Abstract
Chlamydia pecorum (designated 22–58) was isolated in 2010 in HmLu-1 cells from the jejunum of a calf which died of necrotizing enterocolitis in Yamaguchi Prefecture, Japan. Immunohistochemical staining identified C. pecorum positive reactions in the jejunal villi. C. pecorum, designated 24–100, was isolated from the feces of a calf with diarrhea in another farm in Yamaguchi Prefecture in 2012. A significant increase in neutralizing antibody titers against C. pecorum was confirmed in paired sera. Nucleotide sequence identities of omp1 genes of the 2 isolates were 100%. The isolates were genetically and antigenically more closely related to C. pecorum Bo/Yokohama strain isolated from cattle with enteritis in Japan than to the other prototype strains, Bo/Maeda isolated from cattle with pneumonia and Ov/IPA isolated from sheep with polyarthritis. These results indicate that C. pecorum strains similar to 22–58 and 24–100 might be endemic in Yamaguchi Prefecture and cause enteric disease in cattle.
Keywords: cattle, Chlamydia pecorum, diarrhea
Chlamydiaceae are obligate intracellular bacteria that have a unique developmental cycle which includes morphological changes. They cause a wide range of diseases in animals and humans [20, 21]. The most important mode of transmission is thought to be fecal shedding by carrier animals. Chlamydiae may be shed in vaginal, ocular and nasal discharges, uterine fluid, placental tissue, urine and semen. Chlamydial infection can be acquired by direct contact between animals or by indirect transmission, such as the fecal-oral route or via inhalation of contaminated air [18]. Clarkson et al. [4] reported that Chlamydia was isolated from lamb’s feces on 26 farms in England and Wales, and the prevalence of infection varied from 5–50% on individual farms. Isolates were identified as enteric type rather than abortion type and thus were classified as Chlamydia pecorum rather than Chlamydia psittaci. In cattle, C. pecorum is known to cause encephalomyelitis, pneumonia and enteritis, but subclinical and persistent infections are more common [8]. DeGraves et al. [5] reported that low-level of C. psittaci and C. pecorum genital infections was detected in 53% of virgin heifers by quantitative PCR, suggesting predominantly extra-genital transmission of Chlamydia in cattle. Reinhold et al. [17] reported that natural infections with Chlamydia spp. in calves were associated with subclinical chronic effects on animal health. Recently, it was reported that asymptomatic endemic C. pecorum infections reduced growth rates in calves by up to 48% [16]. However, the pathogenesis of C. pecorum is still unclear. In Japan, there are only a few reports of C. pecorum strains isolated from affected cattle [6,7,8].
In 2010 and 2012, two C. pecorum strains were isolated from cattle with enteritis in Yamaguchi Prefecture and were genetically and antigenically characterized.
MATERIALS AND METHODS
Clinical symptoms in diseased calves: Case 1: In May 2010, a Holstein calf (female, 84 days old) showed anorexia, pyrexia and diarrhea in a depositary raising farm in Yamaguchi Prefecture. Despite treatment with antibiotics (kanamycin and tylosin), sulfamonomethoxine and antipyretic, the calf died 20 days after the onset of diarrhea. Case 2: In July 2012, a Japanese black calf (female, 246 days old) on another farm in Yamaguchi Prefecture repeated diarrhea despite treatment with antibiotics (kanamycin) and a probiotic product after the birth, and then, feces were collected during the acute phase for diagnosis. Paired serum samples were collected during the acute and convalescent phases.
Isolation of microorganisms: Tissue specimens obtained from major organs including cerebrum, jejunum, ileum, cecum, colon and ideal contents of the dead calf (case 1) and feces of the second calf (case 2) were minced and homogenized in serum-free Eagle’s minimum essential medium with kanamycin (MEM) (Nissui Pharmaceutical Co., Tokyo, Japan). After centrifugation of the 10% homogenate, the supernatant was filtered through a 450 nm membrane (Merck Millipore Ltd., Carrigtwohill, Ireland), and 0.15 ml of the supernatants were inoculated onto hamster lung (HmLu-1) cells, Mardin-Darby bovine kidney (MDBK) cells, bovine testicular (BT) cells, human rectal adenocarcinoma (HRT-18) cells and Vero cells in 24-well plates. After adsorption for 60 min at 37°C, the cells were washed with MEM, and then, 0.5 ml of MEM containing 0.1% bovine serum albumin (Bovogen Biologicals, Williams Avenue, Australia) was added to each well. The cells were incubated at 37°C, and cytopathic effects (CPE) were observed. After incubation for 10 days, cells were frozen and thawed once and then centrifuged. Subsequent passages were carried out at least twice in the same manner with 0.15 ml of the supernatant. Gimenez stain was used to identify CPE. DHL Agar, Columbia agar with 5% sheep blood and GAM agar with 5% yolk and 0.1% cysteine were used for bacterial isolation. The gene of Clostridium perfringens toxin isolated from small-intestinal contents was identified by PCR [2].
Identification of isolates by PCR, sequence and phylogenetic analysis of C. pecorum omp1 gene: C. pecorum-infected cells and the supernatants from organs described above were used for DNA extraction. Total DNA was extracted using DNeasy Blood & Tissue Kit (QIAGEN, Hiden, Germany). PCR was carried out with TaKaRa Ex Taq Hot Start Version (TaKaRa Bio Inc., Otsu, Japan). Primer pairs targeting fragments of genus-specific and species-specific Chlamydia omp1 genes were used for PCR as described by Kaltenböck et al. [13]. PCR products were electrophoresed on 2.0% agarose gel and visualized using ethidium bromide staining. PCR products were purified using MiniElute PCR Purification Kit (QIAGEN, Germantown, MD, U.S.A.) and directly sequenced using a BigDye Terminator Cycle Sequencing Kit v3.1 (Applied Biosystems, Austin, TX, U.S.A.). Phylogenetic analysis was performed using the MEGA 5 program. Sequence data were aligned using ClustalW method [23]. Genetic distances were calculated using the Tamura-Nei model [22]. Phylogenetic trees were constructed using neighbor-joining methods [19], and the reliability of the branch was evaluated by bootstrapping with 1,000 replicates.
Antigenic analysis of C. pecorum isolates: The antigenicity of isolates were compared by immunoblot analysis using rabbit antisera [6] to Bo/Yokohama [6], Bo/Maeda [7] and Ov/IPA [6] strains of C. pecorum. Antisera to Bo/Maeda and Ov/IPA strains were prepared as described by Fukushi and Hirai [6]. HmLu-1 cells were infected with C. pecorum 22–58, Bo/Yokohama, Bo/Maeda and Ov/IPA strains and C. psittaci Cal10 [9] strain and then incubated at 37°C in 5% CO2 until CPE was observed. The cells were recovered from dishes with 0.02% EDTA in phosphate-buffered saline (PBS). After centrifugation at 200 × g for 5 min at 4°C, the supernatant was removed, and the cells were resuspended in PBS. Cells were then mixed with an equal volume of 2 × concentrated sample buffer consisting of 6.25 mM Tris-HCl pH 6.8, 2% sodium dodecyl sulfate (SDS), 20% glycerol and 0.001% bromophenol blue. Samples were boiled for 3 min, placed on ice for 3 min and centrifuged at 13,000 × g for 3 min at room temperature. Cell lysates were electrophoresed using 12% SDS-PAGE and transferred to a PVDF membrane (Immobilon-P; Millipore, Billerica, MA, U.S.A.). After blocking with Tris-buffered saline (TBS) containing 3% gelatin (EIA Grade Reagent Gelatin; Bio-Rad, CA, U.S.A.) for 45 min at 37°C, the membrane was washed three times with TBS containing 0.05% Tween 20 (T-TBS). After incubation with diluted rabbit antisera for 45 min at 37°C, the membrane was washed 3 times with T-TBS. Then, the membrane was reacted with peroxidase-conjugated purified recombinant protein A/G (Thermo Fischer Scientific, Rockford, IL, U.S.A.) for 45 min at 37°C. After washing the membrane with T-TBS and TBS three times each, specific bands were visualized using 3,3′-diaminobenzidine tetrahydrochloride (DAB; Wako Pure Chemical Industries, Ltd., Osaka, Japan).
Rabbit antisera described above were used for the cross-neutralization test. Sera were serially diluted twofold in MEM in 96-well microplates. Each dilution was mixed with an equal volume of 200 TCID50/0.05 ml of C. pecorum strains and incubated at 37°C for 1 hr. Then, 0.1 ml (approximately 6 × 103 cells) of HmLu-1 cells in MEM containing 10% fetal bovine serum was added to each well. After incubation at 37°C for 7 days, antibody titers were expressed as the reciprocal of the highest dilution of serum which inhibited CPE completely.
Histological and immunohistochemical analyses: Tissue specimens obtained from major organs, intestines and central nervous system of the dead calf (case 1) were fixed in 10% neutral buffered formalin and embedded in paraffin wax. Paraffin-embedded tissues were cut at 2 µm and stained with hematoxylin-eosin.
The sections were also used for immunohistochemical detection of C. pecorum antigens. After deparaffinization and rehydration of the paraffin sections, endogenous peroxidase activity was blocked by incubation in 3% H2O2 for 10 min. Sections were incubated with 10% normal goat serum for 30 min and then with a rabbit anti-C. pecorum Bo/Yokohama strain antiserum [6] (1:2,000) for 30 min. Sections were rinsed three times in PBS containing 0.2% Tween 20 for 10 min and then incubated with Histofine Simple Stain MAX-PO (R) (Nichirei Biosciences, Inc., Tokyo, Japan) for 30 min. The sections were rinsed three times in PBS containing 0.2% Tween 20 for 10 min. The sections were incubated with 3,3′-diaminobenzidine tetrahydrochloride under the microscope to control color development, washed in tap water for 10 min and rinsed with distilled water. The sections were counterstained with Meyer’s hematoxylin.
RESULTS
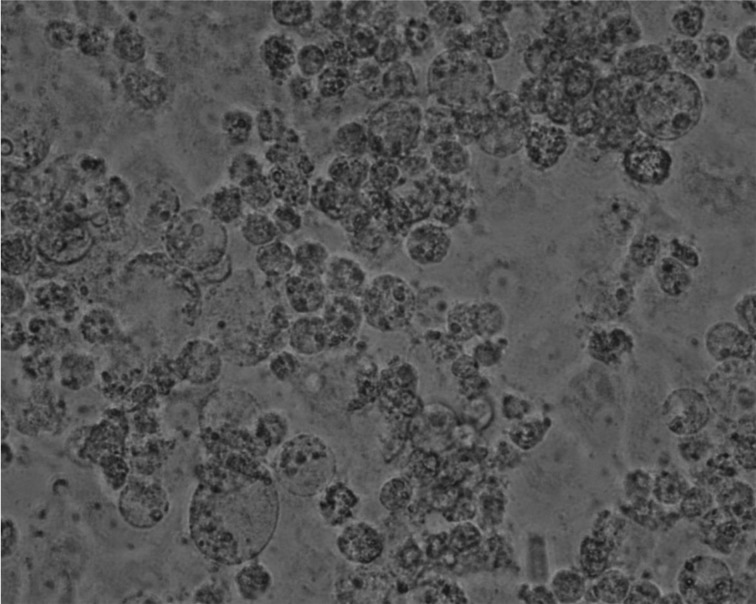
Isolation of microorganisms and PCR for virus detection: Characteristic CPE appeared in HmLu-1 cells inoculated with jejunum homogenate from case 1 (22–58 strain) at the first passage (Fig. 1). CPE was observed seven days after inoculation. On the other hand, at the second passage, CPE similar to that of 22–58 strain appeared in HmLu-1 cells inoculated with calf feces from case 2 (24–100 strain). Gimenez staining of HmLu-1 cells infected with these strains revealed many small purplish-red particles. C. pecorum-specific genes were detected from two isolates. In addition, C. pecorum-specific genes were detected from jejunum, ileum, cecum and colon of case 1. Clostridium perfringens was isolated from jejunal and ileal contents of case 1 at approximately 105 CFU/g. The gene of α-toxins was shown in these isolates by PCR, and isolates were identified as type A (data not shown).
Fig. 1.
Characteristic CPE observed in HmLu-1 cells inoculated with homogenized jejunum supernatant from case 1.
No other viruses were isolated from specimens, and PCR was also negative for all specimens from cases 1 and 2 when tested for bovine viral diarrhea virus [27], bovine coronavirus [26], rotavirus [3, 10, 25], bovine torovirus [11], bovine enterovirus [12], paramyxoviruses [24], bovine adenovirus [14] and bovine herpesvirus 4 [1].
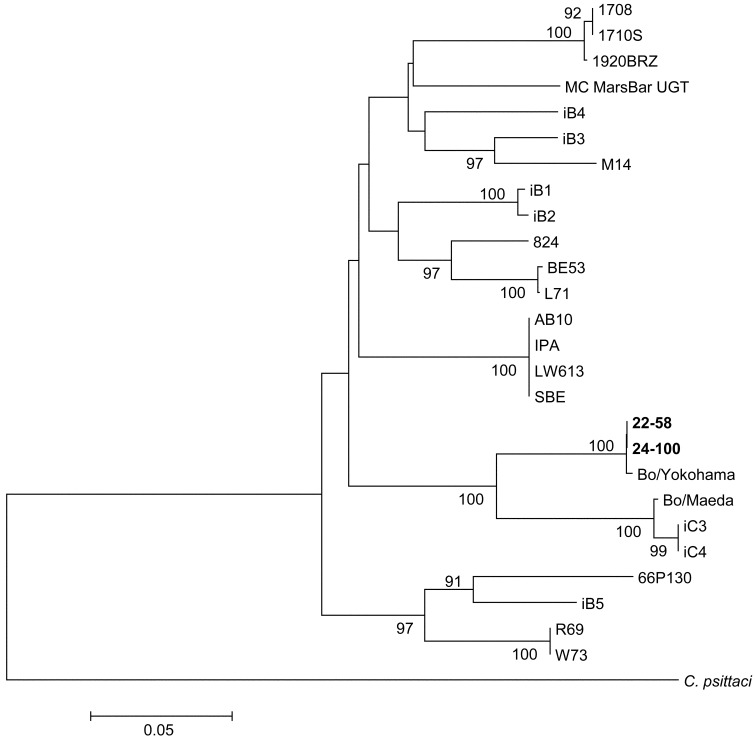
Identification of isolates by PCR, sequence and phylogenetic analysis: Two isolates (22–58 and 24–100) were PCR positive when genus-specific and species-specific omp1 genes of C. pecorum were targeted. Nucleotide sequences of genus-specific PCR products were determined and compared with those of published C. pecorum omp1 genes. Omp1 gene partial sequence of the 2 isolates was 100% identical and showed the highest identity (99.8%) to the Bo/Yokohama strain isolated from cattle with enteritis in Japan. Nucleotide similarities of the omp1 gene between the isolates and other published C. pecorum isolates were 89.2%, 85.2% and 82.0% for Bo/Maeda strain which was isolated from cattle with pneumonia in Japan, Ov/IPA strain which was isolated from sheep with polyarthritis in United States, and 66P130 strain which was isolated from cattle with enteritis in United States, respectively (Fig. 2, Table 1).
Fig. 2.
Phylogenetic tree of C. pecorum strains based on the nucleotide sequence of the omp1 gene (534 nucleotides). The sequences of reference strains were obtained from GenBank. GenBank accession number are 22-58 (LC021417), 24-100 (LC021418), Bo/Yokohama (LC021422), Bo/Maeda (LC021419), Ov/IPA (LC021421), L71 (AF269280), LW613 (AJ440240), SBE (EU684916), AB10 (EU684917), M14 (EU684920), 824 (EU684922), BE53 (EU684923), iB1 (EU684924), iB2 (EU684925), iB3 (EU684926), iB4 (EU684927), iB5 (EU684928), W73 (EU684929), R69 (EU684930), iC3 (EU684932), iC4 (EU684933), 1710S (GQ228167), 1920BRZ (GQ228168), 66P130 (GQ228180), 1708 (GQ228194) and MC MarsBar UGT (HQ457473). The nucleotide sequence of C. psittaci CPX0308 strain (AB284064) was used as an outgroup to root the tree. The percentage bootstrap values calculated from 1,000 replications are indicated above the internal nodes.
Table 1. Nucleotide sequence identities (%) of omp1 genes among C. pecorum strains.
| Strain | 22–58 | 24–100 | Bo/Yokohama | Bo/Maeda | Ov/IPA | 66P130 |
|---|---|---|---|---|---|---|
| 22–58 | - | 100 | 99.8 | 89.2 | 85.2 | 82.0 |
| 24–100 | - | 99.8 | 89.2 | 85.2 | 82.0 | |
| Bo/Yokohama | - | 89.0 | 85.0 | 81.8 | ||
| Bo/Maeda | - | 84.2 | 83.0 | |||
| Ov/IPA | - | 80.2 | ||||
| 66P130 | - |
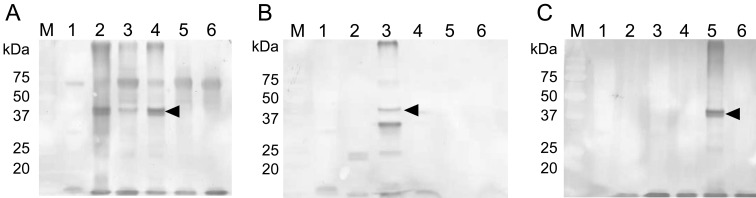
Antigenic characterization of C. pecorum isolates: The 38 to 40 kDa major outer membrane protein [6] of 22–58 strain strongly reacted with rabbit antiserum raised against the Bo/Yokohama strain. On the other hand, no specific reaction was detected by immunoblot analysis with rabbit antisera to Bo/Maeda or Ov/IPA strains (Fig. 3). Antigenicity between isolates (22–58 and 24–100) and the 3 strains (Bo/Yokohama, Bo/Maeda and Ov/IPA) was compared using cross-neutralization tests with rabbit antisera. Isolates 22–58 and 24–100 were antigenically similar to the Bo/Yokohama strain, but were a little different from the Bo/Maeda and Ov/IPA strains (Table 2).
Fig. 3.
Immunoblot analysis of isolates using rabbit antiserum against C. pecorum. Antiserum against Bo/Yokohama (A), Bo/Maeda (B) and Ov/IPA (C) strains was used for each figure. Lane 1: mock-infected HmLu-1 cells; Lane 2, 22–58 strain-infected HmLu-1 cells; Lane 3, Bo/Maeda strain-infected HmLu-1 cells; Lane 4, Bo/Yokohama strain-infected HmLu-1 cells; Lane 5, Ov/IPA strain-infected HmLu-1 cells; Lane 6, C. psittaci Cal10 strain-infected HmLu-1 cells. Arrows indicate a specific band of major outer membrane protein (38–40 kDa).
Table 2. Cross-reactivity among C. pecorum strains determined by neutralization test.
| Strain | Antibody titer to antisera against | ||
|---|---|---|---|
| Bo/Yokohama | Bo/Maeda | Ov/IPA | |
| 22–58 | 8a) | <2 | <2 |
| 24–100 | 4 | <2 | <2 |
| Bo/Yokohama | 16 | 4 | <2 |
| Bo/Maeda | 4 | 8 | <2 |
| Ov/IPA | <2 | <2 | 4 |
a) The reciprocal of the highest dilution of serum which inhibited CPE completely.
Neutralization test for paired sera of case 2: A significant increase in antibody against 24–100 strain was confirmed by neutralizing tests using paired sera. Neutralizing titers in sera collected during the acute phase of infection were less than 1:2, but increased to 1:4 during the convalescent phase.
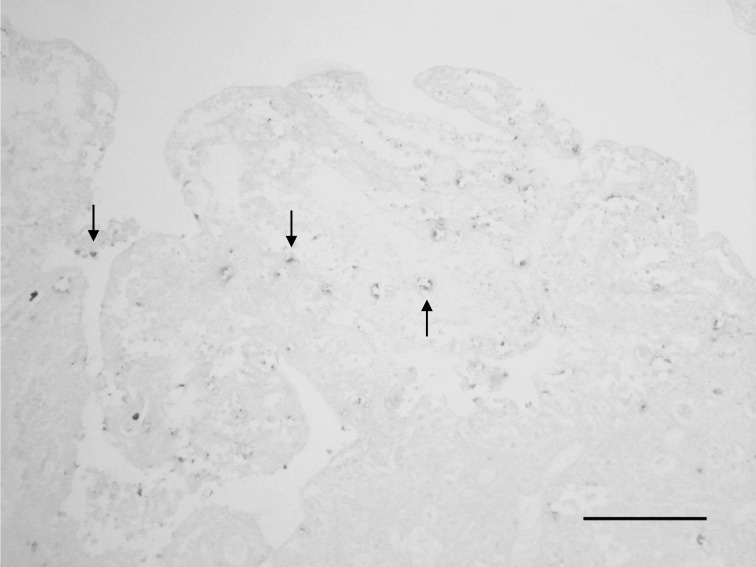
Histopathology and immunohistochemical staining for C. pecorum antigens in tissues from case 1: Systemic hyperemia and congestion together with necrosis of villi with short or long rod-shaped bacteria in the upper part of the small intestine were observed in tissue sections. Hemorrhagic ileitis with edematous and granulomatous changes was observed in the intestinal crypts of the ileum. Hemorrhagic colitis was observed in the cecum and colon. Multiple hemorrhages were observed in the mesenteric lymph nodes. In some organs, historrhexis and decreasing staining properties due to postmortem changes were observed. Positive reactions were observed in the jejunal villi by immunohistochemical staining with rabbit anti-C. pecorum antiserum (Fig. 4).
Fig. 4.
Detection of C. pecorum antigens in the jejunum. C. pecorum-positive reactions are observed in the jejunal villi (arrows). Immunohistochemistry. Bar=100 µm
DISCUSSION
In this study, C. pecorum was isolated from specimens obtained from 2 calves with diarrhea. In case 1, the bacterium was isolated from jejunum, and C. pecorum specific omp1 genes were detected from several locations in the intestines. Additionally, C. pecorum antigens were observed in the jejunal villi by immunohistochemical staining. Necrotizing enterocolitis due to Clostridium perfringens infection was also observed. Therefore, we speculated that C. pecorum might exacerbate the disease caused by Clostridium perfringens.
In these 2 cases, it is possible that C. pecorum was either the primary pathogen or an exacerbating factor causing diarrhea. On the other hand, it is thought that C. pecorum causes asymptomatic infections [8]. Recently, Poudel et al. [16] reported that asymptomatic endemic C. pecorum infections reduce growth rates in calves by up to 48%. They considered that the mechanism of growth suppression by subclinical chlamydial infection was malabsorption of nutrients due to a local inflammatory response to intestinal mucosal infection. Additionally, despite the absence of clinical signs, chlamydial infection was associated with reduced serum iron concentrations and lower hematocrit values, and infected calves were leukopenic [17]. Mohamad and Rodolakis [15] reported that the persistence of C. pecorum strains in the intestines and vaginal mucus of ruminants could cause long-term sub-clinical infection which may affect the animal’s health. This may explain the poor weight gain observed in case 2 after recovery from diarrhea. Further studies on the pathogenesis of C. pecorum infections are required.
Although the standard method for detecting antibodies to Chlamydiaceae spp. in animals is still the complement fixation test [18], our study showed that a neutralization test was also a useful method for diagnosis of C. pecorum infections. In addition, in the dead calf, it was confirmed that immunohistochemistry for C. pecorum antigens was also useful.
Genetic and antigenic analysis showed that 22–58 and 24–100 strains were more closely related to Bo/Yokohama strain isolated from cattle with enteritis than to Bo/Maeda strain isolated from cattle with pneumonia and Ov/IPA strain isolated from sheep with polyarthritis. Bo/Yokohama-like C. pecorum strains might cause enteritis more than other serotypes. However, the 2 isolates showed higher sequence identities to Bo/Maeda and Ov/IPA strains than to 66P130 strain isolated from cattle with enteritis in United States. Thus, sequence identities of C. pecorum omp1 gene might vary according to their geographical background.
The isolation of similar strains from different locations in separate years suggests that C. pecorum might be spreading among the cattle population in Yamaguchi Prefecture. An epidemiological study of C. pecorum is being conducted currently to clarify the seroprevalence and relationship with disease.
In conclusion, this study showed that C. pecorum isolates similar to Bo/Yokohama might be endemic in Yamaguchi Prefecture and cause enteric diseases in cattle.
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan and from the Ministry of Health, Labour and Welfare of Japan.
REFERENCES
- 1.Asano A., Inoshima Y., Murakami K., Iketani Y., Yamamoto Y., Sentsui H.2003. Latency and persistence of bovine herpesvirus type 4, strain B11-41, in bovine nervous tissues. J. Vet. Med. Sci. 65: 87–93. doi: 10.1292/jvms.65.87 [DOI] [PubMed] [Google Scholar]
- 2.Baums C. G., Schotte U., Amtsberg G., Goethe R.2004. Diagnostic multiplex PCR for toxin genotyping of Clostridium perfringens isolates. Vet. Microbiol. 100: 11–16. doi: 10.1016/S0378-1135(03)00126-3 [DOI] [PubMed] [Google Scholar]
- 3.Chinsangaram J., Akita G. Y., Osburn B. I.1994. Detection of bovine group B rotaviruses in feces by polymerase chain reaction. J. Vet. Diagn. Invest. 6: 302–307. doi: 10.1177/104063879400600304 [DOI] [PubMed] [Google Scholar]
- 4.Clarkson M. J., Philips H. L.1997. Isolation of faecal chlamydia from sheep in Britain and their characterization by cultural properties. Vet. J. 153: 307–310. doi: 10.1016/S1090-0233(97)80064-0 [DOI] [PubMed] [Google Scholar]
- 5.DeGraves F. J., Gao D., Hehnen H. R., Schlapp T., Kaltenboeck B.2003. Quantitative detection of Chlamydia psittaci and C. pecorum by high-sensitivity real-time PCR reveals high prevalence of vaginal infection in cattle. J. Clin. Microbiol. 41: 1726–1729. doi: 10.1128/JCM.41.4.1726-1729.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukushi H., Hirai K.1988. Immunochemical diversity of the major outer membrane protein of avian and mammalian Chlamydia psittaci. J. Clin. Microbiol. 26: 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukushi H., Hirai K.1989. Genetic diversity of avian and mammalian Chlamydia psittaci strains and relation to host origin. J. Bacteriol. 171: 2850–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukushi H., Hirai K.1995. Chlamydia pecorum—the fourth species of genus Chlamydia. J. Jpn. Vet. Med. Assoc. 48: 1–6(in Japanese). doi: 10.12935/jvma1951.48.1 [DOI] [PubMed] [Google Scholar]
- 9.Francis T., Jr, Magill T. O.1938. An unidentified virus producing acute meningitis and pneumonitis in experimental animals. J. Exp. Med. 68: 147–160. doi: 10.1084/jem.68.2.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gouvea V., Glass R. I., Woods P., Taniguchi K., Clark K. F., Forrester B., Fang Z. Y.1990. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 28: 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoet A. E., Smiley J., Thomas C., Nielsen P. R., Wittum T. E., Saif L. J.2003. Association of enteric shedding of bovine torovirus (Breda virus) and other enteropathogens with diarrhea in veal calves. Am. J. Vet. Res. 64: 485–490. doi: 10.2460/ajvr.2003.64.485 [DOI] [PubMed] [Google Scholar]
- 12.Jiménez-Clavero M. A., Escribano-Romero E., Mansilla C., Gómez N., Córdoba L., Roblas N., Ponz F., Ley V., Sáiz J. C.2005. Survey of bovine enterovirus in biological and environmental samples by a highly sensitive real-time reverse transcription-PCR. Appl. Environ. Microbiol. 71: 3536–3543. doi: 10.1128/AEM.71.7.3536-3543.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaltenböck B., Schmeer N., Schneider R.1997. Evidence for numerous omp1 alleles of porcine Chlamydia trachomatis and novel Chlamydial species obtained by PCR. J. Clin. Microbiol. 35: 1835–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maluquer de Motes C., Clemente-Casares P., Hundesa A., Martín M., Girones R.2004. Detection of bovine and porcine adenoviruses for tracing the source of fecal contamination. Appl. Environ. Microbiol. 70: 1448–1454. doi: 10.1128/AEM.70.3.1448-1454.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohamad K. Y., Rodolakis A.2010. Recent advances in the understanding of Chlamydophila pecorum infections, sixteen years after it was named as the fourth species of the Chlamydiaceae family. Vet. Res. 41: 27. doi: 10.1051/vetres/2009075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poudel A., Elsasser T. H., Rahman K. S., Chowdhury E. U., Kaltenboeck B.2012. Asymptomatic endemic Chlamydia pecorum infections reduce growth rates in calves by up to 48 percent. PLoS ONE 7: e44961. doi: 10.1371/journal.pone.0044961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinhold P., Jaeger J., Liebler-Tenorio E., Berndt A., Bachmann R., Schubert E., Melzer F., Elschner M., Sachse K.2008. Impact of latent infections with Chlamydophila species in young cattle. Vet. J. 175: 202–211. doi: 10.1016/j.tvjl.2007.01.004 [DOI] [PubMed] [Google Scholar]
- 18.Reinhold P., Sachse K., Kaltenboeck B.2011. Chlamydiaceae in cattle: Commensals, trigger organisms, or pathogens? Vet. J. 189: 257–267. doi: 10.1016/j.tvjl.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 19.Saitou N., Nei M.1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406–425. [DOI] [PubMed] [Google Scholar]
- 20.Storz J.1988. Overview of animal diseases induced by chlamydial infections. pp. 167–192. In: Microbiology of Chlamydia. (Barron, A. L. ed.), CRC Press, Boca Raton. [Google Scholar]
- 21.Storz J., Kaltenboeck B.1993. Diversity of Chlamydia-induced diseases. pp. 27–64. In Rickettsial and Chlamydial diseases of domestic animals. (Woldehiwet, Z. and Ristic, M. eds.), Pergamon Press, Oxford. [Google Scholar]
- 22.Tamura K., Nei M.1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10: 512–526. [DOI] [PubMed] [Google Scholar]
- 23.Thompson J. D., Higgins D. G., Gibson T. J.1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22: 4673–4680. doi: 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong S., Chern S. W., Li Y., Pallansch M. A., Anderson L. J.2008. Sensitive and broadly reactive reverse transcription-PCR assays to detect novel paramyxoviruses. J. Clin. Microbiol. 46: 2652–2658. doi: 10.1128/JCM.00192-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsunemitsu H., Jiang B., Saif L. J.1996. Sequence comparison of the VP7 gene encoding the outer capsid glycoprotein among animal and human group C rotaviruses. Arch. Virol. 141: 705–713. doi: 10.1007/BF01718328 [DOI] [PubMed] [Google Scholar]
- 26.Tsunemitsu H., Smith D. R., Saif L. J.1999. Experimental inoculation of adult dairy cows with bovine coronavirus and detection of coronavirus in feces by RT-PCR. Arch. Virol. 144: 167–175. doi: 10.1007/s007050050493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vilcek S., Herring A. J., Herring J. A., Nettleton P. F., Lowings J. P., Paton D. J.1994. Pestiviruses isolated from pigs, cattle and sheep can be allocated into at least three genogroups using polymerase chain reaction and restriction endonuclease analysis. Arch. Virol. 136: 309–323. doi: 10.1007/BF01321060 [DOI] [PubMed] [Google Scholar]