisation and radiosurgery (4-7). All treatment modalities aim at the total obliteration of the AVM while preserving normal brain function. To date, radiosurgery is either performed using a conventional linear accelerator, a gamma knife or a CyberKnife setup (8-10). Past reports have shown that radiosurgical treatment of AVM is safe in adults and children (11-13). Complete obliteration after radiosurgery occurs in 60-90% of patients with a time latency of 1-3 years during which the risk of intracerebral hemorrhage remains elevated.
The technical details of Helical Tomotherapy (HT) have been discussed in detail before. Essentially, a HT unit is a hybrid of a 6 MV linear accelerator and a helical CT scanner. Treatment is performed using a rotating fan beam. With the patient being moved through the gantry bore, the treatment beam forms a helix (14). The beam is modulated by a very fast moving, pneumatically driven binary multi leaf collimator and can be shaped into different beam widths depending on the opening angle of the secondary collimator (jaws). In an inverse treatment planning process, the MLC conformation is optimised to obtain highly conformal radiation doses to the target (15). For the treatment of small malignant brain tumours, especially in close proximity to critical organs at risk, Yartsev et al. have shown that HT achieves better target dose uniformity and similar sparing of organs at risk compared to other photon techniques (3D conformal radiotherapy, stereotactic arc therapy and stepand-shoot IMRT) (16). HT allows the delivery of dose in a single fraction as in other radiosurgery protocols. However, due to a maximum gantry rotation period of 60 seconds and a minimal beam thickness of 1 cm, treatment delivery must often be broken into two or more delivery runs. When comparing intracranial stereotactic radiotherapy plans for coplanar and non-coplanar step-and-shoot IMRT with HT plans, Han et al. found a better dose conformity and dose gradients in HT plans. Yet, treatment times for HT were significantly longer (17). In a publication by Soisson et al. (18) that compared HT radiosurgery to linac-based radiosurgery with round collimators, HT yielded better conformity and similar homogeneity. While the volume of brain exposed to 12 Gy or more was slightly higher for HT, the predicted risk of symptomatic radiation necrosis was comparable to the clinically observed ranges.
Compared to gamma knife stereotactic radiosurgery of brain lesions, a limitation of HT is the exposure of larger volumes of healthy tissue to low radiation doses, in other words, the formation of a bigger dose penumbra (19, 20). This penumbra is caused by the fact that the jaws are constantly open and that dose delivery starts as soon as the inferior border of the jaws reaches the superior border of the treatment volume and does not stop until the superior jaw border reaches the inferior PTV border. The dimension of the craniocaudal penumbra is defined by the chosen field width (21, 22). In addition, since dose is delivered from 360°, the lateral penumbra is also larger than with techniques that use defined beam angles.
Recent prototypes in HT technique attempt to address this drawback: The running-start-stop (RSS) mode uses a dynamic opening of the jaws at the superior and inferior PTV borders while maintaining a constant field width during passage of the PTV. This has already been proposed by Mackie et al. in the initial Tomotherapy publication in 1993 (23). This way, the dose penumbra can be reduced to the order of regular beams and bigger field widths can be chosen, resulting in shorter treatment times. In dynamic jaw/ dynamic couch (DJDC) Mode, constantly moving jaws create dynamic field widths and sharp dose gradients. Combined with variable couch travel speed, treatment times can be shortened while maintaining high dose conformality and sharp dose gradients (24, 25). The Axial Mode is a non helical delivery mode of Tomotherapy and addresses the problem of long treatment times: For radiosurgery of target volumes not larger than the field width, treatment can be delivered with a dynamic secondary collimator while the couch remains static. Thus, a substantial decrease in treatment time can be obtained.
Therefore, we conducted a plan comparison study on 10 patients with AVM with a broad range of sizes and shapes to highlight advantages and drawbacks of “regular” HT delivery and future Tomotherapy techniques, that are currently not available for clinical treatments, compared with linac-based stereotactic radiosurgery.
Materials and Methods
Patients
Ten consecutive patients with AVM were treated with 3D-conformal radiosurgery on a Linac at our facility in 2008. This patient selection was made with the intent to include a broad spectrum of AVMs concerning size (minimum 0.1 ml, maximum 17.23 ml, average 3.58 +/— 5.41 ml), localisation and shape (see Table I).
Table I.
AVM characteristics: Localisation, volume and prescription dose (80% dose = prescription isodose).
| Localisation | Volume (ml) | 80% dose (Gy) |
|---|---|---|
| Left cerebellar | 0.1 | 19 |
| Right occipital | 0.2 | 18 |
| Left basal ganglia | 0.3 | 18 |
| Left thalamic | 0.7 | 16 |
| Left temporo-occipital | 0.7 | 18 |
| Quadrigeminal lamina and pineal gland | 0.9 | 18 |
| Left temporo-occipital | 2.9 | 20 |
| Left basal ganglia | 3.2 | 17 |
| Right temporal periventricular | 8.8 | 18 |
| Left parietal | 17.2 | 18 |
Radiotherapy Planning
Planning was based on a contrast enhanced high-flow CTangiography with 2 mm slice thickness. A fusion with digital subtraction angiography was performed for target volume definition. Fixation was conducted under local anesthesia with an invasive stereotactic head frame.
Flow characteristics and size of the AVM nidus were detected and the nidus was included in the PTV. A DICOM export of these data to the planning CT used for HT planning was not possible due to technical reasons, so PTVs were copied meticuously by hand.
For physical reasons, dose distribution in 3D radiosurgery on a Linac is arranged concentrically around a dose maximum in the middle with a dose gradient to the edge. Traditionally, radiation dose is prescribed to the 80% isodose which should enclose the PTV. This dose distribution is not typical for HT which is designed to achieve a homogenous dose distribution. In order to exactly reproduce the classical radiosurgery dose distribution, we used a internal structure within the PTV generated by 3 mm isotropic 3D subtraction. 100% of the dose was prescribed to the center volume and 80% to the external ring to create similar dose gradients as in Linacbased radiosurgery. Depending on AVM size, the prescribed doses varied from 16 to 20 Gy on the enclosing 80% isodose (see Table I), resulting in maximum doses of 20 to 25 Gy.
Planning for Linac-based radiosurgery was done on a commercial planning system (STP, Stryker-Leibinger, Freiburg, Germany). Eight patients with complex nidus shapes were treated using a manually driven Micro-Multileaf collimator (MMLC, Brandis Medizintechnik V ertriebs GmbH, Weinheim, Germany) with a minimal leaf width of 1.8 mm in the isocenter. Treatment was delivered via 10-11 individually shaped beams. For the two patients with small spherical AVMs that could be covered with one round collimator, treatment was performed with a round collimator and 9 arcs in order to speed up treatment. If a spherical AVM was too large to be covered by one round collimator, the MMLC was used.
Inverse planning for regular HT delivery was done with the Tomotherapy planning station version 3.1.2.9. Three different plans with field widths of 0.6 cm, 1 cm and 2.5 cm (Reg 0.6, Reg 1 and Reg 2.5) were created. To date, the 0.6 cm field width is a research beam and not available for clinical use. Although the 2.5 cm field width was not considered to be clinically suitable due to the big 2.5 cm dose penumbra, it was still planned in order to provide plans for direct comparison with the advanced techniques that all use maximum field widths bigger than 1 cm. In all three plans, a pitch of 0.1 was chosen to enable multiple rotations for a single voxel and to have enough time for radiosurgical high single dose application.
Inverse planning for advanced HT delivery was performed on the research planning station version 6.1.0.10. For each patient a RSS plan with 2.5 cm and 5 cm field width (RSS 2.5, RSS 5) were created. For the respective DJDC and Axial plans, a maximum field width of 5 cm (DJDC 5 and Axial 5) was chosen; the actual jaw opening was modulated during the planning process by the software.
Plan Evaluation
The dose to the organs at risk was evaluated by comparing their maximum or mean dose, depending on serial or parallel organisation of the OAR.
The integral dose to the brain could not be calculated for technical reasons in the two cases treated with round collimators, so we used V10%, V20%, V40% and V60% (volumes receiving 10-60% of the prescribed dose, in ml) for the evaluation of brain exposure. For each patient, the brain exposure in the HT plans were normalized to the linac plan, based on D99.
As the dose distribution in radiosurgery is traditionally not homogenous, we evaluated D1 and D99 (dose to 1% and 99% of the PTV, in Gy) for the different PTVs instead of Uniformity index.
Statistical Analysis
Calculations of statistical significance were performed with a paired Student’s t-test. Ap-value of p < 0.05 was considered statistically significant.
Results and Discussion
Treatment Time
Dose delivery was fastest using the Axial Mode, resulting in a significant reduction of average beam on time of 85% compared to treatment on a Linac (4:47 min vs. 32:42 min, p < 0.001) at the cost of high dose exposure of the normal brain. Except for Reg 0.6, all modalities achieved significantly shorter beam on times than Linac-based treatment (see Table II). Treatment took longest with Reg 0.6 Mode (39:24 min), but not significantly longer than the Linac plan.
Table II.
Average treatment times for all delivery modes (in min:sec). Axial mode was significantly shorter than treatment on a Linac (p < 0.001).
| Reg 2.5 | Reg 1 | Reg 0.6 | RSS 5 | RSS 2.5 | DJDC 5 | Axial 5 | Linac | |
|---|---|---|---|---|---|---|---|---|
| Min:sec | 11:30 +/— 5:30 | 19:42 +/— 9:16 | 39:24 +/— 19:03 | 9:03 +/— 1:17 | 10:22 +/— 8:35 | 6:30 +/— 1:59 | 4:47 +/— 1:19 | 32:42 +/— 8:46 |
Reg = “Regular” HT delivery; RSS = Running-Start-Stop; DJDC = Dynamic Jaw/Dynamic Couch.
PTV Coverage
The dose distribution typical for linac-based radiosurgery with a dose peak in the center and a concentric dose fall-off could be reproduced with all HT techniques with a deviation of +/— 0.2 Gy of the surrounding 80% isodose (data not shown). Using two concentric rings for PTV definition in HT plans, similar dose gradients compared to Linac plans could be realised.
Brain Exposure
Of all plans, Reg 0.6 Mode yielded minimal low dose exposure of normal brain tissue (see Table III): V10% could be reduced by 16% to 167 ml compared to treatment on a Linac. However, this reduction was not significant and was accompanied by a 21% increase in treatment time. In addition, all other dose levels examined showed a higher exposure of brain tissue in Reg 0.6 Mode compared to Linac (V20%: 63 ml vs. 44 ml; V40%: 21 ml vs. 15 ml; not significant). As high-dose exposure of healthy brain tissue is potentially harmful, V60% is the most critical parameter for the evaluation of brain sparing. For all HT techniques, V60% was higher than for Linac treatment, although the difference was not significant. The fastest treatment delivery (Axial Mode) produced the highest brain exposure in all dose levels examined (e.g. V60% 21 ml vs. 9 ml). Reg 2.5 and DJDC 5 resulted in the second highest brain exposure. RSS Mode is in an interim position between brain sparing and treatment time (RSS 2.5: Vw* 258 ml, V2()% 98 ml, V^% 33 ml, VM% 17 ml, treatment time 10:22 min).
Table III.
Brain exposure. Average volume of brain tissue exposed to 10%-60% of the prescribed dose. Differences in brain exposure between the different treatment modes were not statistically significant.
| Brain volume (ml) | Reg 2.5 | Reg 1 | Reg 0.6 | RSS 5 |
|---|---|---|---|---|
| V10% | 288 ± 176 | 191 ± 137 | 167 ± 126 | 259 ± 181 |
| V20% | 107 ± 86 | 71 ± 63 | 63 ± 60 | 98 ± 85 |
| V40% | 34 ± 32 | 23 ± 23 | 21 ± 21 | 33 ± 34 |
| V60% | 16 ± 16 | 11 ± 12 | 10 ± 11 | 17 ± 18 |
Reg = “Regular” HT delivery; RSS = Running-Start-Stop; DJDC = Dynamic Jaw/Dynamic Couch.
Exposure of other Organs at Risk
In most cases, exposure of organs at risk other than the brain was identical in lincal and HT plans. All planning modalities resulted in excellent sparing of brain stem, optic chiasm, optic nerve, eyes and lenses (data not shown).
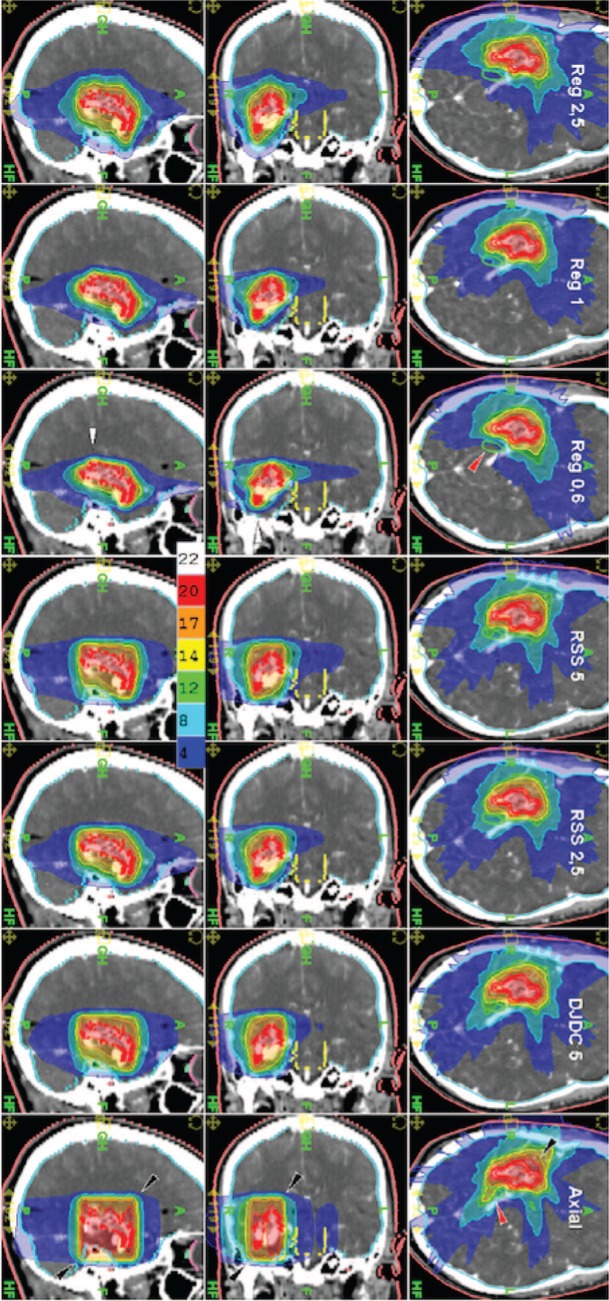
Figures 1 and 2 show an exemplary case of a large AVM with irregular geometry near the skull base. The cylindrical dose distribution in Axial Mode resulted in high brain exposure, the sharpest dose gradients could be realised with Reg 0.6 Mode. In this AVM situated at the skull base near the right optic tract, sparing of the latter was superior with Reg 0.6 Mode compared to all other HT modes but not to the Linac plan.
Figure 1:
Example of an AVM with irregular geometry near the skull base in sagittal, coronal and axial sections (from left to right) for Reg 2.5, Reg 1, Reg 0.6, RSS 5, RSS 2.5, DJDC 5 and Axial Mode (Reg = “Regular” HT delivery, RSS = Running-Start-Stop, DJDC = Dynamic Jaw/Dynamic Couch).
Figure 2:
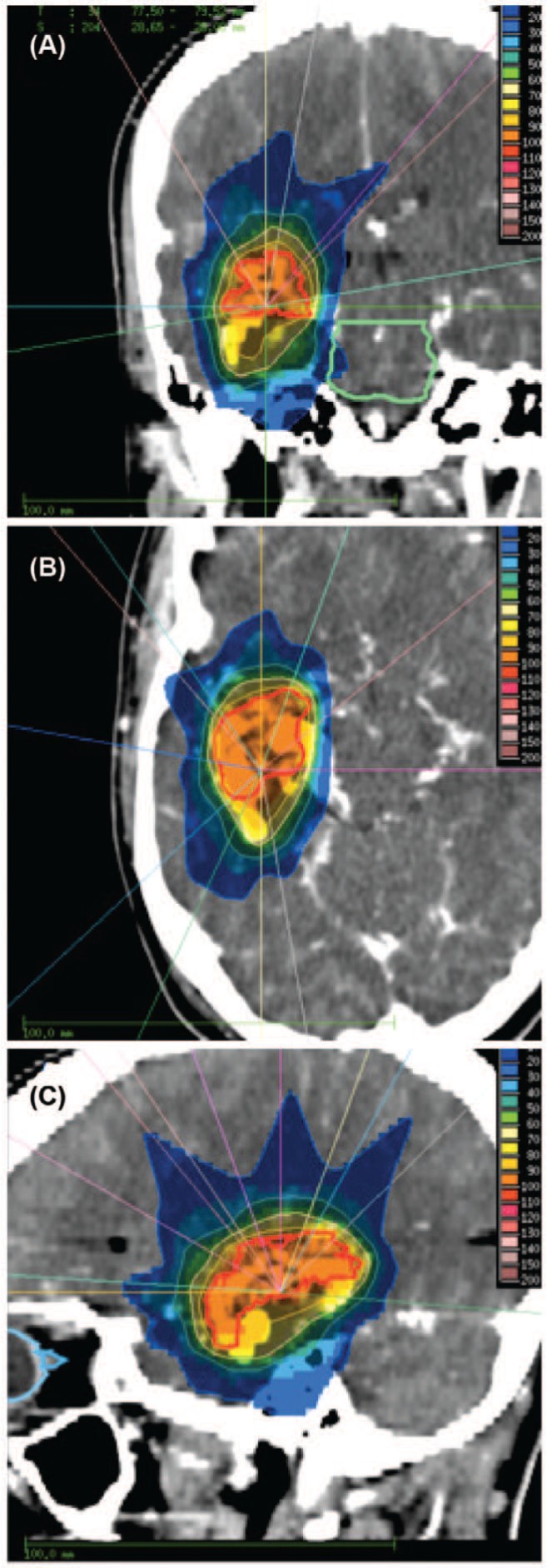
Example of a linac-based treatment plan for the same patient as in Figure 2 in coronal (A), axial (B) and sagittal (C) section.
To our knowledge, this study is the first to show a comprehensive comparison of Linac-based radiosurgery to treatment with state-of-the art HT and future delivery techniques such as RSS and DJDC. The cases included in this study were specifically chosen to represent a broad spectrum regarding size, localisation and shape. This heterogeneity might account for the broad standard deviation in average treatment times and normal tissue exposure.
The Tomotherapy planning software is designed to create a homogenous dose distribution in the target volume as opposed to the dose gradients typical for Linac-based radiosurgery. We used hull structures as described in previous publications (26, 27) to mimic that typical dose distribution with an intratarget dose gradient, as the aim of this study was a plan comparison between HT techniques and Linac-based plans in terms of treatment speed and sparing of organs at risk. A topic of discussion beyond the scope of this article, however, could be if the “classical” inhomogeneous dose pattern that is caused by technical requirements of linac-based radiosurgery is still contemporary. As modern radiotherapy devices become more and more widely available, radiosurgery with homogeneous dose to the target without a central dose peak is technically possible. If, however, this translates into a better brain sparing, both dosimetrically and clinically, remains to be investigated.
Currently used Tomotherapy machines are equipped with a binary MLC with a leaf width of 6.25 mm in the isocenter, whereas the MMLC used for brain radiosurgery in our department has a minimal leaf width of 1.8 mm in the isocenter. These dimensions make the limitations of HT in the treatment of very small AVMs in terms of lateral dose gradients evident. In addition, a dose penumbra above and below the target has to be taken into account in “regular” HT. Future HT techniques such as RSS and DJDC promise to eliminate the latter drawback as previously shown for the treatment of nasopharyngeal cancer (28). Axial Mode which operates with a static couch appears to be the ideal tool for the fast treatment of cylindrical lesions.
It is important to emphasise that these technologies and refinements of the Tomotherapy delivery are currently not available for clinical use. This was a purely theoretical planning comparison to explore the potential of these methods for small volume radiosurgery.
However, in this heterogeneous group of AVMs, HT could not provide substantial advantages compared to Linac-based treatment, neither in Regular Mode nor in more recently developed techniques. Even the research beam Reg 0.6, which provided the best brain sparing on low-dose levels, did not show substantial advantages compared to Linac plans in terms of treatment time. Most clinically important, all HT plans exposed bigger brain volumes to doses >60% of the prescription dose (average 12.75 Gy). It has been demonstrated that the risk of permanent postradiosurgery sequelae is influenced by the localisation and the volume of brain receiving 12 Gy or more (29).
In this plan comparison study, we could not show a superiority of future HT over Linac-based treatments of AVM. Yet, in some AVMs, HT could come up with superior plans: The patient with the best HT results had a C-shaped convex AVM directly above the brain stem. Reg 0.6 could reduce brain stem exposure and treatment time by more than 50%. In cases like this with a complex lesion directly above a critical structure but at some distance laterally, the coplanar dose application with HT shows benefits compared to non-coplanar linac-based treatment plans. Other AVMs with specific anatomy might benefit from the new techniques: For example, Axial with the barrel-shaped dose distribution (see Figure 1, lowest row) caused by the non-helical delivery with a static couch seems to be the ideal treatment mode for AVMs with a cylindrical shape not bigger than the maximum field width. In such a situation, treatment time might be reduced dramatically.
Very small field dosimetry during the opening and the closing of the jaws represents a major challenge for precise dose calculation and also for the technical realisation. These difficulties, however, are beyond the scope of this paper.
While treatment in a stereotactic headframe is possible with HT, the built-in MV fan beam CT allows for precise position correction before treatment and therefore facilitates the treatment of patients that cannot tolerate the rigid fixation needed for stereotactic setup systems. With a thermoplastic mask, an evaluation in our department suggests an expected mean setup error of 0.2 mm in x-, 0.5 mm in y- and 1.1 mm in z-direction (30). A more recent publication on frame-less image-guided radiosurgery (31) reported an intrafractional average 3D error of 0.9 mm, causing decreased target coverage and conformity. This effect could be abolished in the majority of cases by a safety margin of 1.0 mm. Thus, we believe that with modern IGRT devices, immobilisation with thermoplastic masks provides sufficient setup accuracy for radiosurgery.
Unlike the DJDC treatment of nasopharyngeal cancer, this study failed to demonstrate the superiority of advanced HT techniques compared to Linac-based radiosurgery in the treatment of very small volumes. With the current versions of Reg, RSS, Axial and DJDC HT delivery, we could not demonstrate a benefit for radiosurgery with HT in young, healthy patients with a benign tumour and a long life expectancy. Patient with brain metastases might benefit from faster treatments taking into account a slightly higher brain exposure.
Conclusions
In this study, neither current nor future HT techniques outperformed linac-based radiosurgery in terms of brain exposure. Yet, HT radiosurgery might be beneficial for AVM with special geometry as well as patients with brain metastases that benefit from shorter treatment times at the cost of a slightly higher brain exposure.
Acknowledgements
S. Krause receives funding by the Medical Faculty of the University Hospital Heidelberg, Germany.
Abbreviations:
- AVM:
Arteriovenous Malformation
- DJDC:
Dynamic Jaw/Dynamic Couch
- HT:
Helical Tomotherapy
- IMRT:
Intensity-Modulated Radiotherapy
- MMLC:
Micro-Multileaf-Collimator
- MV-CT:
Megavoltage-CT
- PTV:
Planning Target Volume
- Reg:
Regular HT Delivery
- RSS:
Running-Start-Stop.
Footnotes
Conflict of Interest: This work was supported by Accuray Incorporated, Sunnyvale, CA, USA. Accuray Inc. is the manufacturer of Tomotherapy equipment.
References
- 1.Patel P. N., Vyas R. K., Bhavsar D. C., Suryanarayan U. K., Pelagade S., Patel D. Analysis of X-knife and surgery in treatment of arteriovenous malformation of brain. J Cancer Res Ther 4, 169–172 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Bollet M. A., Anxionnat R., Buchheit I., Bey P., Cordebar A., Jay N., Desandes E., Marchal C., Lapeyre M., Aletti P., Picard L. Efficacy and morbidity of arc-therapy radiosurgery for cerebral arteriovenous malformations: a comparison with the natural history. Int J Radiat Oncol Biol Phys 58, 1353–1363 (2004). DOI: 10.1016/j.ijrobp.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Brown R. D., Jr., Wiebers D. O., Forbes G., O'Fallon W. M., Piepgras D. G., Marsh W. R., Maciunas R. J. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg 68, 352–357 (1988). DOI: 10.3171/jns.1988.68.3.0352. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y. C., Tseng C. K., Chang C. N., Wei K. C., Liao C. C., Hsu P. W. LINAC radiosurgery for intracranial cavernous malformation: 10-year experience. Clin Neurol Neurosurg 108, 750–756 (2006). DOI: S0303-8467(06)00053-9 [pii]. [DOI] [PubMed] [Google Scholar]
- 5.Loh Y., Duckwiler G. R. A prospective, multicenter, randomized trial of the Onyx liquid embolic system and N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations. Clinical article. J Neurosurg 113, 733–741 (2010). DOI: 10.3171/2010.3.JNS09370. [DOI] [PubMed] [Google Scholar]
- 6.Lv X., Wu Z., Jiang C., Li Y., Yang X., Zhang Y., Lv M., Zhang N. Endovascular treatment accounts for a change in brain arteriovenous malformation natural history risk. Interv Neuroradiol 16, 127–132 (2010). DOI: IN.v16.i2.p127 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollock B. E., Garces Y. I., Stafford S. L., Foote R. L., Schomberg P. J., Link M. J. Stereotactic radiosurgery for cavernous malformations. J Neurosurg 93, 987–991 (2000). DOI: 10.3171/jns.2000.93.6.0987. [DOI] [PubMed] [Google Scholar]
- 8.Pan D. H., Guo W. Y., Chung W. Y., Shiau C. Y., Chang Y. C., Wang L. W. Gamma knife radiosurgery as a single treatment modality for large cerebral arteriovenous malformations. J Neurosurg 93 (Suppl 3), 113–119 (2000). DOI: 10.3171/jns.2000.93.supplement3.0113. [DOI] [PubMed] [Google Scholar]
- 9.Zabel A., Milker-Zabel S., Huber P., Schulz-Ertner D., Schlegel W., Debus J. Treatment outcome after linac-based radiosurgery in cerebral arteriovenous malformations: retrospective analysis of factors affecting obliteration. Radiother Oncol 77, 105–110 (2005). DOI: S0167-8140(05)00172-6 [pii]. [DOI] [PubMed] [Google Scholar]
- 10.Colombo F., Cavedon C., Casentini L., Francescon P., Causin F., Pinna V. Early results of CyberKnife radiosurgery for arteriovenous malformations. J Neurosurg 111, 807–819 (2009). DOI: 10.3171/2008.10.JNS08749. [DOI] [PubMed] [Google Scholar]
- 11.Bois A. Zabel-du, Milker-Zabel S., Huber P., Schlegel W., Debus J. Stereotactic linac-based radiosurgery in the treatment of cerebral arteriovenous malformations located deep, involving corpus callosum, motor cortex, or brainstem. Int J Radiat Oncol Biol Phys 64, 1044–1048 (2006). DOI: S0360-3016(05)02715-X [pii]. [DOI] [PubMed] [Google Scholar]
- 12.Bois A. Zabel-du, Milker-Zabel S., Huber P., Schlegel W., Debus J. Pediatric cerebral arteriovenous malformations: the role of stereotactic linac-based radiosurgery. Int J Radiat Oncol Biol Phys 65, 1206–1211 (2006). DOI: S0360-3016(06)00242-2 [pii]. [DOI] [PubMed] [Google Scholar]
- 13.Bois A. Zabel-du, Milker-Zabel S., Huber P., Schlegel W., Debus J. Risk of hemorrhage and obliteration rates of LINAC-based radiosurgery for cerebral arteriovenous malformations treated after prior partial embolization. Int J Radiat Oncol Biol Phys 68, 999–1003 (2007). DOI: S0360-3016(07)00133-2 [pii]. [DOI] [PubMed] [Google Scholar]
- 14.Welsh J. S., Patel R. R., Ritter M. A., Harari P. M., Mackie T. R., Mehta M. P. Helical tomotherapy: an innovative technology and approach to radiation therapy. Technol Cancer Res Treat 1, 311–316 (2002). DOI: d=3007&c=4087&p=11179&do=detail [pii]. [DOI] [PubMed] [Google Scholar]
- 15.Shepard D. M., Olivera G. H., Reckwerdt P. J., Mackie T. R. Iterative approaches to dose optimization in tomotherapy. Phys Med Biol 45, 69–90 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Yartsev S., Kron T., Cozzi L., Fogliata A., Bauman G. Tomotherapy planning of small brain tumours. Radiother Oncol 74, 49–52 (2005). DOI: S0167-8140(04)00523-7 [pii]. [DOI] [PubMed] [Google Scholar]
- 17.Han C., Liu A., Schultheiss T. E., Pezner R. D., Chen Y. J., Wong J. Y. Dosimetric comparisons of helical tomotherapy treatment plans and step-and-shoot intensity-modulated radiosurgery treatment plans in intracranial stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 65, 608–616 (2006). DOI: S0360-3016(06)00252-5 [pii]. [DOI] [PubMed] [Google Scholar]
- 18.Soisson E. T., Mehta M. P., Tome W. A. A comparison of helical tomotherapy to circular collimator-based linear-accelerator radiosurgery for the treatment of brain metastases. Am J Clin Oncol 34, 388–394 (2011). DOI: 10.1097/COC.0b013e3181e9c0ee. [DOI] [PubMed] [Google Scholar]
- 19.Clark B., McKenzie M., Robar J., Vollans E., Candish C., Toyota B., Lee A., Ma R., Goddard K., Erridge S. Does intensity modulation improve healthy tissue sparing in stereotactic radiosurgery of complex arteriovenous malformations? Med Dosim 32, 172–180 (2007). DOI: S0958-3947(06)00179-8 [pii]. [DOI] [PubMed] [Google Scholar]
- 20.Penagaricano J. A., Yan Y., Shi C., Linskey M. E., Ratanatharathorn V. Dosimetric comparison of helical tomotherapy and Gamma Knife stereotactic radiosurgery for single brain metastasis. Radiat Oncol 1, 26 (2006). DOI: 1748-717X-1-26 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kissick M. W., Flynn R. T., Westerly D. C., Mackie T. R., Hoban P. W. On the making of sharp longitudinal dose profiles with helical tomotherapy. Phys Med Biol 52, 6497–6510 (2007). DOI: S0031-9155(07)53757-7 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterzing F., Schubert K., Sroka-Perez G., Kalz J., Debus J., Herfarth K. Helical tomotherapy. Experiences of the first 150 patients in Heidelberg. Strahlenther Onkol 184, 8–14 (2008). DOI: 10.1007/s00066-008-1778-6. [DOI] [PubMed] [Google Scholar]
- 23.Mackie T. R., Holmes T., Swerdloff S., Reckwerdt P., Deasy J. O., Yang J., Paliwal B., Kinsella T. Tomotherapy: a new concept for the delivery of dynamic conformal radiotherapy. Med Phys 20, 1709–1719 (1993). [DOI] [PubMed] [Google Scholar]
- 24.Yang J. N., Mackie T. R., Reckwerdt P., Deasy J. O., Thomadsen B. R. An investigation of tomotherapy beam delivery. Med Phys 24, 425–436 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Chen Y., Chen Q., Chen M., Lu W. Dynamic tomotherapy delivery. Med Phys 38, 3013–3024 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Fuss M., Salter B. J. Intensity-modulated radiosurgery: improving dose gradients and maximum dose using post inverse-optimization interactive dose shaping. Technol Cancer Res Treat 6, 197–204 (2007). DOI: d=3029&c=4232&p=15924&do=detail [pii]. [DOI] [PubMed] [Google Scholar]
- 27.Fuss M., Shi C., Papanikolaou N. Tomotherapeutic stereotactic body radiation therapy: techniques and comparison between modalities. Acta Oncol 45, 953–960 (2006). DOI: W8596QR773X550JM [pii]. [DOI] [PubMed] [Google Scholar]
- 28.Sterzing F., Uhl M., Hauswald H., Schubert K., Sroka-Perez G., Chen Y., Lu W., Mackie R., Debus J., Herfarth K., Oliveira G. Dynamic jaws and dynamic couch in helical tomotherapy. Int J Radiat Oncol Biol Phys 76, 1266–1273 (2010). DOI: S0360-3016(09)02776-X [pii]. [DOI] [PubMed] [Google Scholar]
- 29.Flickinger J. C., Kondziolka D., Lunsford L. D., Kassam A., Phuong L. K., Liscak R., Pollock B. Development of a model to predict permanent symptomatic postradiosurgery injury for arteriovenous malformation patients. Arteriovenous Malformation Radiosurgery Study Group. Int J Radiat Oncol Biol Phys 46, 1143–1148 (2000). DOI: S0360301699005131 [pii]. [DOI] [PubMed] [Google Scholar]
- 30.Sterzing F., Kalz J., Sroka-Perez G., Schubert K., Bischof M., Roder F., Debus J., Herfarth K. Megavoltage CT in helical tomotherapy — clinical advantages and limitations of special physical characteristics. Technol Cancer Res Treat 8, 343–352 (2009). DOI: d=3036&c=4295&p=17733&do=detail [pii]. [DOI] [PubMed] [Google Scholar]
- 31.Guckenberger M., Roesch J., Baier K., Sweeney R. A., Flentje M. Dosimetric consequences of translational and rotational errors in frame-less image-guided radiosurgery. Radiat Oncol 7, 63 (2012). DOI: 1748-717X-7-63 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]