Abstract
The large Japanese field mouse, Apodemus speciosus, is a potential indicator of environmental stress, but this function has not been confirmed by histological studies. Since environmental stress affects the reproductive function of mice, we determined the reproductive characteristics of this species at two locations: Toyama (36°35ʹN, 137°24ʹE) and Aomori (40°35ʹN, 140°57ʹE). Mice were captured during May–November (n=119) and July–November (n=146) at these locations, respectively. We classified the breeding season from the numbers of pregnant females and young, in addition to the spermatogenic cycle and seasonal changes in seminiferous tubule morphology of males. Testicular weight was measured, and seminiferous tubule morphology was examined histologically. Fourteen stages were found in the seminiferous epithelium cycle based on acrosome formation and spermatid head morphology. At both locations, the breeding season peaked from late summer to early autumn and possibly in spring. Spermatogenic activity was classified into 4 periods from June to November: resting around June and October–November; resumptive around July; active around August; and degenerative around September. During the resting period, the seminiferous tubules consisted of Sertoli cells, spermatogonia and spermatocytes. Spermatogenesis began during the resumptive period, and spermatids were observed. During the active period, active spermatogenesis and a broad lumen were observed. During the degenerative period, spermatogenesis ended, and Sertoli cells, spermatogonia, spermatocytes and degenerating exfoliated round spermatids were observed. This study provides scientific information about the testicular histopathological evaluations of the large Japanese field mouse for its use as an index species of environmental pollution.
Keywords: Apodemus speciosus, histological observation, seasonal change, spermatogenesis, testis
The large Japanese field mouse (Apodemus speciosus) is a small mammal that is endemic to Japan, with its distribution ranging from Hokkaido to Kyusyu. This species inhabits a wide range of environments, including forests, plantations, riverside fields, paddy fields and cultivated fields [27]. Many ecological studies have been performed on the wild populations of this species, with a focus on reproduction, morphology, behavior, food preferences and genetics [11, 25, 31, 32, 35]. In addition, there is a wealth of comparable biological data available for closely related laboratory rat and mouse species.
Species belonging to the genus Apodemus have attracted attention as reference animals for studies on environmental pollution [41]. One study used wild A. speciosus to evaluate dioxin pollution [13]. Several studies have used A. flavicollis to monitor heavy metal pollution [5, 12, 24]. Biological data have been reported for A. agrarius, as an indicator of environmental disturbance [41]. Miyazaki University (Japan) attempted to use A. speciosus as an environmental indicator under laboratory conditions [34]. The large Japanese field mouse could potentially be used to monitor several environmental stresses in the field, including chemical, heavy metal and radioactive contamination.
Environmental stress affects the reproductive function of mice, with the male testis being particularly sensitive to stress. For example, cadmium chloride is a ubiquitous environmental heavy metal toxicant that causes the localized necrosis of the seminiferous epithelium in rats [37]. The testes are also the most radiation-sensitive organs [23, 42]. Few histological studies of the testes of large Japanese field mice have been conducted [6, 29, 30], with no published descriptions detailing the spermatogenic cycle of this species. Spermatogenesis is a complex biological process of the cellular transformation of spermatogonial cells into spermatozoa [10]. It is essential to distinguish environmental stress-induced histological changes from normal variation. Thus, it is necessary to obtain knowledge about the testicular characteristics and the histological patterns of spermatogenic stages in terms of testicular pathology [37]. Pathological microscopy should be performed as a qualitative examination within the context of the spermatogenic cycle [19].
Like other mammals that are distributed in temperate zones, the large Japanese field mouse is a seasonal breeder [25]. Consequently, the morphology of the seminiferous tubules is subject to dramatic seasonal variation [30]. The large Japanese field mouse has 3 different breeding seasons depending on living climates, specifically, one peak in the summer (Hokkaido [16] and Oze District [39]), one peak in winter (Fukuoka [30]) and two peaks in spring and autumn (Saitama [40], Shizuoka [38], Aichi [36] and Kyoto [25]). Few histological studies on the seasonal changes to the seminiferous tubules have been conducted on the large Japanese field mouse [6, 18, 30]. And, these observations remain a matter of examination. For instance, little histological information is available, except for the peaks of breeding and non-breeding periods. Therefore, in this study, we divided the breeding season into 4 periods based on spermatogenesis activity (resting, resumptive, active and degenerative [14]) and examined seasonal changes in the histology of the seminiferous epithelium.
This study aimed to identify the breeding season of the large Japanese field mouse in two geographically distinct locations in Japan; Toyama and Aomori. In addition, we aimed to classify the spermatogenic cycle and seasonal changes in seminiferous tubule morphology based on histopathological observations. The results are expected to provide baseline information about the large Japanese field mouse, facilitating its use as a robust model species for environmental pollution analyses.
MATERIALS AND METHODS
Animals: One-hundred and nineteen large Japanese field mice were captured using Sherman traps in Tateyama, Toyama Prefecture (36°35ʹN, 137°24ʹE; altitude: 580–620 m) from May to November 2012 and 2013. A further 146 mice were captured in Towada, Aomori Prefecture (40°35ʹN, 140°57ʹE; altitude: 450–490 m) from July to November 2012 and 2013. The animals were sacrificed by cervical dislocation or CO2 asphyxiation, and the body weights were measured. The testes of the males were removed surgically and weighed (Toyama, n=56; Aomori, n=61). Females were checked for pregnancy by autopsy (Toyama, n=36; Aomori, n=59).
Histological examination: The testes were immediately fixed with Bouin’s fixative. The specimens were dehydrated in graded ethanol, embedded in paraffin wax and sliced into 4-µm sections before staining with hematoxylin and eosin (HE) or periodic acid Schiff (PAS)-hematoxylin. Subsequently, the testes were observed under a light microscope (BZ-9000, KEYENCE, Osaka, Japan). The diameters (minor axis) of 20 randomly selected round-shaped sections of seminiferous tubules (Toyama, n=55; Aomori, n=43) were measured using the analysis application software of BZ-9000.
Spermatogenic cycle: PAS-hematoxylin staining samples from 10 males (Toyama, n=4; Aomori, n=6) captured in August (breeding season, described below) were used for the spermatogenic cycle study. These males had a body weight of over 30 g, and the two testes of each male weighed over 1.0 g. These values indicate that these males were sexually mature [25]. Classification of the stages of the spermatogenic cycle was based on the characteristics of acrosomic granules and cap formation, in addition to the shape of the spermatid head, according to the method of Russell et al. [33]. Relative frequencies were estimated for each stage by counting randomly selected round tubules. The round tubules numbered 147–185 in each animal, with 1,685 in total.
Numbers of pregnant females and young: To estimate the mating and birthing periods, the numbers of pregnant females and young were examined. The rate of pregnant females was represented by (the number of captured pregnant females) / (the number of captured females in total) × 100. The estimated gestation period ranges from 19 to 21 days in this species [29]. Thus, pregnant females were assumed to deliver after approximately 10 days, for descriptive purposes.
The rate of young mice was represented by (the number of captured young)/ (the number of captured individuals in total) × 100. Young mice were defined as individuals with a body weight under 25 g [25, 29] in this paper. The date of birth was estimated from body weight, with the young being divided into 2 classes; from 20 g to 25 g and under 20 g. Captive large Japanese field mice of 30 and 40 days of age have body weights of about 22 g and 28 g, respectively [29]. In general, captive animals grow faster than free-ranging ones, due to sufficient food supply [1]. Thus, individuals, weighing 20–25 g and under 20 g, were assumed to have been born about 40 and 30 days previously, respectively, for descriptive purposes.
Statistics: Values are represented as means. Statistical differences among values at different periods were examined by one-way analysis of variance using SAS 9.3 (SAS Institute Inc., Cary, NC, U.S.A.).
RESULTS
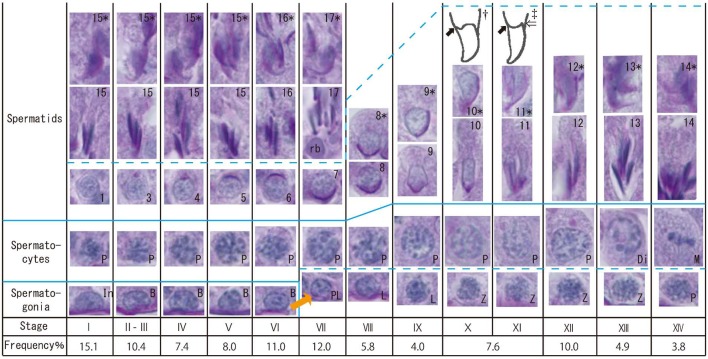
Spermatogenic cycle: The spermatogenic cycle was divided into 14 stages for the large Japanese field mouse. The characteristics of each stage are shown in Figs. 1 and 2. The relative frequencies of stages II–III and stages X–XI were combined for descriptive purposes, because it was difficult to distinguish between these stages (Fig. 2).
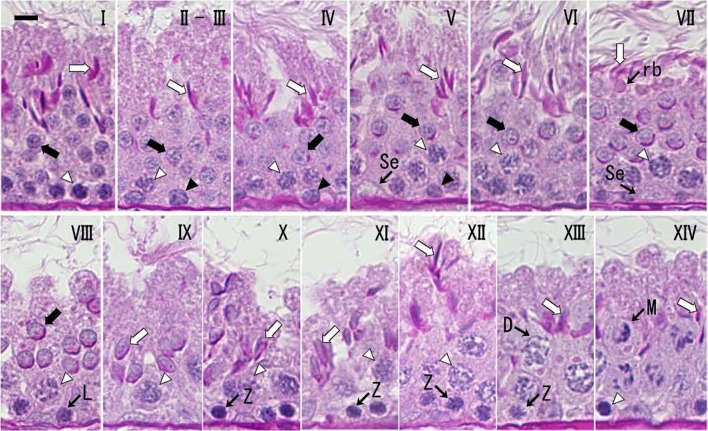
Fig. 1.
The 14 morphological stages of the spermatogenic cycle in the seminiferous tubules of the large Japanese field mouse during the breeding season. Roman numerals indicate each stage. Open arrow, elongated spermatid; Solid arrow, round spermatid; Open arrowhead, Pachytene spermatocyte; Solid arrowhead, Spermatogonium; Se, Sertoli cell; rb, residual body; L, leptotene spermatocyte; Z, zygotene spermatocyte; D, diplotene spermatocyte; M, meiosis. PAS-hematoxylin staining. Scale bar=10 µm.
Fig. 2.
Morphology of the germ cell nuclei at each spermatogenic stage of the large Japanese field mouse. PAS-hematoxylin staining. In, type intermediate spermatogonia; B, type B spermatogonia; PL, preleptotene spermatocytes; L, leptotene spermatocytes; P, pachytene spermatocytes; Di, diplotene spermatocytes; M, meiosis; rb, residual bodies. Arabic numerals (inside of spermatid figures) indicate each step of spermatids. Arabic numerals with an asterisk at the same stage indicate the appearance of spermatids from the other side. †, ‡, Illustrations of spermatid nuclei at stages X (†) and XI (‡). Solid arrow, ventral angle. Open arrow, dorsal angle.
Stage I: Round spermatids (step 1) were observed in the middle layer, and acrosomes were not observable by light microscopy. Spermatogonia were observed in the basal position at all stages. Pachytene spermatocytes were observed in the lower layer at all stages, except stage XIII. Developing elongated spermatids (steps 15–17) were observed in the upper layer from stages I to VII. Stages II–III: The proacrosomal (II) and acrosomal (III) granules began to appear in the round spermatids (steps 2 and 3). The acrosomal vesicle was not yet sufficiently flattened on the nuclear surface in the round spermatids. Stage IV: The acrosomal vesicle began to form a hemisphere on the nuclear surface in the round spermatids (step 4). The angle subtended by the spreading acrosome was <40°. Stage V: The angle subtended by the acrosome was 40–95° in the round spermatids (step 5). Stage VI: The angle subtended by the acrosome was 95–120° in the round spermatids (step 6). Elongated spermatids (step 16) had a small cytoplasm, and residual bodies were rarely observed. Stage VII: The angle subtended by the acrosome was >120° in the round spermatids (step 7). Elongated spermatids (step 17) had almost no cytoplasm. Residual bodies derived from the cytoplasm were found between the round spermatids and the elongated spermatids. Preleptotene spermatocytes were observed in the basal position. Elongated spermatid heads had a sharply curved fish-hook shape. Stage VIII: The matured spermatids and residual bodies disappeared from the epithelium, with only a few partially remaining. The spermatid (step 8) began to elongate, and its nucleus moved to the cell surface. Leptotene spermatocytes were observed in the lower layer at stages VIII and IX. Stage IX: The ventral surface of the spermatid (step 9) nucleus was flattened, and the nucleus was slightly elongated. Stage X: The spermatid (step 10) nucleus underwent continued lateral flattening and elongation, and the ventral angle appeared [33] (Fig. 2). The dorsal angle [33] (Fig. 2) had not yet appeared. The dorsal and ventral aspects of the spermatid nucleus became thinner by approximately half. Zygotene spermatocytes were observed in the lower layer from stages X to XIII. Stage XI: The spermatid (step 11) nucleus had ventral and dorsal angles and a curved dorsal surface. The dorsal and ventral aspects of the spermatid nucleus became approximately a quarter of the width. Stage XII: The spermatid (step 12) nucleus stained slightly darker than it did at stage XI and showed further lateral flatting. Stage XIII: The spermatid (step 13) nucleus stained darker than at stage XII. Diplotene spermatocytes appeared in the middle layer. Stage XIV: The spermatid (step 14) nucleus stained dark like a mature spermatid. Spermatocyte meiosis was observed. Sometimes, meiosis was mixed with diplotene spermatocytes, secondary spermatocytes and spermatids.
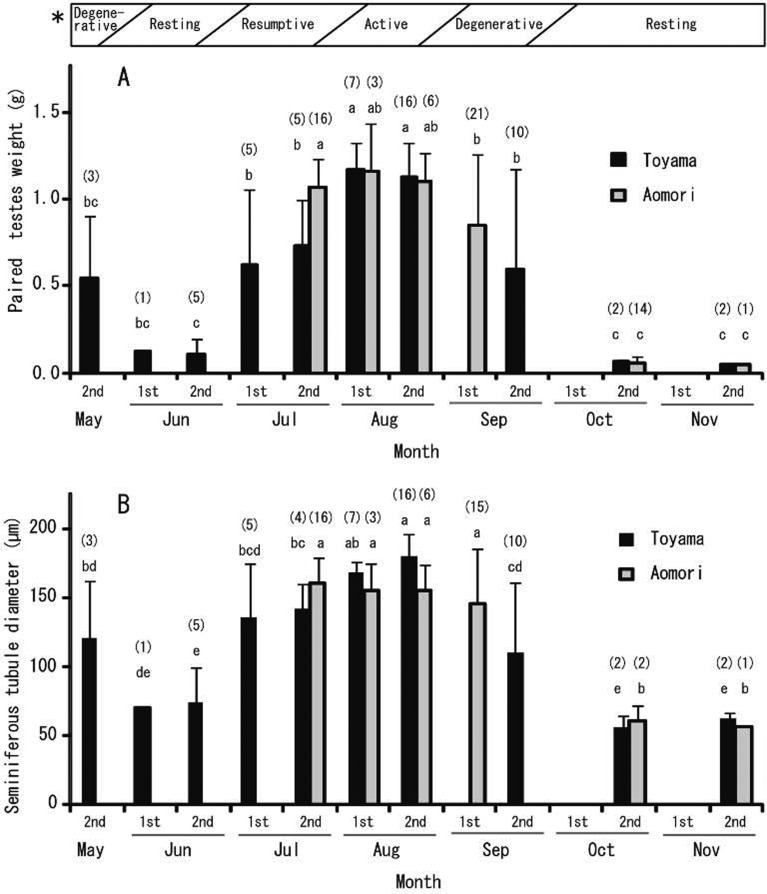
Seasonal change (testes and seminiferous tubules): Seasonal changes in testicular weight and seminiferous tubule diameter are shown in Fig. 3. Testicular weights and seminiferous tubule diameter changed significantly (P<0.05) with season. From May to November in Toyama, the highest testicular weight was recorded in August (first half, 1.17 g; second half, 1.13 g), while the lowest weight was recorded in June (second half, 0.11 g), followed by October (second half, 0.06 g) and November (second half, 0.05 g). From July to November in Aomori, the highest testicular weight was recorded in July (second half, 1.07 g) and August (first half, 1.16 g; second half, 1.10 g), while the lowest weight was recorded in October (second half, 0.06 g) and November (second half, 0.05 g).
Fig. 3.
Seasonal changes of testicular weight (A) and seminiferous tubule diameter (B) of sexually matured large Japanese field mice distributed in Toyama and Aomori. Within the same data set, values with different superscripts are significantly different (P<0.05). Sample sizes are shown in parentheses. Error bars represent standard deviations. *, Spermatogenic activity. Each month is divided into first half (1st) and second half (2nd).
From May to November in Toyama, the highest seminiferous diameter was recorded in August (first half, 169 µm; second half, 180 µm), while the lowest diameter was recorded in June (first half, 71 µm; second half, 74 µm), followed by October (second half, 56 µm) and November (second half, 62 µm). From July to November in Aomori, the highest seminiferous diameter was recorded in July (second half, 161 µm), August (first half, 155 µm; second half, 155 µm) and September (second half, 146 µm), while the lowest diameter was recorded in October (second half, 61 µm) and November (second half, 56 µm).
Male large Japanese field mice showed similar seasonal changes in spermatogenesis at both study sites (Toyama and Aomori). Based on the observed spermatogenic activity of these males, we classified the breeding season into 4 periods between June and November: resting around June and October–November; resumptive around July; active around August; and degenerative around September (Fig. 3).
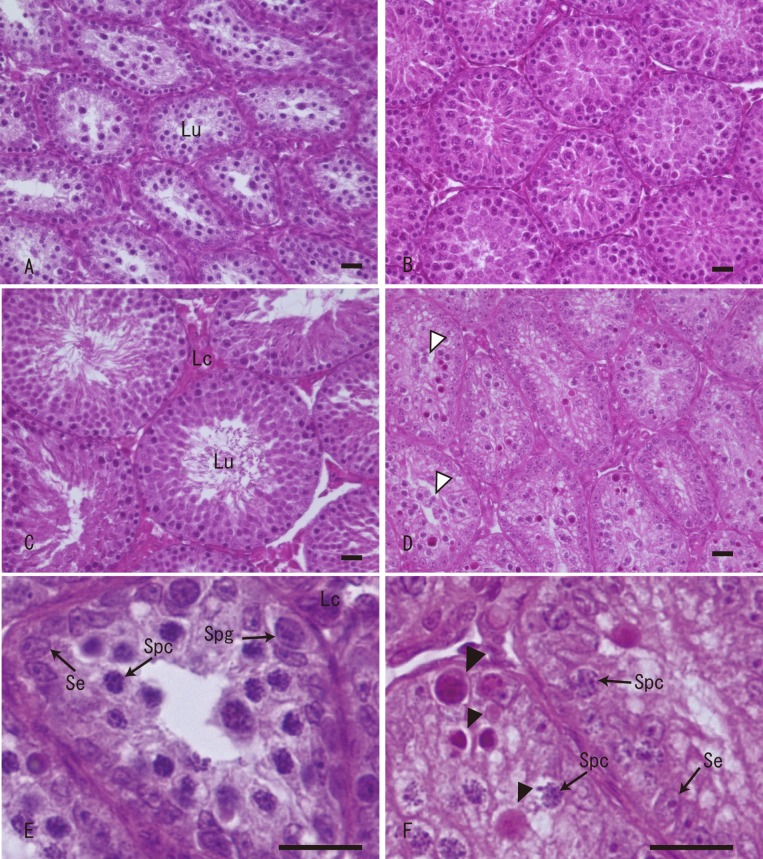
Seasonal change (seminiferous tubule morphology): During the examined period, seminiferous tubule morphology of sexually mature large Japanese field mice changed with season (Fig. 4). During the resting period, the seminiferous tubules had almost no lumen and consisted of Sertoli cells and spermatogonia in the lower layer, and spermatocytes in the internal layer (Fig. 4A and 4E). During the resumptive period, the lumens of the seminiferous tubules were narrow, at which point spermatogenesis began, with Sertoli cells, spermatogonia, spermatocytes and spermatids being observed (Fig. 4B). During the active period, active spermatogenesis and broad lumen were observed (Fig. 4C). Further information about seminiferous tubule morphology during the active period was described in the previous section (Spermatogenic cycle). During the degenerative period (around May and September), spermatogenesis disappeared, and the number of seminiferous epithelial cells decreased, with Sertoli cells, spermatogonia and spermatocytes, in addition to degenerating exfoliated round spermatids, being observed (Fig. 4D and 4F). Elongated or mature spermatids were not observed. Apoptotic germ cells, assumed to be spermatogonia or spermatocytes, were frequently observed.
Fig. 4.
Seasonal changes of seminiferous tubule structures of sexually matured large Japanese field mice in Toyama. (A) Resting period (June); Seminiferous tubules contain Sertoli cells, spermatogonia and primary spermatocytes. (B) Resumptive period (July); Spermatogenesis begins, and elongated spermatids are observed. (C) Active period (August); Active spermatogenesis is observed, and seminiferous tubules are enlarged. (D) Degenerative period (September); The number of germ cells decreases, and apoptotic germ cells are observed. (E) Resting period (high magnification of (A)). (F) Degenerative period (high magnification of (D)). Lu, lumen; Lc, Leydig cells; Se, Sertoli cells; Spg, spermatogonia; Spc, spermatocytes; Open arrowhead, degenerating spermatids; Solid arrowhead, apoptotic germ cells (spermatogonia or spermatocytes). HE staining. Scale bars=20 µm.
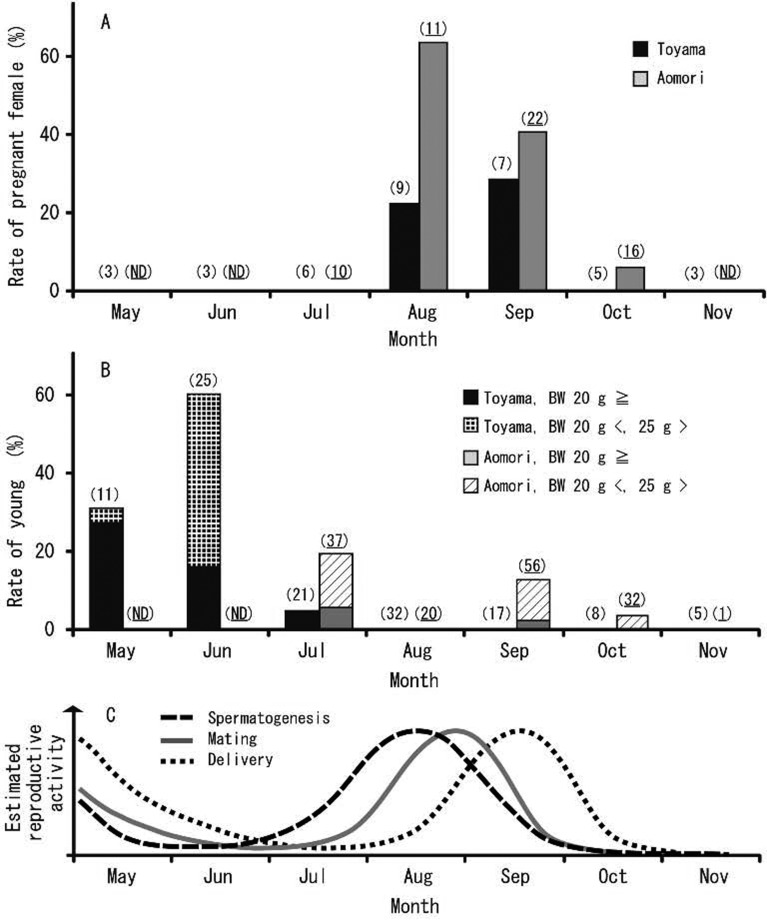
Seasonal change (appearance of young and pregnant females): In Toyama, pregnant females were only captured in August and September during the examined period. In Toyama, the rate of pregnant females was 22% in August and 29% in September (Fig. 5A). In Aomori, pregnant females were captured from July to October, with rates peaking in August (64%) and declining in September (41%), with a few being documented until early October (6%) (Fig. 5A).
Fig. 5.
Seasonal changes in the capture rate of pregnant females (A) and young (B), along with an estimate of reproductive activity (C) of large Japanese field mice distributed in Toyama and Aomori. Rate of pregnant female: (the number of captured pregnant females)/ (the number of captured females in total) × 100. Rate of young: (the number of captured young)/ (the number of captured individuals in total) × 100. Sample sizes are shown in parentheses; plain text, Toyama, and underline, Aomori. ND, no data.
The young were captured over a relatively expanded period in both Toyama and Aomori. In Toyama, young, weighing <20 g, were captured from May to July, with capture rates peaking in June (60%) (Fig. 5B). In Aomori, young, weighing <20 g, were captured in July and September during the examined period (Fig. 5B).
DISCUSSION
The seminiferous epithelium consists of different types of germ cells at each stage of the spermatogenic cycle, with species specificity [10]. It is important to determine the spermatogenic cycle of each species for histopathological observation. One reason for this need is that radiation sensitivity differs between spermatogonia and spermatocytes and also differs among the various stages of the spermatogenic cycle [21]. There are many studies on the spermatogenic cycle of animals [2, 3, 7, 20, 28]. However, few studies have reported the spermatogenic cycle of wild animals in Japan [8, 15, 26]. Fourteen- and 12-stage spermatogenic cycles are observed in rats and mice, respectively [33]. The large Japanese field mouse has 14 spermatogenic cycle stages, resembling that of rats. The main difference between the two species was that the elongated spermatid is removed from the epithelium at stage VIII in the large Japanese field mouse, but is observed until stage VIII in the rat.
Many previous studies on the reproductive biology of the large Japanese field mouse indicate that this species is clearly a seasonal breeder [16, 25, 36, 38,39,40]. Honshu is the main island of Japan and includes Toyama and Aomori. In most of Honshu, this species is considered to breed in spring and autumn [25, 36, 38, 40]. In Kyoto, high numbers of sexually active males and females of the large Japanese field mouse have been observed in March-April and around September, with minor year-to-year variation [25]. In contrast, in the highlands of Honshu, like Oze District, the breeding season tends to occur during summer for this species [39]. In this study, we used seasonal changes in testes weight, seminiferous tubule diameter and the appearance of pregnant females and young to confirm that the breeding season of the large Japanese field mouse peaks in late summer and early autumn in both Toyama and Aomori. In addition, breeding activity might occur in spring due to the appearance of young from May to July (Fig. 5B and 5C). Large Japanese field mice from Toyama and Aomori exhibited a seasonal reproductive pattern similar to that recorded by previous studies in most parts of Honshu Island. Toyama and Aomori have mild climates, similar to that of Saitama, Shizuoka, Aichi and Kyoto [25, 36, 38, 40], despite being slightly colder. More specifically, spermatogenesis activity starts earlier and ends earlier (by 1–2 weeks) at Aomori, compared to Toyama during the autumn breeding season. Ambient temperature and the day/night cycle are important factors for mammalian reproduction [1]. Temperature is also considered one of the most important environmental factors regulating the reproductive cycle of the large Japanese field mouse [22, 25]. Aomori has a slightly colder climate than Toyama; thus, the pup caring period should end before the onset of the severe cold season.
In the present study, we classified the breeding season into 4 periods from June to November based on spermatogenic activity (Fig. 3). In addition, we described the seasonal changes in seminiferous tubule morphology based on this classification (Fig. 4). Spermatogenesis is regulated by the hypothalamo-pituitary-testes axis [18], leading to seasonal changes in the structure of the seminiferous tubules. The cells that form the seminiferous tubule during the active and resting periods are similar to the cells recorded in other seasonal breeders [9, 14, 17]. Testicular regression during the degenerative period is regulated by apoptosis [43]. In this study, we observed seminiferous tubule morphology during the resumptive and degenerative periods, with many apoptotic cells being observed in HE stained sections during the degenerative period. In contrast, TdT-mediated dUTP nick end labeling (TUNEL) confirmed the presence of many apoptotic cells during the resting period in the Iberian mole (Talpa occidentalis), with a few apoptotic cells being observed during the degenerative period [4]. TUNEL is a commonly used method for detecting DNA fragmentation by apoptosis. Due to the clearly different results obtained in these 2 species, further research with TUNEL technology is required to obtain a clear understanding about the appearance of apoptotic cells in the large Japanese field mouse.
In conclusion, we found that the breeding season of the large Japanese field mouse peaked in late summer and early autumn at Toyama and Aomori, and possibly in spring. Furthermore, this study classifies the spermatogenic cycle and seasonal changes in seminiferous tubule morphology from June to November for the large Japanese field mouse. This study is expected to support testicular histopathological evaluations of the large Japanese field mouse, for its potential use as an index species of environmental pollution.
Acknowledgments
The authors thank Mr. A. Yasuda, Mr. H. Ishida and other members of the Laboratory of Wildlife Conservation, Graduate School of Science and Engineering, University of Toyama, Japan, for assistance with the capture and the sampling of animals.
REFERENCES
- 1.Bronson F. H.1985. Mammalian reproduction: An ecological perspective. Biol. Reprod. 32: 1–26. doi: 10.1095/biolreprod32.1.1 [DOI] [PubMed] [Google Scholar]
- 2.Clermont Y.1963. The cycle of the seminiferous epithelium in man. Am. J. Anat. 112: 35–51. doi: 10.1002/aja.1001120103 [DOI] [PubMed] [Google Scholar]
- 3.Clermont Y., Leblond C. P.1959. Differentiation and renewal of spermatogonia in the monkey, Macacus rhesus. Am. J. Anat. 104: 237–273. doi: 10.1002/aja.1001040204 [DOI] [PubMed] [Google Scholar]
- 4.Dadhich R. K., Real F. M., Zurita F., Barrionuevo F. J., Burgos M., Jiménez R.2010. Role of apoptosis and cell proliferation in the testicular dynamics of seasonal breeding mammals: a study in the Iberian mole, Talpa occidentalis. Biol. Reprod. 83: 83–91. doi: 10.1095/biolreprod.109.080135 [DOI] [PubMed] [Google Scholar]
- 5.Damek-Poprawa M., Sawicka-Kapusta K.2003. Damage to the liver, kidney, and testis with reference to burden of heavy metals in yellow-necked mice from areas around steelworks and zinc smelters in Poland. Toxicology 186: 1–10. doi: 10.1016/S0300-483X(02)00595-4 [DOI] [PubMed] [Google Scholar]
- 6.Endo H., Yoshiyuki M.1996. Seminiferous tubules of the Japanese wood mouse (Apodemus speciosus) in Abukuma Mountains (Fukushima Prefecture). Mem. Natn. Sci. Mus., Tokyo 29: 147–151. [Google Scholar]
- 7.Foote R. H., Swierstra E. E., Hunt W. L.1972. Spermatogenesis in the dog. Anat. Rec. 173: 341–351. doi: 10.1002/ar.1091730309 [DOI] [PubMed] [Google Scholar]
- 8.Hayakawa D., Sasaki M., Suzuki M., Igota H., Kitamura N.2009. Classification of the seminiferous epithelial cycle in the Sika deer (Cervus nippon). Mammal Study 34: 41–45. doi: 10.3106/041.034.0107 [DOI] [Google Scholar]
- 9.Hayakawa D., Sasaki M., Suzuki M., Tsubota T., Igota H., Kaji K., Kitamura N.2010. Immunohistochemical localization of steroidogenic enzymes in the testis of the sika deer (Cervus nippon) during developmental and seasonal changes. J. Reprod. Dev. 56: 117–123. doi: 10.1262/jrd.09-102T [DOI] [PubMed] [Google Scholar]
- 10.Hess R. A., de Franca L. R.2009. Spermatogenesis and cycle of the seminiferous epithelium. pp. 1–15. In: Molecular Mechanisms in Spermatogenesis (Cheng, C. Y. ed.), Springer, New York. [Google Scholar]
- 11.Hirota T., Hirohata T., Mashima H., Satoh T., Obara Y.2004. Population structure of the large Japanese field mouse, Apodemus speciosus (Rodentia: Muridae), in suburban landscape, based on mitochondrial D-loop sequences. Mol. Ecol. 13: 3275–3282. doi: 10.1111/j.1365-294X.2004.02324.x [DOI] [PubMed] [Google Scholar]
- 12.Ieradi L. A., Zima J., Allegra F., Kotlanova E., Campanella L., Grossi R., Cristaldi M.2003. Evaluation of genotoxic damage in wild rodents from a polluted area in the Czech Republic. Folia Zool.-Praha- 52: 57–66. [Google Scholar]
- 13.Ishiniwa H., Sakai M., Tohma S., Matsuki H., Takahashi Y., Kajiwara H., Sekijima T.2013. Dioxin pollution disrupts reproduction in male Japanese field mice. Ecotoxicology 22: 1335–1347. doi: 10.1007/s10646-013-1120-7 [DOI] [PubMed] [Google Scholar]
- 14.Komatsu T., Tsubota T., Yamamoto Y., Atoji Y., Suzuki Y.1997. Seasonal changes in the immunolocalization of the steroidogenic enzymes in the testes of the Japanese black bear (Ursus thibetanus japonicus). J. Vet. Med. Sci. 59: 521–529. doi: 10.1292/jvms.59.521 [DOI] [PubMed] [Google Scholar]
- 15.Komatsu T., Yamamoto Y., Tsubota T., Atoji Y., Suzuki Y.1996. Spermatogenic cycle in the testis of the Japanese black bear (Selenarctos thibetanus japonicus). J. Vet. Med. Sci. 58: 329–335. doi: 10.1292/jvms.58.329 [DOI] [PubMed] [Google Scholar]
- 16.Kondo N., Abe H.1978. Reproductive activity of Apodemus speciosus ainu. Mem. Fac. Agric. Hokkaido Univ. 11: 159–165(in Japanese with English summary). [Google Scholar]
- 17.Kurohmaru M., Saruwatari T., Kimura J., Mukohyama M., Watanabe G., Taya K., Hayashi Y.2002. Seasonal changes in spermatogenesis of the Japanese lesser horseshoe bat, Rhinolophus cornutus from a morphological viewpoint. Okajimas Folia Anat. Jpn. 79: 93–100. doi: 10.2535/ofaj.79.93 [DOI] [PubMed] [Google Scholar]
- 18.Kuwahara S., Mizukami T., Omura M., Hagihara M., Iinuma Y., Shimizu Y., Tamada H., Tsukamoto Y., Nishida T., Sasaki F.2000. Seasonal changes in the hypothalamo-pituitary-testes axis of the Japanese wood mouse (Apodemus speciosus). Anat. Rec. 260: 366–372. doi: [DOI] [PubMed] [Google Scholar]
- 19.Lanning L. L., Creasy D. M., Chapin R. E., Mann P. C., Barlow N. J., Regan K. S., Goodman D. G.2002. Recommended approaches for the evaluation of testicular and epididymal toxicity. Toxicol. Pathol. 30: 507–520. doi: 10.1080/016128401750063376 [DOI] [PubMed] [Google Scholar]
- 20.Leblond C. P., Clermont Y.1952. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann. N. Y. Acad. Sci. 55: 548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x [DOI] [PubMed] [Google Scholar]
- 21.Liu G., Gong P., Zhao H., Wang Z., Gong S., Cai L.2006. Effect of low-level radiation on the death of male germ cells. Radiat. Res. 165: 379–389. doi: 10.1667/RR3528.1 [DOI] [PubMed] [Google Scholar]
- 22.Maehata I.1996. Fauna of Muridae and the seasonal changes in the number and reproductive status of Apodemus speciosus in the evergreen and deciduous broad-leaved forests. Bull. Center Nat. Environ. Educ. Nara Univ. Educ. 1: 21–32(in Japanese with English summary). [Google Scholar]
- 23.Meistrich M. L., Hunter N. R., Suzuki N., Trostle P. K., Withers H. R.1978. Gradual regeneration of mouse testicular stem cells after exposure to ionizing radiation. Radiat. Res. 74: 349–362. doi: 10.2307/3574894 [DOI] [PubMed] [Google Scholar]
- 24.Metcheva R., Teodorova S., Topashka-Ancheva M.2003. A comparative analysis of the heavy metal loading of small mammals in different regions of Bulgaria I: monitoring points and bioaccumulation features. Ecotoxicol. Environ. Saf. 54: 176–187. doi: 10.1016/S0147-6513(02)00051-9 [DOI] [PubMed] [Google Scholar]
- 25.Murakami O.1974. Growth and development of the Japanese wood mouse (Apodemus speciosus) I. The breeding season in the field. Jap. J. Ecol. 24: 194–206(in Japanese with English summary). [Google Scholar]
- 26.Nagato Y., Enomoto T., Matsubayashi K.1994. Observation on the cycle of the seminiferous epithelium in the Japanese macaques (Macaca fuscata) using semithin sections. Primates 35: 455–464. doi: 10.1007/BF02381954 [DOI] [Google Scholar]
- 27.Nakata K., Saitoh T., Iwasa M. A.2009. Apodemus speciosus (Temminck, 1844). pp. 169–171. In: The Wild Mammals of Japan (Ohdachi, S., Ishibashi, Y., Iwasa, M. A. and Saitoh, T. eds.), Shoukadoh, Kyoto. [Google Scholar]
- 28.Oakberg E. F.1956. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am. J. Anat. 99: 507–516. doi: 10.1002/aja.1000990307 [DOI] [PubMed] [Google Scholar]
- 29.Oh S. H., Mori T.1998. Growth, development and reproduction in captive of the large Japanese field mouse, Apodemus speciosus (Rodentia, Muridae). J. Fac. Agr. Kyushu Univ. 43: 397–408. [Google Scholar]
- 30.Oh S. H., Mori T.1998. Histological studies on reproductive organs of the large Japanese field mouse, Apodemus specious. Mammal. Sci. 32: 103–113(in Japanese with English summary). [Google Scholar]
- 31.Oka T.1992. Home range and mating system of two sympatric field mouse species, Apodemus speciosus and Apodemus argenteus. Ecol. Res. 7: 163–169. doi: 10.1007/BF02348495 [DOI] [Google Scholar]
- 32.Renaud S., Millien V.2001. Intra- and interspecific morphological variation in the field mouse species Apodemus argenteus and A. speciosus in the Japanese archipelago: the role of insular isolation and biogeographic gradients. Biol. J. Linn. Soc. Lond. 74: 557–569. doi: 10.1111/j.1095-8312.2001.tb01413.x [DOI] [Google Scholar]
- 33.Russell L. D., Ettlin R. A., Hikim A. P. S., Clegg E. D.1990. The classification and timing of spermatogenesis. pp. 41–58. In: Histological and Histopathological Evaluation of the Testis. Cache River Press, Clearwater. [Google Scholar]
- 34.Sakai Y., Sakamoto S. H., Kato G. A., Iwamoto N., Ozaki R., Eto T., Shinohara A., Morita T., Koshimoto C.2013. Rearing method to induce natural mating of the large Japanese field mouse, Apodemus speciosus. Mammal Sci. 53: 57–65(in Japanese with English summary). [Google Scholar]
- 35.Shimada T., Saitoh T.2003. Negative effects of acorns on the wood mouse Apodemus speciosus. Popul. Ecol. 45: 7–17. [Google Scholar]
- 36.Takada Y.2002. Growth, reproduction, and age structure of large Japanese field mice, Apodemus speciosus in two habitats: riverbanks and hills. J. Growth 41: 95–103. [Google Scholar]
- 37.Takahashi M., Matsui H.1993. Mechanisms of testicular toxicity. J. Toxicol. Pathol. 6: 161–174. doi: 10.1293/tox.6.161 [DOI] [Google Scholar]
- 38.Takanaka K., Ando M., Amano T., Ogawa H.2009. Relationship between the season of falling into side gutter and the breeding season of the large field mouse Apodemus speciosus. J. Agric. Sci. Tokyo Univ. Agric. 54: 15–19. [Google Scholar]
- 39.Tateishi T.2007. Reproductive activity of the large Japanese field mouse (Apodemus speciosus) in the Oze district in northern Honshu, Japan. Mammal Sci. 47: 215–220(in Japanese with English summary). [Google Scholar]
- 40.Tateishi T., Shiraishi S.1992. Distribution and reproductive activity of the Japanese large field mouse, Apodemus speciosus around the lake Syayama, Saitama Prefecture. Sci. Bull. Fac. Agr. Kyushu Univ. 47: 85–92. [Google Scholar]
- 41.Velickovic M.2007. Measures of the developmental stability, body size and body condition in the black-striped mouse (Apodemus agrarius) as indicators of a disturbed environment in northern Serbia. Belg. J. Zool. 137: 147–153. [Google Scholar]
- 42.Withers H. R., Hunter N., Barkley H. T., Jr. Reid B. O.1974. Radiation survival and regeneration characteristics of spermatogenic stem cells of mouse testis. Radiat. Res. 57: 88–103. doi: 10.2307/3573759 [DOI] [PubMed] [Google Scholar]
- 43.Young K. A., Zirkin B. R., Nelson R. J.2000. Testicular regression in response to food restriction and short photoperiod in white-footed mice (Peromyscus leucopus) is mediated by apoptosis. Biol. Reprod. 62: 347–354. doi: 10.1095/biolreprod62.2.347 [DOI] [PubMed] [Google Scholar]