Abstract
Facioscapulohumeral muscular dystrophy (FSHD) is caused by the aberrant expression of the DUX4 transcription factor in skeletal muscle. The DUX4 retrogene is encoded in the D4Z4 macrosatellite repeat array, and smaller array size or a mutation in the SMCHD1 gene results in inefficient epigenetic repression of DUX4 in skeletal muscle, causing FSHD1 and FSHD2, respectively. Previously we showed that the entire D4Z4 repeat is bi-directionally transcribed with the generation of small si- or miRNA-like fragments and suggested that these might suppress DUX4 expression through the endogenous RNAi pathway. Here we show that exogenous siRNA targeting the region upstream of the DUX4 transcription start site suppressed DUX4 mRNA expression and increased both H3K9 methylation and AGO2 recruitment. In contrast, similarly targeted MOE-gapmer antisense oligonucleotides that degrade RNA but do not engage the RNAi pathway did not repress DUX4 expression. In addition, knockdown of DICER or AGO2 using either siRNA or MOE-gapmer chemistries resulted in the induction of DUX4 expression in control muscle cells that normally do not express DUX4, indicating that the endogenous RNAi pathway is necessary to maintain repression of DUX4 in control muscle cells. Together these data demonstrate a role of the endogenous RNAi pathway in repeat-mediated epigenetic repression of the D4Z4 macrosatellite repeat, and show that enhancing the activity of this pathway by supplying exogenous siRNA oligonucleotides represents a potential therapeutic approach to silencing DUX4 in FSHD.
Introduction
Facioscapulohumeral dystrophy (FSHD) is caused by the inefficient epigenetic repression of the DUX4 retrogene encoded in the D4Z4 macrosatellite repeats, resulting in the mis-expression of the DUX4 protein in skeletal muscle (1). Inefficient epigenetic repression can be caused by a contraction of the D4Z4 array from a range of 11–100 units to between one and 10 repeat units (FSHD1), or by a mutation in the SMCHD1 gene (FSHD2) (2), a gene previously shown to be necessary for repeat-mediated epigenetic repression (3,4). Although very small amounts of DUX4 mRNA are detected in FSHD muscle, the strict association of FSHD with a specific haplotype of chromosome 4 (the 4qA haplotype) that contains a polymorphic poly-adenylation sequence for the DUX4 mRNA provides strong genetic evidence that the expression of DUX4 is the cause of FSHD (5), and, consequently, that preventing DUX4 expression should prevent FSHD.
The molecular mechanisms associated with repeat-mediated epigenetic repression in mammalian cells remain poorly understood. Although the transcription of a full-length poly-adenylated DUX4 mRNA is relatively specific to FSHD muscle cells, RNA transcripts can be detected throughout the D4Z4 array in both FSHD and control cells. Previously, we demonstrated that the D4Z4 repeats are bi-directionally transcribed in somatic tissues (fibroblasts and muscle cells) of control and FSHD individuals, generating both long non-polyadenylated stretches of RNA as well as small RNA fragments consistent with si- or mi-RNAs, and suggested that these might have a role in an RNA-mediated epigenetic silencing of the array (6). Transcriptional gene silencing by siRNA has been mechanistically well characterized in model organisms (7). In fission yeast, siRNAs recruit an RNAi transcriptional silencing (RITS) complex that leads to subsequent H3K9 methylation and chromatin repression (8). Similar small RNA-directed chromatin repression has been described in other model organisms, including C. elegans, Tetrahymena and Arabidopsis (9–11), including the demonstration that an ortholog of the mammalian SMCHD1 participates in this pathway in Arabidopsis (12). Several studies have also shown RNA-mediated transcriptional gene silencing in vertebrate cells. For example, si- or miRNAs targeting ncRNAs have been shown to modulate gene expression in mammalian cells (13,14), and studies have implicated Dicer-dependent mechanisms of maintaining constitutive heterochromatin in vertebrates and silencing LINE-1 retrotransposons through naturally occurring endo-siRNAs in mammals (15,16). Therefore, we investigated the possibility that the small si- and mi-like RNAs generated from the D4Z4 bi-directional transcripts might contribute to the epigenetic silencing of the D4Z4 macrosatellite repeat.
In this study, we show that siRNA targeting D4Z4 regions upstream of the DUX4 mRNA, many of them in regions where clusters of endogenous small RNAs were identified, efficiently silenced DUX4 expression in FSHD cells. The silencing by the upstream siRNA had delayed kinetics compared with siRNA targeting the DUX4 mRNA and was correlated with increased H3K9me2 and AGO2 recruitment in the targeted D4Z4 regions, whereas 2′-O-methoxyethyl (MOE)-gapmers that degrade RNA through the RNase H pathway targeted to the same regions did not repress DUX4 expression. Interfering with the endogenous siRNA pathway by DICER or AGO2 knockdown, using either siRNA or MOE-gapmer antisense oligonucleotides, resulted in the de-repression of DUX4 in non-FSHD muscle cells that normally do not express DUX4. These studies demonstrate that the RNAi transcriptional silencing pathway is necessary for the somatic repression of DUX4 and the D4Z4 region, and suggest oligonucleotide-directed strategies for therapeutic epigenetic silencing of DUX4 in FSHD.
Results
siRNAs upstream of the DUX4 TSS suppress DUX4 expression in FSHD cells
Previously we demonstrated that sense and antisense RNA transcripts extend through the D4Z4 repeats and generate small si- or mi-like RNA fragments (6). We suggested that these small RNA fragments might regulate DUX4 expression either by modulating DUX4 mRNA stability or translation, or possibly through an RNA-mediated epigenetic silencing or a combination of these mechanisms. To determine whether small RNAs from the D4Z4 region were effective at modulating DUX4 expression, we synthesized siRNAs that were perfect or near mimics of the endogenously generated small RNAs as well as a series of additional siRNA spanning the D4Z4 sequence (all designed to target a ‘sense’ transcript of D4Z4 relative to the DUX4 open-reading frame (ORF) (Fig. 1A and Supplementary Material, Table S1), and tested them for suppression of DUX4 expression when transfected into FSHD muscle cells in culture.
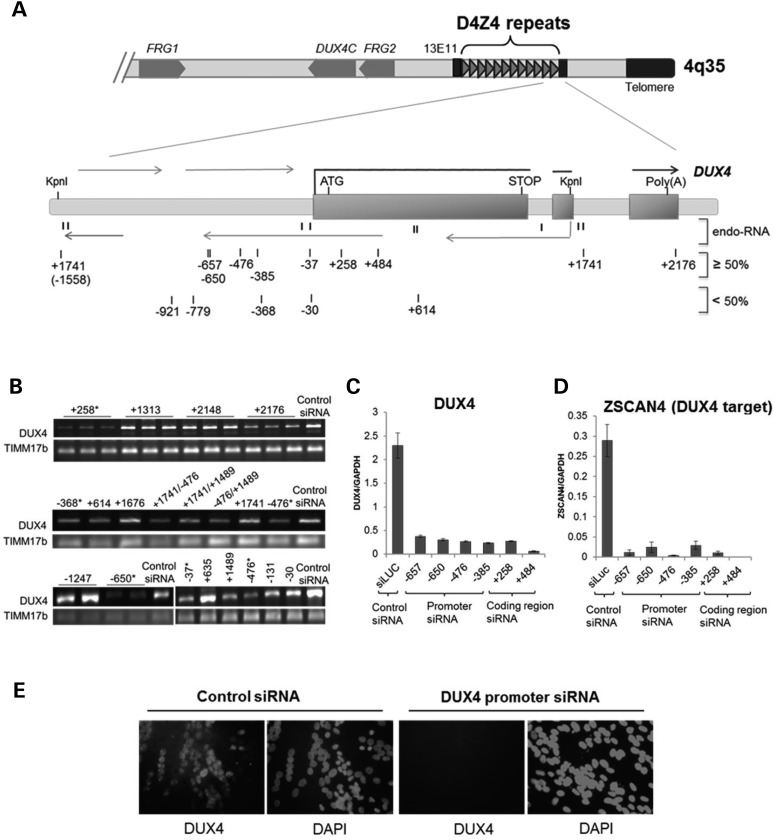
Figure 1.
Inhibition of DUX4 expression in FSHD cells by siRNAs targeting D4Z4 regions. (A) Schematic diagram of the distal tip of 4q35 showing the D4Z4 repeat region and enlargement of the distal D4Z4 unit flanked by KpnI sites and the adjacent pLAM sequence with the imbedded DUX4 gene. Exons 1–3 of DUX4 are indicated by rectangles. Thick horizontal lines correspond to the DUX4 mRNA, whereas thin horizontal lines depict multiple sense and antisense D4Z4 transcripts. Direction of transcription is indicated with arrows. Previously identified D4Z4-derived endogenous small RNAs (6) are shown by short vertical lines and indicated as endo-RNA. Numbered vertical marks show the location of exogenous duplex siRNAs targeting the D4Z4 region. siRNA numbering indicates location of the 5-prime nucleotide of the targeted sequences relative to the most promoter proximal DUX4 transcription start site (TSS = 1) (6) with all siRNAs in this figure designed to target the sense sequence relative to the DUX4 transcript (see Supplementary Material, Table S1 for each targeted sequence). Top lane shows siRNAs that efficiently reduce DUX4 mRNA levels (≥50%), and bottom lane shows less-efficient siRNAs (<50%) as determined by RT- and qRT–PCR analysis of DUX4 and DUX4 target gene expression (B and C). (B) Representative sample of RT–PCR analysis of DUX4 mRNA abundance following transient transfection of FSHD1 muscle cells with duplex siRNAs targeting D4Z4 regions as depicted in (A). TIMM17b RT–PCR on each sample serves as internal control. Biological replicates are indicated by horizontal lines above lanes; otherwise, single representative PCR results from multiple experiments are shown. Asterisk mark indicates siRNAs that were verified by qRT–PCR and selected for further investigation. (C and D) DUX4 and DUX4-target mRNA levels in FSHD2 muscle cells following transfection with the siRNAs, targeting coding and non-coding DUX4 sequences, were quantified by real-time qRT–PCR analysis (see Supplementary Material, Fig. S1 for details). DUX4 and DUX4-target qRT–PCR data were analyzed by standard curve, normalized to GAPDH and presented as mean ± SEM based on triplicate PCR reactions for each sample. (E) Immunofluorescence analysis of FSHD2 muscle cells transfected with control (siLuc) and promoter (−650) targeting siRNAs. Cells were stained with DUX4 antibody and DAPI, as indicated. Single representative images of FSHD muscle cells transfected with the control and promoter targeting siRNAs that were validated by qRT–PCR in (C) are shown. For all experiments presented in Figure 1, DUX4 or DUX4-target expression was analyzed in FSHD muscle cells 96 h post siRNA transfection and 48 h post-induction to differentiation (MB to MT 4 days time point) (see also Supplementary Material, Figs. S1 and S2).
Figure 1A and B summarize the results of the initial screen based on RT–PCR for DUX4 mRNA. As expected, siRNAs targeting the DUX4 mRNA sequence, e.g. the siRNA initiating at position +258 relative to the DUX4 transcriptional start site (TSS), robustly decreased DUX4 mRNA abundance. The TSS refers to the most promoter-proximal of several 5-prime capped ends of DUX4 mRNA transcripts identified by 5-prime RACE (6) and the siRNAs are named based on the position of the 5-prime nucleotide of their targeted sequence relative to this TSS. In addition to the siRNAs targeting the DUX4 transcript, several of the siRNAs designed to target the regions upstream of the DUX4 mRNA TSS, such as −385, −476 and −650, also strongly suppressed DUX4 expression.
To further test the efficiency of the suppression of DUX4 expression by siRNAs targeted to the region upstream of the TSS, which we will refer to as the ‘promoter’ region, compared with the mRNA-targeting siRNAs, we used quantitative RT–PCR to measure DUX4 mRNA levels and the levels of genes induced by the DUX4 transcription factor, including ZSCAN4 and RPFL2 (17). We focused on the region between −350 and −675 upstream of the transcription start site because this region was relatively far removed from the DUX4 mRNA and yet siRNA to this region showed robust suppression of the DUX4 mRNA, similar to siRNA directed to the coding region of the DUX4 mRNA. Also, while the initial screen shown in Figure 1A and B was performed in an FSHD1 muscle cell line, subsequent studies were performed in an FSHD2 muscle cell line to facilitate studies of chromatin modifications because the D4Z4 arrays on both chromosomes 4q and 10q exhibit chromatin relaxation in FSHD2 (1). Supplementary Material, Figures S1 and S2 show that the expression of the full length DUX4 mRNA and its targets can be quantitatively measured in these cells and also validates the efficiency of the siRNA transfections. Quantitative RT–PCR confirmed that siRNAs directed to the −350 to −675 region suppressed expression of the DUX4 mRNA as robustly as siRNA directed at the DUX4 coding region (Fig. 1C), and both also suppressed the expression of the DUX4 regulated gene ZSCAN4 (Fig. 1D), and this correlated with decreased DUX4 protein in the nuclei of differentiated FSHD myotubes (Fig. 1E and Supplementary Material, Fig. S2D).
H3k9me2 and AGO2 association with the D4Z4 region increases with exogenous siRNA repression
A time course showed that the siRNAs directed to the DUX4 mRNA region decreased DUX4 mRNA abundance as early as 12–24 h after transfection, whereas the siRNA directed to the promoter region upstream of the DUX4 transcript began to suppress DUX4 mRNA abundance later with efficient knockdown observed at 3 and 4 days post-transfection (Fig. 2A and data not shown), and the decrease in a DUX4 target gene, RFPL2, also showed that the siRNAs targeting the coding region inhibited DUX4 activity more rapidly than the siRNAs targeting the upstream region (Fig. 2B). The delayed kinetics of DUX4 mRNA suppression by the upstream siRNA suggested that they might be suppressing the transcription of the DUX4 mRNA, whereas the coding region siRNA might be directly degrading the mRNA.
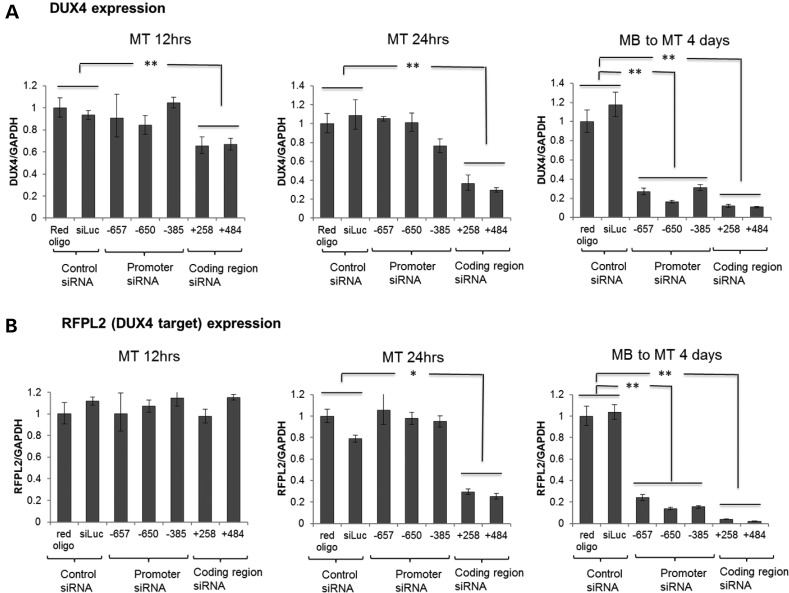
Figure 2.
Delayed kinetics of promoter targeting siRNA compared with coding region siRNA. qRT–PCR analysis of DUX4 mRNA (A) and DUX4-target gene, RFPL2 (B) expression in FSHD2 muscle cells at different time points following transient transfection with DUX4 promoter siRNAs or coding region siRNAs targeting the corresponding D4Z4 sequences numbered relative to the DUX4 TSS (see Supplementary Material, Table S1). siRNAs were transfected into cells and total RNA samples were prepared at 12 h (MT 12 h), 24 h (MT 24 h) and 4 days (MB to MT 4 days) post-transfection. For each time point, FSHD myoblasts (MB) were induced to differentiation into myotubes (MT) for 48 h prior RNA isolation. Relative DUX4 and RFPL2 expression normalized to GAPDH are presented as mean ± SEM based on triplicate PCR reactions for each sample. P-values were calculated by comparing DUX4 or RFPL2 expression levels in cells transfected with the three promoter or the two coding region siRNAs to the corresponding expression levels in the two controls by using a Student's t-test. All relative expressions with a P < 0.05 (*) or <0.01 (**) are indicated. Coding region siRNAs showed DUX4 and RFPL2 (DUX4-target) decreased at early time points, but promoter region siRNAs showed decrease only at the late time point (4 days post-transfection).
siRNA-mediated transcriptional gene silencing in model organisms and mammalian cells is associated with Argonaute (AGO) recruitment and increased H3K9 di-methylation (18–20). Therefore, we used ChIP to examine H3K9me and AGO binding in the upstream region and near the DUX4 transcription start site following siRNA transfection. While in both control and FSHD2 cells, H3K9me2 increased at both the upstream region and near the TSS during differentiation of myoblasts to myotubes, H3K9 methylation levels were slightly lower in the FSHD2 cells, consistent with the chromatin de-repression of the D4Z4 locus in FSHD (21) (Supplementary Material, Fig. S3). The siRNA directed to −650 region upstream of the DUX4 TSS increased H3K9me2 in FSHD2 myotubes at 12 and 24 h following transfection and in FSHD2 myoblasts at 24 h post-transfection (Fig. 3B), which was prior to the decrease in DUX4 mRNA (see Fig. 2A). The siRNA targeting the +258 region encompassing the mRNA transcript did not increase H3K9me2 at the upstream region in myoblasts or myotubes, and had a modest variable effect near the TSS.
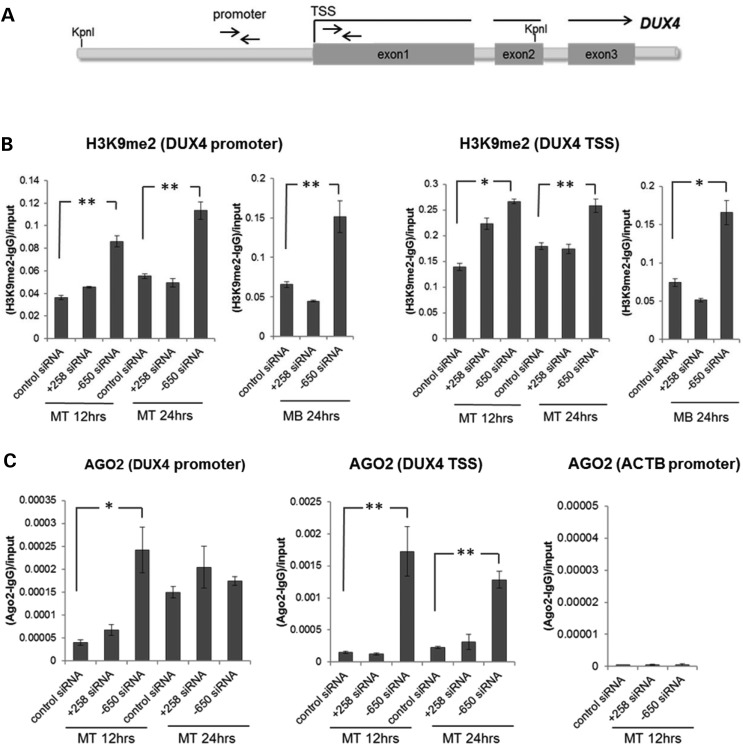
Figure 3.
Exogenous siRNA targeting the DUX4 promoter region increase D4Z4 H3K9me2 and AGO2 enrichment. (A) Schematic representation of the D4Z4 repeat with the location of promoter and TSS PCR primers indicated by arrows. (B and C) Enrichment of H3K9me2 (B) and AGO2 (C) at the promoter region and near the transcription start site (TSS) of the DUX4 gene was analyzed by chromatin immunoprecipitation (ChIP) followed by qPCR. As a negative control for AGO2 enrichment, the beta-actin (ACTB) promoter region was analyzed. Control siRNA and siRNAs targeting coding (+258) and non-coding (−650) D4Z4 sequences were transfected into FSHD2 myoblasts (MB) and myotubes (MT) and chromatin samples were prepared at 12 h and 24 h post-transfection time points. Relative amounts of precipitated DNA were calculated as ratios of the real-time PCR signals obtained for IP with the H3K9me2 or AGO2 antisera minus those obtained for mock IP with IgG to input signals. Data are presented as mean ± SEM based on triplicate PCR reactions for each sample. ChIP enrichments with a P < 0.05 (*) or <0.01 (**) based on a t-test comparing the enrichment in the cells transfected with the DUX4 promoter or coding region siRNA to the corresponding enrichment in the cells transfected with the control siRNA (see Materials and Methods) are indicated. The promoter siRNA targeting the region D4Z4 upstream of DUX4 (termed promoter region) showed increased H3K9me2 and AGO2 binding at the promoter region and near the TSS region of the DUX4 gene (see also Supplementary Material, Fig. S3).
The increased H3K9me2 following transfection with the −650 siRNA correlated with increased AGO2 binding at these regions as determined by AGO2 ChIP (Fig. 3C). In addition, AGO2 association at the D4Z4 repeat was higher in all samples relative to a control region of the genome (ACTB promoter) and also increased at the upstream region in control siRNA between 12 and 24 h of differentiation, correlating with the increased H3K9me2 at these regions with differentiation (see Supplementary Material, Fig. S3), demonstrating that the exogenous siRNA recruit AGO2 to epigenetically silence the D4Z4 and suggesting that the endogenous siRNAs might have a similar role in the normal silencing of this region. ChIPs with antisera to AGO1 did not show enrichment at the D4Z4 repeat (data not shown).
Together, these results showed a correlation between AGO2 binding at the D4Z4 repeat and epigenetic repression of DUX4 mRNA production by exogenous promoter siRNAs, and suggested that the RNAi silencing pathway might normally function to maintain epigenetic silencing of DUX4 in somatic tissues.
The endogenous RNAi pathway is necessary to maintain epigenetic silencing of DUX4
To determine whether the RNAi pathway is necessary to maintain the epigenetic silencing of DUX4 in somatic cells, we knocked down DICER, AGO1 and AGO2 in control myoblast cell lines from two different unaffected individuals with 13 unit D4Z4 arrays on a chromosome 4qA161 haplotype that contained the polymorphisms encoding the DUX4 polyadenylation site (5). FSHD1 is associated with fewer than 11 D4Z4 repeat units and 13 units are at the lower range of the non-FSHD1 population. Knockdown of DICER or AGO2 using siRNAs resulted in the induction of DUX4 mRNA expression in the muscle cells from these two non-FSHD individuals (Fig. 4A and Supplementary Material, Fig. S4). DUX4 de-repression in these cells was associated with the secondary induction of DUX4 target genes and a decrease in the endogenous AGO2 associated with the DUX4 promoter region (Fig. 4B and C, results in control line 1 are shown). In contrast, we did not see DUX4 re-activation in a control muscle cell line with a much larger (∼74 units) D4Z4 repeat (Fig. 4A and Supplementary Material, Fig. S4), suggesting that the smaller repeat sizes might be more susceptible to DUX4 de-repression following AGO or DICER knockdown.
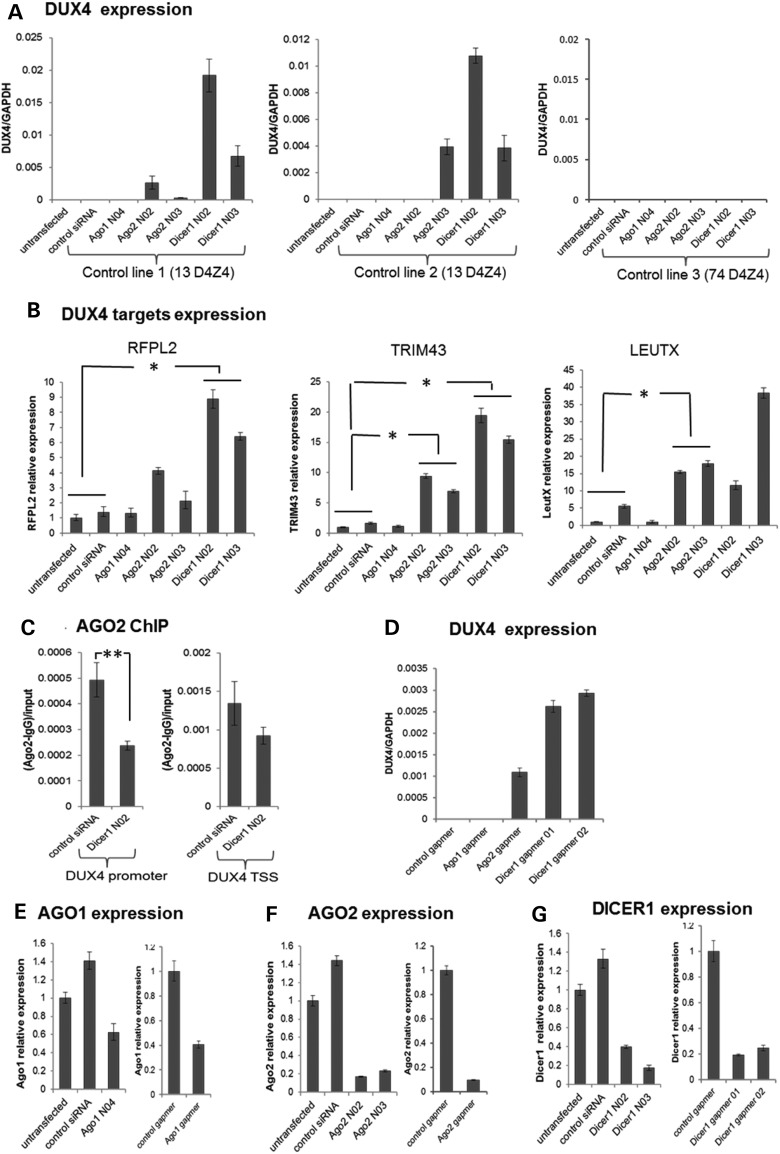
Figure 4.
AGO2 or DICER1 knockdown in control cells de-repress DUX4 expression. (A) qRT–PCR analysis of DUX4 mRNA in three control muscle cell lines following siRNA-mediated depletion of AGO1, AGO2 and DICER1. (B) qRT–PCR analysis of DUX4-target gene transcripts (RFPL2, TRIM43, LEUTX) in Control Line 1 following siRNA-mediated depletion of AGO1, AGO2 and DICER1. Myoblasts were transfected twice with the validated siRNAs targeting AGO1 (AGO1 N04), AGO2 (AGO2 N02 and N03) or DICER1 (DICER1 N02 and N03) mRNA using a double-transfection protocol with the first transfection at −96 h followed by the second transfection and induction of differentiation at −48 h prior to RNA isolation (MB to MT 4 days time point). qRT–PCR data were analyzed using standard curve method for DUX4 expression and ddCt for DUX4 target gene expression, normalized to GAPDH levels and presented as mean ± SEM based on triplicate PCR reactions for each sample. (C) ChIP-qPCR analysis of AGO2 enrichment at the DUX4 promoter and near the TSS region in control myotubes [Control line 1 in (A)] following transfection with control siRNA or DICER1 N02 siRNA. (D) qRT–PCR analysis of DUX4 mRNA in control muscle cell line 1 following depletion of AGO1, AGO2 and DICER1 using MOE-gapmers. Myoblasts were transfected twice with the gapmers using a double-transfection protocol with the first transfection at −96 h followed by the second transfection and induction of differentiation at −48 h prior to RNA isolation. qRT–PCR data were analyzed using standard curve method, normalized to GAPDH levels and presented as mean ± SEM. (E–G) AGO1, AGO2 and DICER1 mRNA levels in control line 1 following siRNA or MOE-gapmer knockdowns were analyzed by qRT–PCR by ddCt method, normalized to GAPDH and presented as mean ± SEM (see also Supplementary Material, Fig. S4). Data plotted in all panels represent the mean ± SEM based on triplicate PCR reactions. *P < 0.05; **P < 0.01. (A) and (D) lacked measurable signal in the control samples, so P-values were not calculated, see Supplementary Material, Figure S4 for Ct values from (A). (E–G) show the degree of target knockdown for the control line 1 experiment presented and P-values were not calculated. See Supplementary Material, Figure S4B for degree of AGO1, AGO2 and DICER knockdown in the control lines 2 and 3 experiments presented.
As an alternative method to determine whether DICER and AGO2 were necessary to maintain repression of DUX4 expression, we used single stranded 2′-O-methoxyethyl antisense oligonucleotides (MOE-gapmers) designed to degrade the DICER or AGO target RNA through an RNase H mechanism. Similar to the siRNA, MOE-gapmers that knockdown DICER or AGO2 also resulted in the induction of DUX4 mRNA expression in the control muscle cells with shorter D4Z4 repeat arrays (Fig. 4D, data shown for control line 1). Knockdown of AGO2 by either MOE-gapmer or siRNA also prevented the upstream exogenous siRNAs from suppressing DUX4 expression in FSHD cells, confirming that DUX4 silencing by the upstream exogenous siRNAs was AGO2-dependent (Supplementary Material, Fig. S5). AGO1 knockdown using either siRNA or MOE-gapmer chemistries did not de-repress DUX4 expression in any of the cell lines, but the knockdown was less efficient than the AGO2 knockdown and we cannot conclude that this is an AGO2-specific pathway (Fig. 4E–G).
Endogenous chromatin- and AGO-associated D4Z4 siRNA suppress DUX4 expression
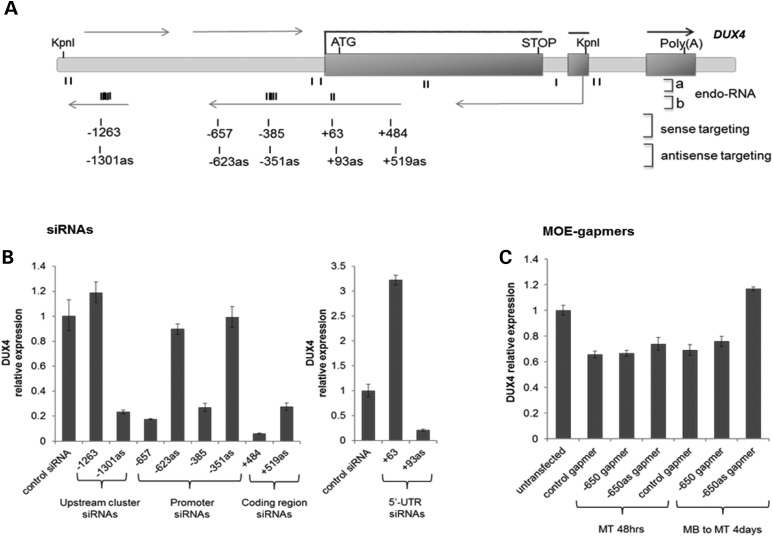
Our original identification of endogenous small si/mi-like RNAs mapping to the D4Z4 repeat used northern analysis and early studies of small-RNA sequencing in tumor cells (6). Two recent publications have performed genome-wide studies of AGO-mediated transcriptional gene silencing in human cells that included small RNA sequencing of chromatin-associated RNA. Consistent with our ChIP data (see Figs. 3C and 4C), Benhamed et al. (20) identified the DUX4 promoter as one of the AGO-associated promoters in their Supplementary Material, Table S1 and their small RNA-seq included a 23 nucleotide RNA that was a perfect match to the D4Z4 repeat at position +62 relative to our benchmark TSS (see Supplementary Material, Table S2), although this siRNA was not directly shown to be AGO-associated. Amayer-Azouza et al. (22) identified an AGO-associated small RNA that matched the D4Z4 sequence at position +59, as well as multiple additional chromatin-associated small RNAs not directly shown to be AGO-associated that were clustered near the KpnI site in the D4Z4 repeat and near the DUX4 promoter up to ∼ 500 nucleotides upstream of the DUX4 TSS (Fig. 5A and Supplementary Material, Table S2). All of these small RNA sequences were in the sense orientation relative to the DUX4 ORF.
Figure 5.
Strand-specific suppression of DUX4 by siRNA but not MOE-gapmers in FSHD cells. (A) Schematic diagram depicting the D4Z4 region and the structure of the DUX4 gene. endo-RNA: (a) The location of previously identified D4Z4-derived small endogenous RNAs (6) and (b) the location of the newly identified endogenous chromatin-associated D4Z4 small RNAs from publicly available small RNA sequencing data sets (20,22) indicated as clusters of short bold vertical marks (see Supplementary Material, Table S2 for sequences). Rows of numbered vertical marks show the location of additional siRNAs tested corresponding to the clustered regions of chromatin-associated small RNAs. Numbering is from the 5-prime end of the targeted sequence relative to the DUX4 TSS. siRNA designed to target the transcript in the antisense orientation relative to the DUX4 mRNA are annotated ‘as' (i.e. siRNA −657 targets the sense sequence starting at −657 relative to the TSS, whereas siRNA −623as targets the antisense sequence with the 5-prime end beginning at −623 and providing the complementary overlap to the −657 siRNA). (B) DUX4 siRNAs were transfected into FSHD2 myoblasts and induced to differentiation 2 days after transfection. Total RNA was prepared at 4 days post-transfection time point. Relative expression of DUX4 mRNA was analyzed by qRT–PCR, normalized to GAPDH, and presented as mean ± SEM based on triplicate PCR reactions. (C) qRT–PCR analysis of DUX4 mRNA expression in FSHD2 muscle cells following transfection with MOE-gapmers targeting D4Z4 sequences upstream of the DUX4 TSS. Gapmer numbering corresponds to the position of their target sequences relative to the DUX4 transcription start site (TSS = 1). Gapmers targeting antisense sequence are noted as ‘as’. Cells were either transfected once with the gapmers and induced to differentiation −48 h prior RNA isolation (MT 48 h time point) or twice (double-transfection protocol) with the first transfection at −96 h followed by the second transfection and induction of differentiation at −48 h prior to RNA isolation (MB to MT 4 days time point). DUX4 mRNA expression data were normalized to GAPDH expression and presented as mean ± SEM (see also Supplementary Material, Fig. S5). Data plotted in all panels represent the mean ± SEM based on triplicate PCR reactions. See Supplementary Material, Figure S6 for replication and additional controls.
Transfection of FSHD2 cells with siRNA mimics of representative members of the cluster near KpnI site (−1301as) and the AGO-associated siRNA (+93as) near the TSS (i.e. siRNA designed to target the antisense sequence beginning at +93, which is the putative target region for the small sense-strand RNAs identified with a 5-prime end at +63) (see Supplementary Material, Table S1 for sequences) efficiently reduced DUX4 mRNA abundance (Fig. 5B). Both the −1301as and the +93as were designed to target the antisense sequence relative to DUX4, i.e. mimicking the sense small RNA identified by sequencing. Similarly positioned siRNA designed to target the sense sequence (−1263 and +63) did not suppress DUX4 expression, and the +63 siRNA increased DUX4 mRNA abundance (see also Supplementary Material, Fig. S6), whereas siRNA in the region between −1263 and the TSS showed the opposite strand activity with siRNA targeting the sense sequence suppressing DUX4 (−657 and −385) but not siRNA targeting the antisense strand (−623as and −351as) (Fig. 5B). Although based on a small number of siRNAs, these results suggest that sense or antisense non-coding D4Z4 RNA transcripts, which have been previously identified in these regions (6,23), might be targeted by the siRNA. It is unlikely, however, that simple degradation of these transcripts account for the biological activity of the siRNA because single-stranded antisense oligonucleotides using 2′-O-methoxyethyl chemistry (MOE-gapmers) designed to target these non-coding upstream RNA sequences and degrade them through an RNase H mechanism failed to suppress DUX4 expression, whether designed to degrade the sense or antisense transcript (Fig. 5C and Supplementary Material, Fig. S6).
Discussion
Together, these data indicate that endogenous small RNAs derived from the D4Z4 macrosatellite repeats normally function to epigenetically suppress DUX4 expression in muscle cells, and likely other somatic tissues, and that exogenous siRNA to the D4Z4 region can enhance the epigenetic repression of DUX4 expression in FSHD muscle cells. Previously, we demonstrated that DUX4 mRNA can be efficiently knocked-down using siRNA that target the coding strand of the mRNA (17). The current study shows that oligonucleotides targeting D4Z4 regions upstream of the DUX4 mRNA TSS, some more than 1 kb upstream of the DUX4 TSS, also efficiently suppress DUX4 expression. Several lines of evidence indicate that these exogenous upstream siRNA suppress DUX4 through epigenetic transcriptional gene silencing dependent on the DICER/AGO pathway. First, the kinetics of silencing by the upstream siRNA is delayed relative to siRNA targeting the coding region. Secondly, silencing is correlated with increased AGO2-association and H3K9me2 levels in the targeted upstream region. Thirdly, MOE-gapmers to the same regions do not suppress DUX4 expression, indicating that simply targeting non-coding RNA transcripts in these upstream regions for degradation is not sufficient to suppress DUX4 expression. Fourth, AGO2 knockdown using either siRNA or MOE-gapmer chemistries prevent the suppression of DUX4 by the upstream siRNA. These results show that the AGO pathway is necessary for the exogenous promoter-targeted siRNA to suppress DUX4 expression through a mechanism that is independent of RNA degradation and is associated with regional increase in repressive chromatin marks, consistent with prior studies of transcriptional gene silencing by si- and mi-RNAs.
Many of the exogenous siRNAs used in this study either closely mimic or are in the same region as endogenous siRNA previously identified (6) or identified in this study by the analysis of publically available small RNA sequencing data sets (20,22) (see Supplementary Material, Table S2). A role for these endogenous siRNA in epigenetic repression of the D4Z4 macrosatellite array is strongly supported by the induction of DUX4 expression in control cells following knockdown of either DICER or AGO2, using either siRNA or MOE-gapmer chemistries. DICER or AGO knockdown de-repressed the expression of DUX4 mRNA in myoblast cell cultures from two different individuals with 13 repeats of the D4Z4 unit on a 4qA FSHD-permissive allele, only slightly above the 10 repeat cut-off that is the genetic criteria for FSHD1, whereas DICER or AGO knockdown did not de-repress DUX4 in a muscle culture with ∼74 4qA repeats. This suggests that even within the normal range, the lower number of repeats do not establish the same degree of epigenetic repression. This is also consistent with our recent demonstration that the degree of D4Z4 methylation on chromosomes 4 and 10 correlates with the total number of D4Z4 units on both chromosomes (24), suggesting that there might be both a large effect in cis, and a smaller effect in trans for repeat-mediated epigenetic repression. Further research will be necessary to determine the relative contributions of the different mechanisms that repress the D4Z4 regions.
The AGO2 ChIP showed enhanced enrichment of AGO2 following transfection of siRNA to the region upstream of DUX4, but also showed baseline enrichment of AGO2 at the D4Z4 relative to a control locus in control cells, findings consistent with a normal role of AGO2 in silencing the D4Z4 region that can be enhanced by additional exogenous siRNA. A normal role for AGO2 in the repression of DUX4 is further supported by the demonstration of de-repression of DUX4 expression following either DICER or AGO2 knockdown, concomitant with a decreased association of AGO2 with the D4Z4 after DICER knockdown, as demonstrated by ChIP (see Fig. 3A–D). The conclusion that the endogenous siRNA actively suppresses the D4Z4 repeats through a DICER/AGO-dependent pathway is further supported by a ChIP-on-chip demonstration that an anti-pan-AGO antibody immunoprecipitated the DUX4 promoter region as one of the ∼4500 potential AGO-promoter binding sites in senescent WI38 primary human fibroblasts (20). In addition, an RNA sequencing of chromatin-associated complexes in HeLa cells (22) identified an AGO-associated RNA that mapped to D4Z4 position +63 relative to the DUX4 TSS with a single mismatch to the sequence of the last 4qA repeat, as well as additional clusters of chromatin-associated small RNAs with perfect or high matches to the D4Z4 repeat outside of the DUX4 transcript (see Supplementary Material, Table S2). These findings from other studies provide additional support for chromatin-associated D4Z4-generated small RNAs and the AGO proteins participating in the regulation of DUX4 and possibly other non-coding D4Z4 RNAs. Together with the current study, these data indicate that the D4Z4 repeats are epigenetically repressed through a DICER/AGO-dependent siRNA pathway in a length-dependent manner, and that exogenously supplied siRNA to the D4Z4 can enhance the repression of the inefficiently silenced D4Z4 repeats in FSHD by increasing the amount of D4Z4 siRNA.
Similar to our previous study (6), Cabianca et al. (25) identified long non-coding RNA transcripts extending through the D4Z4, but, in contrast to our previous study, they identified the transcription start site in a region centromeric to the D4Z4 repeats rather than within the repeats, naming this long non-coding RNA DBE-T. Because shRNA directed to the DBE-T in regions similar to our promoter-directed siRNA suppressed expression of DUX4 in FSHD cells, they concluded that the DBE-T RNA was necessary for de-repression of DUX4 expression in FSHD cells. Our study suggests that suppression of DUX4 expression by siRNA directed to the D4Z4 transcripts upstream of DUX4 might also be due to an siRNA-directed epigenetic repression of DUX4 expression, particularly since MOE-gapmers to the same region did not suppress DUX4 expression in our study.
siRNA-directed RNAi-mediated epigenetic silencing of transcription has been well characterized in model organisms, and many recent studies have indicated that a similar process can occur in vertebrate and mammalian cells (13,14). In each organism, it is generally thought that promoter or non-coding RNAs in the region serve as a target for the si- or miRNA loaded in the AGO complex. Earlier studies showed that synthetic siRNA directed to non-coding RNA at the promoter or upstream regions of a gene recruited the AGO complex and silenced gene expression in an AGO-dependent manner (18,26), and more recent studies have confirmed the nuclear location and activity of AGO, DICER and siRNA complexes in mammalian nuclei (27–29). Although the biological relevance of si- or miRNA-guided transcriptional signaling in vertebrate cells remains incompletely understood, studies support a role for this pathway in establishing epigenetic repression. For example, the 16 kb constitutive heterochromatin domain near the chicken beta-globin locus was shown to be bound by AGO2 in a DICER-dependent manner and knockdown of DICER resulted in increased histone acetylation, decompaction of the heterochromatin and increased transcript levels (15). In human cancers, decreased expression of endogenous siRNA corresponding to LINE-1 elements correlated with aberrant LINE-1 expression and over-expression of these siRNAs re-established LINE-1 epigenetic repression in the cancer cells (16). And, more recently, AGO-associated small RNAs have been implicated in the epigenetic repression of the DXZ4 macrosatellite repeat (30).
The RNAi pathways are being exploited for potential therapeutic approaches by delivering synthetic siRNA or single-stranded RNA that achieve transcriptional gene silencing by targeting promoter-associated non-coding RNAs (31,32). Our current study identifies a critical regulatory role of siRNA-directed AGO/DICER-dependent epigenetic repression in the repeat-dependent silencing of the human D4Z4 region and the DUX4 retrogene and implicates this pathway as a potential therapeutic target for FSHD. DUX4 mRNA is normally expressed in the testis, most likely in the spermatogonia and primary spermatocytes, and epigenetically repressed in somatic tissue. The number of repeats directly correlates with the degree of epigenetic repression in somatic tissues, with fewer than 11 repeats resulting in low levels of DUX4 expression in skeletal muscle. The low-level variegated expression of DUX4 in the nuclei of FSHD muscle is similar to the variegated repression of the white gene in Drosophila ommatidia with increased copy number (33), one of the first demonstrations that repression of directly repeated genes occurred through epigenetic mechanisms. Our current study demonstrates the role of the endogenous RNAi pathway in repeat-mediated epigenetic repression of D4Z4 and DUX4 and shows that enhancing the activity of this pathway by supplying additional exogenous small RNAs represents a potential therapeutic approach to silencing DUX4 in FSHD.
Materials and Methods
Cell cultures
The Fred Hutchinson Cancer Research Center approved the use of de-identified human cells for this study. Primary myoblasts from both unaffected individuals and FSHD patients were obtained through the Fields Center at the University of Rochester (http://www.urmc.rochester.edu/fields%2Dcenter/) and derived from a needle biopsy of the vastus lateralis (http://www.urmc.rochester.edu/fields-center/protocols/needle-muscle-biopsy.cfm) and established as primary cultures through dispase and collagenase dispersion (http://www.urmc.rochester.edu/fields-center/protocols/myoblast-cell-cultures.cfm). Primary myoblast cell lines were routinely maintained in F-10 Medium (Invitrogen) supplemented with 20% fetal bovine serum (HyClone, UT, USA), 1% Pen/Strep, 10 ng/ml hrFGF (Promega) and 1 µm Dexamethasone (Sigma) (Growth media). Primary myoblasts were induced to differentiation with DMEM, 1% heat-inactivated horse serum, 10 µg/ml insulin (Sigma) and 10 µg/ml transferrin (Sigma) (Differentiation media) for 48 h. Cells were maintained at 37°C in a humidified atmosphere of 5% CO2.
Design and transfection of siRNAs and gapmers
Duplex siRNAs targeting both coding and non-coding regions of the DUX4 gene were obtained from Dharmacon (21 bp synthetic dsRNA) and Life Technologies (Stealth siRNA 25 bp synthetic dsRNA). The siRNA design favors guide-strand loading in the RISC complex and the Stealth siRNA includes modifications on the passenger strand ensuring that the guide strand is preferentially loaded in the RISC complex. DUX4 siRNAs targeting either sense or antisense strand of upstream and DUX4 coding region are indicated in Supplementary Material, Table S1. siRNAs were named according to their target sequence location relative to the most promoter-proximal-mapped transcription start site of DUX4 (6). siRNA transfections into myoblasts and myotubes were carried out using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. Briefly, cells were plated at ∼50% confluence in six-well plates in 2 ml of growth media without antibiotics. Twenty-four hours after plating, cells were transfected with 5 μl Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) and 100 pmol of either a gene-specific siRNA or a non-silencing control siRNA, including Stealth RNAi Negative control Medium GC siRNA (Invitrogen, Carlsbad, CA, USA) or siRNA targeting luciferase gene (siLuc), diluted in 250 μl of Opti-MEM® Reduced Serum Medium. BLOCK-iT red or green fluorescent oligos (Invitrogen, Carlsbad, CA, USA) were used as a transfection efficiency control (Supplementary Material, Fig. S2A). Only experiments with more than 90% transfection efficiency were analyzed further. In all experiments, DUX4 mRNA levels were measured at 48 h after induction to differentiation (Supplementary Material, Fig. S2B). For DUX4 siRNAs, a single transfection protocol was used: at 48 h following DUX4 siRNA transfection, cells were treated with differentiation media and harvested for RNA or protein analysis 48 h after induction to differentiation (96 h total unless otherwise specified).
Single-stranded 2′-O-methoxyethyl antisense oligonucleotides (MOE-gapmers) were designed to target both sense and antisense transcripts at the D4Z4 regions upstream of the DUX4 gene and synthesized by Isis Pharmaceuticals, Inc. (Supplementary Material, Table S1). Gapmer transfections were carried out using Lipofectamine RNAiMAX as described for siRNA transfections. Double-transfection protocol was used to ensure prolonged knockdown by gapmers: at 48 h after the first gapmer transfection, cells were transfected second time and treated with differentiation media followed by RNA and protein analysis at 48 h after the second transfection and induction to differentiation (96 h total unless otherwise specified).
For siRNA-mediated down-regulation of Argonaute 1, Argonaute 2 and Dicer1, Stealth™ siRNA duplexes (Invitrogen, Carlsbad, CA, USA) and single-stranded 2′-O-methoxyethyl antisense oligonucleotides (MOE-gapmers) (Isis Pharmaceuticals) were designed and transfected into primary myoblasts. To control for off-target effects, at least two independent siRNAs targeting different sequences of a gene were used to down regulate each gene. To ensure prolonged knockdown and efficient depletion of pre-existing proteins, cells were transfected with the siRNA gapmers twice: at 48 h after the first siRNA transfection, cells were transfected second time and treated with differentiation media followed by RNA and protein analysis at 48 h after the second transfection and induction to differentiation (96 h total). The efficiency of knockdown was assessed by real-time RT–PCR. Targeting sequences of all AGO1, AGO2 and DICER1 siRNAs and MOE-gapmers designed and used in the study are listed in Supplementary Material, Table S1.
RNA isolation
Total RNA from myoblasts from control and FSHD individuals was isolated using TRIZOL (Invitrogen) according to the manufacturer's instructions. The isolated RNA was treated with DNAase twice to remove contaminating DNA prior cDNA generation. First, isolated RNA was treated with RNase-free DNaseI (Roche) followed by acidic phenol/chloroform extraction, and then treated with TURBO RNase-free DNase (Ambion) followed by inactivation reagent treatment. The DNaseI-treated RNA was tested for genomic DNA contamination by PCR and RNA quality by RNA gel.
cDNA generation
cDNA was generated with Superscript II (Invitrogen) under the following conditions. One microliter of OligodT primer (Invitrogen) was added to 1 µg of RNA and incubated at 65°C for 5 min in a thermocycler. The RNA/primer mixture was chilled on ice. Next, the reaction mixture, nuclease free H2O, 5X buffer, RNase inhibitor, dNTPs, DTT and RT, was added to the RNA/primer mixture and incubated for 1 h at 45°C and for 10 min at 50°C. The RT was inactivated at 70°C for 15 min. Synthesis of cDNA was verified and normalized by amplification of the control transcripts GAPDH and TIMM17b (see Supplementary Material, Table S3 for primer sequences). no-RT reaction was used as a control for DNA contamination and primers were verified by amplification of cDNA or genomic DNA.
Semi-quantitative RT–PCR
For the semi-quantitative RT–PCR, the full length DUX4 mRNA levels were analyzed using PCRx Enhancer System (Invitrogen) and primers DUX4-1AF and DUX4-R to DUX4 exons 1 and 3 and primers DUX4-F and DUX4-R to exons 2 and 3 (Supplementary Material, Table S3). To determine the linear range for each primer set, aliquots of the RT–PCR reaction were collected every two cycles ranging from 20 to 42 cycles. The linear range for the DUX4 primers was established at 36–40 cycles. For the control transcripts, GAPDH and TIMM17b, used for normalization, the linear range was established at 22 to 28 cycles and at 30 to 36 cycles, respectively. The RT–PCR products were separated on 1% standard agarose gel. DUX4 RT–PCR products were cloned into the pCR®4-TOPO vector (Invitrogen) and verified by sequencing using an ABI Prism 3730xl DNA analyzer (Applied Biosystems). The semi-quantitative RT–PCR was repeated for each pair of primers at a minimum of two-three times.
Real-time RT–PCR
Quantification of mRNA levels in control and FSHD myoblasts and myotubes was carried out by real-time qRT–PCR on the automated ABI 7900 PCR machine (Applied Biosystems) using Fast Start SYBR Green Master Mix (ROCHE) with ROX passive reference dye added. cDNA was generated with OligodT primers using 1 µg RNA, then diluted 1:1. One microliter of cDNA was used as template for the real-time reaction. DUX4 mRNA levels were analyzed by qRT–PCR using primers DUX4-F and DUX4-R to DUX4 exons 2 and 3 (see Supplementary Material, Fig. S1 and Supplementary Material, Table S3). PCR cycling was performed at [94°/2 min, (94°/30 s, 60°/30 s, 72°/30 s) ×40] with an additional ramping step added after cycling to calculate dissociation curves and confirm that fluorescence detected was due to full size PCR product and not PCR artifacts. The standard curve assay as described by Applied Biosystems was used for absolute quantification or delta-delta-Ct method was used for relative expression to reference gene. The values calculated for transcripts levels were normalized to those of GAPDH in the same samples. Approximate threshold cycle (Ct) values in FSHD2 myotubes were as follows: DUX4 27–28, and GAPDH 17. Primer sequences used for qRT–PCR analyses are listed in Supplementary Material, Table S3. Expression data from a single representative transfection were presented for each experiment as mean ± standard error of the mean for real-time PCR triplicates. P-values were calculated by comparing relative expression values of a target gene in cells transfected with a gene-specific siRNA or gapmer to the corresponding relative expression in cells transfected with the control siRNA or gapmer by using one-tailed unpaired equal variance Student's t-test. All relative expressions with a P < 0.05 (*) or <0.01 (**) based on a t-test were indicated.
Chromatin immunoprecipitation
The ChIP analyses of control and FSHD myoblasts before and after differentiation were performed as previously described (34). Briefly, cells were cross-linked with formaldehyde at 1.42% final concentration for 15 min at room temperature, quenched and sonicated (Bioruptor UCD-200) to generate 500–200 bp DNA fragments. To ensure reproducibility of the results, DNA was isolated and measured for each chromatin sample, and aliquots of chromatin corresponding to 400 ng (for histone ChIP) or 800 ng (for AGO2 ChIP) of DNA were used for each immunoprecipitation with 1 µl of anti-H3K9me2 (Abcam), -H3K9me3 (Abcam), or -AGO2 (Abcam) antisera. Non-immune IgG fraction was used as a mock control. After reverse cross-linking and DNA purification, the IP products were analyzed by real-time PCR. Primers sequences used for ChIP assay are listed in Supplementary Material, Table S3. Relative amounts of precipitated DNA were calculated as ratios of the real-time PCR signals obtained for IP with the corresponding antibody minus those obtained for mock IP with IgG to input signals [(IP-IgG)/Input]. Representative experiments with the data presented as mean ± SEM for the qPCR triplicates were shown. P-values were calculated by comparing relative enrichments [(IP-IgG)/Input] for each antibody obtained in cells transfected with the DUX4 siRNA or Dicer siRNA to the corresponding relative enrichments obtained for the cells transfected with the control siRNA by using a one-tailed unpaired equal variance Student's t-test. All relative enrichments with a P < 0.05 (*) or <0.01 (**) based on a t-test were indicated.
Immunofluorescence
Cells were plated on eight-chamber slides, grown to 70–80% confluence, and transfected as previously described earlier for DUX4 siRNA experiments. Instead of standard differentiation media, a knockout serum replacer (KSR) media DMEM/F12 (1:1) supplemented with 20% KSR (Life Technologies), 100 µm non-essential amino acids (10 mm/100X stock, Life Technologies) and 1 mm sodium pyruvate (100 mm/100X stock, Life Technologies) (see Supplementary Material, Fig. S2) was used to enhance fusion as described in (35). Cells were fixed for 10 min in 3.7% formaldehyde in PBS at 4 days post-siRNA transfection with the last 2 days in differentiation KSR media. The slides were rinsed with PBS, and the cells were permeabilized for 10 min with 0.5% Triton X-100 in PBS. Non-specific sites were blocked for 1 h using 10% normal goat serum in PBS. The cells were incubated for 2 h with rabbit monoclonal anti-DUX4 antibody (E5.5, ABCAM) and MF20 anti-Myosin Heavy Chain (Developmental Studies Hybridoma Bank, University of Iowa) diluted 1:10 000 and 1:500, respectively, in 5% NGS. Secondary antibodies (Molecular Probe) were conjugated with FITC diluted 1:500 in PBS. Coverslips were mounted with antifade medium containing DAPI (Vector Laboratories) to stain the cell nuclei. Positive staining of cultured cells was visualized on a fluorescent microscope, equipped with a digital camera. Multiple fields were scored for DUX4 positive and negative nuclei.
Supplementary Material
Supplementary Material is available at HMG online.
Conflict of Interest statement. F.R. and C.F.B. are paid employees of Isis Pharmaceuticals; S.J.T. owns common stock in Isis Pharmaceuticals.
Funding
This work was supported by NINDS P01NS069539 and Friends of FSH Research. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Supplementary Material
References
- 1.Tawil R., van der Maarel S.M., Tapscott S.J. (2014) Facioscapulohumeral dystrophy: the path to consensus on pathophysiology. Skeletal Muscle, 4, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemmers R.J., Tawil R., Petek L.M., Balog J., Block G.J., Santen G.W., Amell A.M., van der Vliet P.J., Almomani R., Straasheijm K.R. et al. (2012) Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulohumeral muscular dystrophy type 2. Nat. Genet., 44, 1370–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blewitt M.E., Gendrel A.V., Pang Z., Sparrow D.B., Whitelaw N., Craig J.M., Apedaile A., Hilton D.J., Dunwoodie S.L., Brockdorff N. et al. (2008) SmcHD1, containing a structural-maintenance-of-chromosomes hinge domain, has a critical role in X inactivation. Nat. Genet., 40, 663–669. [DOI] [PubMed] [Google Scholar]
- 4.Blewitt M.E., Vickaryous N.K., Hemley S.J., Ashe A., Bruxner T.J., Preis J.I., Arkell R., Whitelaw E. (2005) An N-ethyl-N-nitrosourea screen for genes involved in variegation in the mouse. Proc. Natl Acad. Sci. USA, 102, 7629–7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemmers R.J., van der Vliet P.J., Klooster R., Sacconi S., Camano P., Dauwerse J.G., Snider L., Straasheijm K.R., van Ommen G.J., Padberg G.W. et al. (2010) A unifying genetic model for facioscapulohumeral muscular dystrophy. Science, 329, 1650–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snider L., Asawachaicharn A., Tyler A.E., Geng L.N., Petek L.M., Maves L., Miller D.G., Lemmers R.J., Winokur S.T., Tawil R. et al. (2009) RNA transcripts, miRNA-sized fragments and proteins produced from D4Z4 units: new candidates for the pathophysiology of facioscapulohumeral dystrophy. Hum. Mol. Genet., 18, 2414–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holoch D., Moazed D. (2015) RNA-mediated epigenetic regulation of gene expression. Nat. Rev. Genet., 16, 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reyes-Turcu F.E., Grewal S.I. (2012) Different means, same end-heterochromatin formation by RNAi and RNAi-independent RNA processing factors in fission yeast. Curr. Opin Genet. Dev., 22, 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grishok A., Sinskey J.L., Sharp P.A. (2005) Transcriptional silencing of a transgene by RNAi in the soma of C. elegans. Genes Dev., 19, 683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson I.R., Jacobsen S.E. (2007) Epigenetic inheritance in plants. Nature, 447, 418–424. [DOI] [PubMed] [Google Scholar]
- 11.Mochizuki K., Gorovsky M.A. (2004) Small RNAs in genome rearrangement in Tetrahymena. Curr. Opin Genet. Dev., 14, 181–187. [DOI] [PubMed] [Google Scholar]
- 12.Kanno T., Bucher E., Daxinger L., Huettel B., Bohmdorfer G., Gregor W., Kreil D.P., Matzke M., Matzke A.J. (2008) A structural-maintenance-of-chromosomes hinge domain-containing protein is required for RNA-directed DNA methylation. Nat. Genet., 40, 670–675. [DOI] [PubMed] [Google Scholar]
- 13.Morris K.V. (2011) Modulation of gene-specific epigenetic states and transcription by non-coding RNAs. Clin. Epigenet., 2, 433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagnon K.T., Corey D.R. (2012) Argonaute and the nuclear RNAs: new pathways for RNA-mediated control of gene expression. Nucleic Acid Therapeutics, 22, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giles K.E., Ghirlando R., Felsenfeld G. (2010) Maintenance of a constitutive heterochromatin domain in vertebrates by a Dicer-dependent mechanism. Nat. Cell Biol., 12, 94.–; suppl. pp. 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L., Dahlstrom J.E., Lee S.H., Rangasamy D. (2012) Naturally occurring endo-siRNA silences LINE-1 retrotransposons in human cells through DNA methylation. Epigenetics, 7, 758–771. [DOI] [PubMed] [Google Scholar]
- 17.Geng L.N., Yao Z., Snider L., Fong A.P., Cech J.N., Young J.M., van der Maarel S.M., Ruzzo W.L., Gentleman R.C., Tawil R. et al. (2012) DUX4 activates germline genes, retroelements, and immune mediators: implications for facioscapulohumeral dystrophy. Dev. Cell, 22, 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janowski B.A., Huffman K.E., Schwartz J.C., Ram R., Nordsell R., Shames D.S., Minna J.D., Corey D.R. (2006) Involvement of AGO1 and AGO2 in mammalian transcriptional silencing. Nat. Struct. Mol. Biol., 13, 787–792. [DOI] [PubMed] [Google Scholar]
- 19.Kim D.H., Villeneuve L.M., Morris K.V., Rossi J.J. (2006) Argonaute-1 directs siRNA-mediated transcriptional gene silencing in human cells. Nat. Struct. Mol. Biol., 13, 793–797. [DOI] [PubMed] [Google Scholar]
- 20.Benhamed M., Herbig U., Ye T., Dejean A., Bischof O. (2012) Senescence is an endogenous trigger for microRNA-directed transcriptional gene silencing in human cells. Nat. Cell Biol., 14, 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng W., de Greef J.C., Chen Y.Y., Chien R., Kong X., Gregson H.C., Winokur S.T., Pyle A., Robertson K.D., Schmiesing J.A. et al. (2009) Specific loss of histone H3 lysine 9 trimethylation and HP1gamma/cohesin binding at D4Z4 repeats is associated with facioscapulohumeral dystrophy (FSHD). PLoS Genet., 5, e1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ameyar-Zazoua M., Rachez C., Souidi M., Robin P., Fritsch L., Young R., Morozova N., Fenouil R., Descostes N., Andrau J.C. et al. (2012) Argonaute proteins couple chromatin silencing to alternative splicing. Nat. Struct. Mol. Biol., 19, 998–1004. [DOI] [PubMed] [Google Scholar]
- 23.Block G.J., Petek L.M., Narayanan D., Amell A.M., Moore J.M., Rabaia N.A., Tyler A., van der Maarel S.M., Tawil R., Filippova G.N. et al. (2012) Asymmetric bidirectional transcription from the FSHD-causing D4Z4 array modulates DUX4 production. PloS one, 7, e35532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemmers R.J., Goeman J.J., van der Vliet P.J., van Nieuwenhuizen M.P., Balog J., Vos-Versteeg M., Camano P., Ramos Arroyo M.A., Jerico I., Rogers M.T. et al. (2014) Inter-individual differences in CpG methylation at D4Z4 correlate with clinical variability in FSHD1 and FSHD2. Hum. Mol. Genet., 24, 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabianca D.S., Casa V., Bodega B., Xynos A., Ginelli E., Tanaka Y., Gabellini D. (2012) A long ncRNA links copy number variation to a polycomb/trithorax epigenetic switch in FSHD muscular dystrophy. Cell, 149, 819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han J., Kim D., Morris K.V. (2007) Promoter-associated RNA is required for RNA-directed transcriptional gene silencing in human cells. Proc. Natl Acad. Sci. USA, 104, 12422–12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gagnon K.T., Li L., Chu Y., Janowski B.A., Corey D.R. (2014) RNAi factors are present and active in human cell nuclei. Cell Reports, 6, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schraivogel D., Meister G. (2014) Import routes and nuclear functions of Argonaute and other small RNA-silencing proteins. Trends Biochem. Sci., 39, 420–431. [DOI] [PubMed] [Google Scholar]
- 29.White E., Schlackow M., Kamieniarz-Gdula K., Proudfoot N.J., Gullerova M. (2014) Human nuclear Dicer restricts the deleterious accumulation of endogenous double-stranded RNA. Nat. Struct. Mol. Biol., 21, 552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pohlers M., Calabrese J.M., Magnuson T. (2014) Small RNA expression from the human macrosatellite DXZ4. G3, 4, 1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts T.C., Andaloussi S.E., Morris K.V., McClorey G., Wood M.J. (2012) Small RNA-Mediated Epigenetic Myostatin Silencing. Mol. Ther. Nucleic Acids, 1, e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsui M., Prakash T.P., Corey D.R. (2013) Transcriptional silencing by single-stranded RNAs targeting a noncoding RNA that overlaps a gene promoter. ACS Chem. Biol., 8, 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorer D.R., Henikoff S. (1994) Expansions of transgene repeats cause heterochromatin formation and gene silencing in Drosophila. Cell, 77, 993–1002. [DOI] [PubMed] [Google Scholar]
- 34.Nelson J.D., Denisenko O., Bomsztyk K. (2006) Protocol for the fast chromatin immunoprecipitation (ChIP) method. Nat. Protoc., 1, 179–185. [DOI] [PubMed] [Google Scholar]
- 35.Block G.J., Narayanan D., Amell A.M., Petek L.M., Davidson K.C., Bird T.D., Tawil R., Moon R.T., Miller D.G. (2013) Wnt/beta-catenin signaling suppresses DUX4 expression and prevents apoptosis of FSHD muscle cells. Hum. Mol. Genet., 22, 4661–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.