Abstract
Blood vessels within tumours represent a key component for cancer cell survival. Disruption of these vessels can be achieved by inducing vascular endothelial-cell apoptosis. Moreover, endothelial cell apoptosis has been proven to be enhanced by ceramide-increasing drugs. Herein, we introduce a novel therapeutic approach which uses ultrasound-stimulated microbubbles used in combination with radiation to cause a rapid accumulation of ceramide in endothelial cells in-vitro. We also test this modality directly with other cell types as a general method of killing cancer cells. Human umbilical vein endothelial cells (HUVEC), acute myeloid leukemia cells (AML), murine fibrosarcoma cells (KHT-C), prostate cancer cells (PC3), breast cancer cells (MDA-MB-231) and astrocytes were used to evaluate this mechanism of inducing cell death. Survival was measured by clonogenic assays, and ceramide content was detected using immunohistochemistry. Exposure of cell types to ultrasound-stimulated bubbles alone resulted in increases in ceramide for all cell types and survivals of 12 ± 2%, 65 ± 5%, 83 ± 2%, 58 ± 4%, 58 ± 3%, 18 ± 7% for HUVEC, AML, PC3, MDA, KHT-C and astrocyte cells, respectively. Results from selected cell types involving radiation treatments indicated additive treatment enhancements and increases in intracellular ceramide content one hour after exposure to ultrasound-activated microbubbles and radiation. Endothelial cell survival decreased from 8 ± 1% after 2 Gy of radiation treatment alone and from 12 ± 2% after ultrasound and microbubbles alone, to 1 ± 1% with combined treatment. In Asmase +/+ astrocytes, survival decreased from 56 ± 2% after 2 Gy radiation alone and from 17 ± 7% after ultrasound and microbubbles alone, to 5 ± 2% when combined. Using ASMase deficient astrocytes (Asmase -/-) and Sphingosine-1-phosphate (S1P), we also demonstrate that ultrasound-activated microbubbles stimulate ASMase activity and ceramide production. These findings suggest that ultrasound-stimulated microbubbles could be used as a new biomechanical method to enhance the effects of radiation.
Keywords: Ceramide, Microbubbles, Ultrasound, Radiation, Asmase
Introduction
Tumours rely on blood vessels for survival (1). Tumour responses to radiotherapy can be affected by pro-angiogenic factors that protect endothelial cells, contributing to tumour radioresistance (2, 3). Radiation can provoke an up-regulation of VEGF to protect endothelial cells against apoptosis (4), which has been demonstrated to occur within 24 hours after radiation (5). These findings suggest that targeting vascular endothelial cells can be an effective strategy to enhance tumour response to radiation (6). In addition to angiogenesis, the survival of cancer cells is often further complicated by the presence of nearby healthy tissue. This often necessitates the use of low doses of radiation in order to avoid radiation toxicity effects. Hence, treatments that can maximize the effects of radiation and yet spare healthy tissue are necessary. The search for these new treatments has led to the development of numerous radiationenhancing cancer-fighting strategies, which involve the combination of radiotherapy with other therapeutic modalities. These include the inhibition of epidermal growth factor receptors, new anti-angiogenic drugs, and more recently, the use of ultrasound and microbubble contrast agents (6).
Microbubbles are often comprised of microscopic lipid or protein shells encapsulating gaseous content such as octafluoropropane that have previously been used for imaging, gene delivery (7, 8), tumour ablation (9) and as disrupting agents (10). Their compressible property allows them to be excited by ultrasound waves at low pressures for imaging, higher pressures for drug delivery, and very high pressures for non-invasive ultrasound surgery (11).
Bubble-based treatments with ultrasound can make use of such intravascular microbubbles, or convert liquid microdroplets (12) into gas bubbles which can form interstitially. Alternatively, at higher powers, ultrasound can cause bubble formation de novo from dissolved gases (7). Previous work reported near ideal pressures for microbubble permeation of the vasculature (8).
We have also recently investigated effects in animal models that have demonstrated a 40 to 50-fold increased sensitivity to radiation enhanced by a priori treatment with ultrasound and microbubbles (13). The factors responsible for the synergistic effect lead to blood vessel collapse and involve endothelial cell apoptosis leading to enhanced cell kill (13). Gene expression data and immunohistochemistry suggest the involvement of ceramide, a well known lipid mediator of apoptosis responsive to cell membrane damage (13).
Studies of endothelial cell apoptosis have revealed that radiation induces pro-apoptotic second messengers such as ceramide which cause apoptosis in endothelial cells (9). Synthesis of ceramide can occur through two distinct pathways: via de novo synthesis, and through sphingomyelinasedependent catabolism of sphingomyelin. Both pathways may further enhance cell kill (10, 14). Kolesnick et al. reported increases in ceramide levels seconds to minutes after irradiation, and hypothesized that this increase in ceramide was due to membrane-bound acid and neutral sphingomyelinases (ASMases/NSMases), whereas several hours later increased ceramide levels were attributed to the activity of ceramide synthase activated through the de novo pathway.
In this study, we undertook in vitro research prompted by results from in vivo experiments (13). Specifically, the experiments here were undertaken in order to characterize the potential for ceramide involvement in microbubble responses and to better understand the role of particular genetic pathways. This is to understand potential effects at a biochemical level and the role of the membrane-bound asmase gene on microbubble effects.
We demonstrate with proof-of-principle experiments that ultrasound and microbubbles can be used to additively enhance radiation effects. We also demonstrate this method results in an accumulation of ceramide in endothelial, leukemia, breast cancer, prostate cancer and murine fibrosarcoma cells. We further test the importance of the asmase pathway using asmase +/+ and asmase —/— astrocytes in addition to drug inhibition of the ceramide pathway. Since microbubbles administered intravenously are likely to damage only vascular endothelial cells, the choice was made to use HUVEC cells for this study. Astrocytes were chosen also as a test system for their relative radio-resistance. They also represent a good target for new ultrasound-stimulated interstitial microbubbles and permitted the investigation of the asmase pathway as stable cultures were available from wild type and asmase knock-out mice. We propose that microbubbles have the potential to maximize the effects of radiation by inducing the synthesis of pro-apoptotic intracellular ceramide. This technique could potentially be used as a radiation enhancer to achieve greater tumour eradication and avoid the use of higher doses of radiation.
Materials and Methods
Cell Cultures
All cells were grown at 37°C with 5% CO2. Primary astrocytes (obtained from asmase + /+ and asmase —/— mouse brains, Sunnybrook Health Sciences Centre, Toronto, ON) were cultured in DMEM with 10% fetal bovine serum (FBS) and 5% Penicillin. HUVEC cells (Sunnybrook Health Sciences Centre, Toronto, ON, Canada) were grown in EBM-2 (Lonza, Walkersville, MD USA) supplemented with 10 ml FBS, 0.2 ml Hydrocortisone, 2 ml hFGF-B, 0.5 ml VEGF, 0.5 ml R3-IGF-1, 0.5 ml ascorbic acid, 0.5 ml hEGF, 0.5 ml GA-1000 and 0.5 ml Heparin using EGM-2 singlequots kits. Breast and PC3 cells were grown in 1640-RPMI medium (Sigma-Aldrich Canada Inc., Oakville, ON, Canada), leukemia (AML, Ontario Cancer Institute, Toronto, ON), and sarcoma cells KHT (Sunnybrook Health Science Centre) were grown in a-MEM (SigmaAldrich Canada Inc., Oakville, ON), all with 10% FBS and 5% penicillin. In order to harvest adherent cells, confluent flasks were PBS washed, after which 0.05% trypsin EDTA (Gibco, Carlsbad, CA USA) was added for 5 minutes to detach cells. After trypsinization, cells were centrifuged at 440 g for 10 minutes and brought to a final cell concentration of 2 X 106 cells/ml of medium with 1.5 ml aliquots used for each treatment (i.e., 3 X 106 cells/sample). In all experiments cells were carefully handled to avoid clumping after typrinsization and this was verified by microscopy in advance of ultrasound treatments.
Treatments
For ultrasound treatments, a pulse was generated by a 2.86 cm-diameter single element transducer (IL0509HP, 500 kHz center frequency, Valpey-Fisher Inc., Hopkinton, MA, USA) connected to a micro-positioning system (8). The set-up also included a cylindrical chamber (10 mm diameter) for cell exposure. The chamber had mylar windows on both sides and a magnetic stirrer to mix cells and bubbles during ultrasound exposure in order to avoid standing wave effects. In order to treat the cells, 50 pL of 45 seconds agitated vialmix Definity microbubbles (Perflutren lipid microspheres, Lantheus Medical Imaging, Billerica, MA USA) and 1.5 ml of the 2 X 106 cells/ml solution were added to the chamber (for a 3.3% v/v bubble concentration). Insonification took place using a peak negative pressure of 570 kPa using a pulse sequence with a 9.6% duty cycle consisting of a 16 cycle tone burst and a pulse repetition frequency of 3 kHz, for a total insonification time of 2,880 milliseconds over 30 seconds. The -6 dB beamwidth for this transducer was 31 mm and the -3 dB beamwidth was 18 mm. Transducer characteristics were measured using a calibrated hydrophone in the absence and presence of the treatment set-up.
Sphingosine-1-Phosphate (S1P) (Biomol International L.P., Plymouth Meeting, PA USA), was used to counteract the mechanisms leading to ceramide-mediated apoptotic cell death (20). In order to treat cells, 1 pM S1P was added to asmase +/+ and asmase —/— culture media one hour before damage treatments with radiation or ultrasound, and was present during trypsinization, and for clonogenic survival assays in media. The rational for this was that any activation of ceramide dependent cell death by treatment may take many hours to manifest and should be inhibited long-term. Treatment of cells with C2-Ceramide (Sigma-Aldrich) was carried out similarly.
Radiation treatments were carried out by exposing samples to X-ray ionizing radiation (Faxitron Cabinet X-ray, Faxitron X-Ray LLC, IL) at a dose rate of 200 cGy/minute. For combined treatments radiation treatments were given within 1-2 minutes of ultrasound exposure.
Immunohistochemistry
Cells were added to generic cyto-spin cuvettes and cyto-spun onto Poly-L-lysine (Sigma-Aldrich Canada Inc., Oakville, ON, Canada) coated slides at 1500 RPM for 2 minutes (Cytospin3, Shandon, Thermo Fisher Scientific Inc, Ottawa, ON, Canada). Cells were fixed with 4% paraformaldehyde (TAAB Laboratories Equipment Ltd, Aldermaston, England). Slides were subsequently immunostained for ceramide using a monoclonal anti-ceramide antibody (MID 15B4, Alexis Biochemicals, Plymouth Meeting, PA USA). Ceramide content was measured from immunohistochemistry by measuring the brown to blue ratio in cell microscopy images (Image J, NIH, Bethesda MD USA). A brown to blue ratio of 8:2 was taken as positive for ceramide staining.
Statistical Analysis
Survival assay were performed in duplicate, and within any one experiment, conditions were all done in triplicate (i.e. 3 culture dishes/sample and 2 samples per condition). Student’s t-test were done to confirm statistical significance (GraphPad Prism 4.0, La Jolla, CA).
Results
Cell Types and Ceramide Formation in Response to Microbubbles
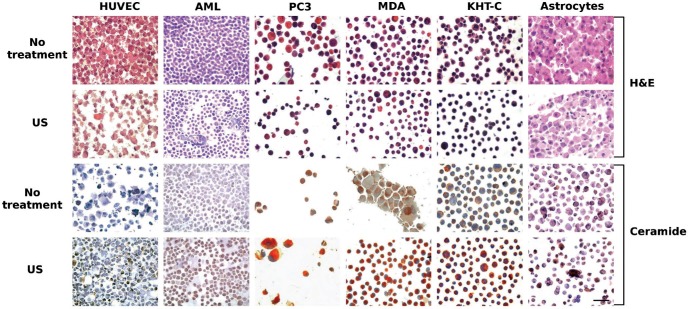
Several cell types were exposed to microbubbles in the presence of ultrasound. Clonogenic assays indicated survivals of 12 ± 2%, 65 ± 5%, 83 ± 2%, 58 ± 4%, 58 ± 3%, 18 ± 7% for HUVEC, AML, PC3, MDA, KHT-C and asmase +/+ astrocyte cells, Histology and immunohistochemistry (Figures 1 and 2) indicated that ceramide was formed in all cell types tested (AML, PC3, MBA231, KHT, HUVEC, astrocytes) in response to ultrasound-stimulated microbubble exposure. Immunohistochemistry using anti-ceramide antibody stained cells for ceramide after ultrasound exposure. This is specific antibody-medicated detection of ceramide with antibodies then stained using a colorimetric process.
Figure 1:
Haematoxylin and Eosin and immunohistochemical staining of HUVEC, AML, PC3, MDA-MB-231, KHT-C and astrocyte (asmase +/+) cells with and without ultrasound and microbubble exposure, one hour after treatment. H&E Staining: In all cell lines, haematoxylin and eosin staining indicated structural changes such as nuclear fragmentation with ultrasound-stimulated microbubble exposure. The decrease in cellular density in HUVEC and astrocytes after treatment indicates potential destruction of the cells by the ultrasound and microbubble treatment. Shrinkage of AML, PC3, MDA-MB-231 and KHT-C cells was also observed. Ceramide staining: ceramide (brown stain) was observed in small amounts in untreated cells as expected, but darker staining was present in the treated cells. The scale bar represents 50 microns.
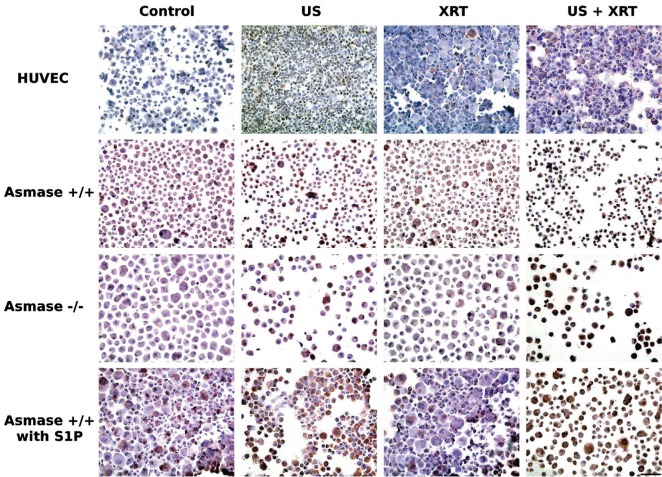
Figure 2:
Immunohistochemical ceramide staining of HUVEC, asmase +/+ astrocytes, asmase —/— astrocytes and S1P-treated asmase +/+ astrocytes one hour after treatments. A similar pattern of effect was observed across all cell lines. Untreated cells and cells treated with radiation only appeared blue, but those treated with ultrasound and microbubbles, with or without radiation, caused cells to shrink and ceramide to be accumulated as shown by the brown staining. The scale bar represents 100 microns.
Two cell types were selected for further analysis including HUVEC to test effects that may be involved in vivo upon endothelial cells subjected to intravascular exposure to ultrasound-stimulated microbubbles. Genetically modified astrocytes were also selected to probe the role of the asmase gene product. In addition, HUVEC cells were selected because they are highly enriched in asmase and sensitive to its activities, whereas astrocytes were selected because of low baseline levels of asmase and relative insensitivity to ceramide.
Ultrasound-Stimulated Microbubble Effects on RadiationInduced Cell Death in Endothelial Cells and Astrocytes
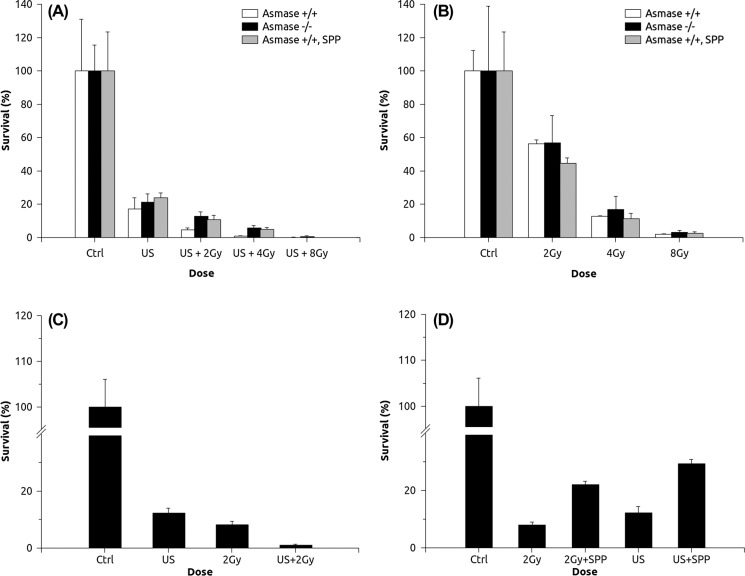
Survival assays of asmase + /+ and asmase -/- astrocytes, in response to ultrasound-activated microbubbles in combination with radiation (Figure 3A), demonstrated a significantly enhanced level of cell death as compared to survival in response to radiation alone (Figure 3B). The combination of the two treatments was additive. Survival decreased to 56 ± 2% in response to 2 Gy radiation alone, whereas bubbles alone caused 17 ± 7% survival. Only 5 ± 2% survival or less in response to 2 Gy radiation with ultrasound-stimulated microbubble treatment was observed. A differential effect was also observed in the survival of asmase +/+ astrocytes and asmase —/— astrocytes in response to the combined treatments only (p < 0.1 for the combination with 2 Gy and p < 0.05 for the combination with 4 Gy), whereas ultrasound and microbubbles treatment alone induced no observable differential effect (p > 0.1). Use of 1 jM S1P demonstrated no protective effect when asmase +/+ astrocytes were treated with either radiation or ultrasound with microbubbles (Figure 3B), but did so when treated with the two modalities combined (Figure 3A). Control astrocytes exhibited a 50 ± 5% survival when treated with ceramide alone as a control. Survival assessment of HUVEC (Figure 3C) revealed an additive effect when treated with ultrasound and microbbubles, combined with 2 Gy radiation. Here 1 jM S1P significantly protected the endothelial cells from apoptosis when the cells were treated with either radiation or ultrasound and microbubbles (p < 0.01) (Figure 3D).
Figure 3:
Survival assays of astrocytes and HUVEC in response to treatments. Ultrasound-stimulated microbubble exposure induced a similar response for wild type, mutant and S1P-treated astrocytes (3A). The combination of ultrasound and microbubbles with radiation, however, induced a greater response in wild type astrocytes; mutant and S1P-treated astrocytes still had a similar response. On the other hand, radiation treatment alone demonstrated a similar response for all astrocytes, which indicates the activation of ceramide synthesizing enzymes by ultrasound and microbubbles (3B). Treatment of HUVEC with the ceramide pathway blocker S1P demonstrated enhanced cell survival, suggesting the involvement of the ceramide pathway in both radiation alone and ultrasound and microbubbles alone treatments (3C). The combination of ultrasound and microbubbles with radiation caused an additive response in HUVEC (3D).
Ultrasound-Activated Microbubble Effects on Intracellular Levels of the Apoptotic 2nd Messenger Ceramide
Ceramide presence after treatment was assessed for HUVEC and astrocytes by immuno-staining. Exposure of HUVEC cells indicated maximum detectable ceramide after treatment. Figure 2 presents representative immunohistochemistry results for HUVEC and astrocytes. HUVEC cells, control and 8 Gy treated cells exhibited no appreciable ceramide staining. For HUVEC cells treated with ultrasound-stimulated microbubbles and ultrasoundactivated microbubbles with 8 Gy, 30-50% of cells exhibited ceramide staining. For astrocyte cells, little difference was observed between control, ultrasound-stimulated microbubble exposed and 8 Gy treated cells. In contrast, ultrasound-stimulated microbubble and radiation exposed cells demonstrated a very high number of brown stained cells (70-80%). Asmase —/— astrocytes exhibited a lower survival response to bubble-induced ceramide compared to wild type astrocytes, but with similar 70-80% staining. We tested the effects of S1P to inhibit ceramide related signalling in astrocytes as it was effective in doing so in HUVEC cells. Results indicated S1P conferred some radio-resistance to wild type astrocytes. Astrocytes treated with S1P and ASMase-deprived astrocytes had a higher and similar survival as compared to wild type astrocytes (Figure 3).
Our results indicated that astrocytes did not exhibit apoptotic cell death with radiation treatment, which is consistent with previous studies (15). In order to assess the inherent sensitivity of cells to ceramide, HUVEC and astrocytes cells were exposed to ceramide. Treatment of astrocytes with 1 jM c2-ceramide yielded no significant cell kill. HUVEC cells did not survive this exposure (data not shown). The apoptotic blocker S1P failed to protect wild type astrocytes from cell death, indicating that these cells died from other types of cell death. Histologically, they exhibited mitotic arrest rather than apoptosis. However, S1P mediated protection was detected (Figure 3A) when wild type astrocytes (+/+) were treated with ultrasound and microbubbles prior to radiation exposure. This suggests that ultrasound and microbubbles induce damage that causes wild type astrocytes to die by apoptosis.
Discussion
Exposing different cell types (leukemia, HUVEC, fibrosarcoma, breast, prostate and astrocytes) to ultrasound and microbubbles indicated that ceramide formation in response to microbubbles mediated cell membrane perturbation was present amongst different cells (Figures 1 and 2). We further investigated bubble exposure on two cell types; HUVEC cells in culture are a closer model to expected cell-types which would be exposed to microbubbles given intravascularly, although still an in vitro model. Such bubbles are already used as radiological contrast agents. Astrocytes were selected to represent a relatively radiation resistant cell line. These could be treated in vivo with microbubbles by contact with liquid nano-droplets which can perfuse into tissue through leaky tumour vasculature that can be turned into gas bubbles by ultrasound exposure. The astrocyte line was also selected since there are asmase +/+ and asmase —/— strains which can be used experimentally.
Data indicated that radiation-induced cell death was enhanced by exposing cells to ultrasound-activated microbubbles. Endothelial cells demonstrated an additive effect in response to the two treatments (Figure 3C), rather than a synergistic one as may occur in vivo, suggesting the involvement of alternate physiologic processes in vivo where vessel collapse has been observed with such treatments. A 12 ± 2% survival in response to ultrasound-activated microbubbles and an 8 ± 1% survival in response to 2 Gy radiation led to about 1 ± 1% survival when the two were combined. It is possible that endothelial cells are very sensitive to the mechanism of death being induced, thereby masking a possible synergistic effect. Wild type astrocytes (asmase + /+ ), mutant type astrocytes (asmase —/—) and wild type astrocytes treated with S1P all had a similar response to 2, 4 and 8 Gy radiation alone, i.e. no differential effect was observed (p > 0.05) consistent with a lack of apoptotic cell death. Two studies have reported expected contrasting related conclusions, but with cell lines that are more sensitive to radiation. One in vivo study attributed acquired resistance to radiation damage to acid sphingomyelinase (AMSase) deficiency in knockout mice (16), whereas another in vitro study reported that loss of neutral sphingomyelinase (NSMase), not ASMase, was responsible for acquired radiation resistance (17). Wong et al. reported reduced radiation-induced apoptosis in astrocytes as compared to other cell lines (18). This is consistent with our study, given that wild type astrocytes treated with an apoptotic blocker (S1P) did not significantly protect cells from radiation (Figure 3B). This was similar for ultrasound and bubble treatments alone but with a trend towards an effect. There was, however, a protective effect, when cells were pre-treated with ultrasound-stimulated microbubbles before radiation treatments. Wild type astrocytes showed a differential effect (p < 0.05) in response to the combined treatment as compared to asmase —/— astrocytes. Asmase 1/1 astrocytes treated with S1P exhibited a protective effect from microbubble damage (Figure 3A). This result points to the involvement of the ASMase and ceramide pathways with ultrasound-microbubble treatments.
It is now well recognized that apoptosis in certain cells occurs mainly through the accumulation of intracellular ceramide, an inducer of apoptosis (9, 14, 16, 19, 20). All cell types here exposed to ultrasound-stimulated microbubbles indicated an increase in ceramide content as observed using immunohistochemistry. Ceramide is a cell-stress related molecule which can be elevated due to cell membrane damage in addition to radiation (21). In the current study, endothelial cells demonstrated a minimum two-fold increase in survival after treatment with ultrasound-stimulated microbubbles in the presence of S1P (Figure 1D), indicating that ultrasound-stimulated microbubble exposure can initiate apoptotic pathways. In order to verify the role of ceramide in ultrasound-stimulated microbubble enhanced cell death, control HUVEC and wild type astrocytes were first treated with 1 μM exogenous ceramide (not shown). Colony assessment indicated no survival in HUVEC cells, which was expected. This was consistent with ceramide generation with the combined effects of exposure to ultrasound-stimulated microbubbles and radiation (Figure 3). Astrocytes, in contrast, were resistant to ceramide-induced cell death, as expected (18). Wild type astrocyte (asmase +/+) survival was also lower than both asmase —/— and S1P treated asmase +/+ astrocytes with ultrasound-stimulated microbubble treatment, suggesting that ultrasound-stimulated microbubble exposure activates other cell death mechanisms within the cell other than radiation-induced classic mitotic arrest. Astrocyte immunohistochemical staining confirmed a high concentration of ceramide one hour after treatment (Figure 2). In particular, the combination of ultrasound-stimulated microbubbles and 8 Gy treatment caused a large accumulation of detectable ceramide in asmase + /+ , asmase —/— and asmase +/+ astrocytes treated with S1P. Cells produce ceramide by numerous processes, among which two of the most studied are sphingosine degradation, and hydrolysis of sphingomyelin by sphingomyelinases (19, 20). Asmase —/— astrocytes lack the ASMase enzyme but can still make ceramide by breaking down sphingosine, and possibly by NSMase activity, which can explain increased ceramide levels and cell death (Figures 2 and 3). Similarly, even though S1P conferred some protection to asmase +/+ astrocytes as above, an increase in ceramide levels was still present in histology (Figure 2). Protection by S1P however, can be thought of as relatively functionally insignificant for astrocytes because these cells are not very sensitive to apoptotic cell death (18, 15). These results suggest that ultrasoundstimulated microbubble-induced stress causes the activation of ceramide production.
The research here was conducted in vitro and was prompted by results from in vivo experiments (13). Specifically, the experiments here were undertaken in order to characterize the potential for ceramide involvement in microbubble responses and to better understand the role of particular genetic pathways. The environment in vitro can be different but here we were interested in the fundamental biochemical changes that could be elicited by microbubbles and how genetic difference in asmase expression (related to the ceramide pathway) could influence microbubble effects in the absolute sense. There is the possibility with in vitro experimentation that standing waves could be created. However, cells were stirred in order to minimize potential heterogenous exposure to such waves. Nevertheless, the activation of ceramide in the different cell types was similar to that detected in vivo (13) using immunohistochemistry with comparable effects also seen in vivo with ceramide inhibition. In addition, care was taken to ensure cells were not aggregated during ultrasound exposure as this could protect cells from microbubble induced damage. No heating was detected in experiments and the power levels used were low similar to those used in color Doppler.
In summary, endothelial cell apoptosis is a key component of radiotherapy response (3). Its importance arises from the relationship between angiogenesis and tumour sustainment, given that endothelial cell death can lead to blood vessel collapse within a tumour (4). Recently, numerous studies have identified the apoptotic messenger ceramide as a useful tool to achieve higher endothelial cell apoptosis levels (9, 17). Our data suggests that ultrasound-stimulated microbubbles in combination with radiation could be used as a radioenhancing modality. Data indicate that ultrasound-activated microbubble exposure causes sufficient ceramide production to cause cell death. It also sensitized a relatively radioresistant cell type. The bioeffect elicited here also represents an excellent alternative to increasing radiation doses to improve cancer therapies, which often is not possible, and thus could enhance treatment effects at clinically relevant radiation doses.
Acknowledgements
This work was supported by funding from NSERC, CCSRI, and the Terry Fox Foundation. Dr. Gregory J. Czarnota is supported by a CCO Research Chair in Experimental Therapeutics and Imaging. We thank the Sunnybrook Health Sciences Centre for infrastructure support.
Footnotes
Conflict of Interest Statement: We certify that regarding this paper, no actual or potential conflicts of interest exist; the work is original, has not been accepted for publication nor is concurrently under consideration elsewhere, and will not be published elsewhere without the permission of the Editor.
References
- 1.Folkman J., Shing Y. Angiogenesis. J Biol Chem 267, 10931–10934 (1992). [PubMed] [Google Scholar]
- 2.Fuks Z., Kolesnick R. Engaging the vascular component of the tumour response. Cancer Cell 8, 89–91 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Barros M., Paris F., Cordon-Cardo C., Lyden D., Rafii S., Haimovitz-Friedman A., Fuks Z., Kolesnick R. Tumour response to radiotherapy regulated by endothelial cell apoptosis. Science 300, 1155–1159 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Gorski D. H., Beckett M. A., Jaskowiak N. T., Calvin D. P., Mauceri H. J., Seetharam S., Koons A., Hari D. M., Kufe D. W., Weichselbaum R. R. Blockage of the vascular endothelial growth factor stress response increases the antitumour effects of ionizing radiation. Cancer Res 59, 3374–3378 (1999). [PubMed] [Google Scholar]
- 5.Shinohara C., Gobbel G. T., Lamborn K. R., Tada E., Fike J. R. Apoptosis in the subependyma of young adult rats after single and fractionated doses of X-rays. Cancer Res 57, 2694–2702 (1997). [PubMed] [Google Scholar]
- 6.Caissie A., Karshafian R., Hynynen K., Czarnota G. J. Ultrasound Contrast Microbubbles: In Vivo Imaging and Potential Therapeutic Applications. In: NanoImaging. Goins B., Phillips W. T. (Eds.), Chicago, USA: Pan Stanford Publishing; (in press) (2009). [Google Scholar]
- 7.McLaughlan J., Rivens I., Leighton T., Ter Haar G. A study of bubble activity generated in ex vivo tissue by high intensity focused ultrasound. Ultrasound Med Biol 36, 1327–1344 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Karshafian R., Bevan P. D., Williams R., Samac S., Burns P. M. Sonoporation by ultrasound-activated microbubble contrast agents: effect of acoustic exposure parameters on cell membrane permeability and cell viability. Ultrasound Med Biol 35, 847–860 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Sathishkumar S., Boyanosky B., Karakashian A. A., Rozenova K., Giltiay N. V., Kudrimoti M., Mohiuddin M., Ahmed M. M., Nikolova-Karakashian M. Elevated sphingomyelinase activity and ceramide concentration in serum of patients undergoing high dose spatially fractionated radiation treatment: implications for endothelial apoptosis. Cancer Biol Ther 4, 979–89 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Reynolds C. P., Maurer B. J., Kolesnick R. N. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett 206, 169–180 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Kolesnick R., Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene 22, 5897–5906 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Zhang P., Porter T. An in vitro study of a phase-shift nanoemulsion: a potential nucleation agent for bubble-enhanced HIFU tumour ablation. Ultrasound Med Biol 36, 1856–1866 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Czarnota C. J., Karshafian R., Burns P. N., Wong S., Lee J., Caissie A., Furukawa M., AL-Mahrouki A., Giles A. Ultrasound Induced Microbubble Enhancement of Tumour Radiation Response. Proceedings National Academy Sciences U.S.A. In Press. [DOI] [PMC free article] [PubMed]
- 14.Jarvis W. D., Grant S., Kolesnick R. N. Ceramide and the induction of apoptosis. Clin Cancer Res 2, 1–6 (1996). [PubMed] [Google Scholar]
- 15.Li Y. Q., Jay V., Wong C. S. Oligodendrocytes in the Adult Rat Spinal cord undergo radiation-induced apoptosis: Cancer Research (1996). [PubMed]
- 16.Santana P., Pena L. A., Haimovitz-Friedman A., Martin S., Green D., McLoughlin M., Cordon-Cardo C., Schuchman E. H., Fuks Z., Kolesnick R. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell 86, 189–199 (1996). [DOI] [PubMed] [Google Scholar]
- 17.Chmura S. J., Nodzenski E., Beckett M. A., Kufe D. W., Quintans J., Weichselbaum R. R. Loss of ceramide production confers resistance to radiation-induced apoptosis. Cancer Res 57, 1270–1275 (1997). [PubMed] [Google Scholar]
- 18.Lu F. G., Wong C. S. Radiation-induced apoptosis of oligodendrocytes and its association with increased ceramide and down-regulated protein kinase B/Akt activity. Int J Radiat Biol 80, 39–51 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Cuvillier O. Sphingosine in apoptosis signaling. Biochim Biophys Acta 1585, 153–162 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Cuvillier O., Pirianov G., Kleuser B., Vanek P. G., Coso O. A., Gutkind S., Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381, 800–803 (1996). [DOI] [PubMed] [Google Scholar]
- 21.Nikolova-Karakashian N. M., Rozenova K. A. Ceramide in Stress Response. Adv Exp Med Biol 688, 86–108 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]