Abstract
The aim of this retrospective study is to evaluate our therapeutic results in patients with paranasal sinus (PNS) or nasal cavity (NC) malignancies treated with robotic stereotactic radiosurgery (SRS). Between August 2007 and October 2008, 27 patients with PNS or NC tumors were treated in our department using SRS. Median age was 53 years (range, 27-84 years). Eleven patients were female and sixteen were male. Most common histopathology was SCC (44%). The disease involved the maxillary sinus in 15 patients (55%). SRS was applied to 6 patients (22%) for reirradiation, while the others received it as a primary treatment. Seven patients had SRS as a boost dose to external beam radiotherapy. SRS was delivered with cyberknife (Accuray Incorporated, Sunnyvale, CA, USA). The median dose to the tumor was 31 Gy (range, 15-37.5 Gy) in median 5 fractions (range, 3-5 fractions). After a median follow-up of 21.4 months (range, 3-59 months), 76% of the patients were free of local relapse. Three patients showed local progression and 3 developed distant metastases. One- and two-year survival rates for the entire group were 95.2% (SEM = 0.046) and 77.1% (SEM = 0.102), respectively. We observed brain necrosis in 2 patients, visual disorder in 2 patients, bone necrosis in 2 patients and trismus in 1 patient as a SRS related late toxicity. Robotic SRS seems to be a feasible treatment strategy for patients with PNS tumors. Further prospective studies with longer follow up times should be performed.
Keywords: Paranasal sinus tumors, Nasal cavity tumors, Robotic stereotactic radiosurgery
Introduction
Cancers of the nasal cavity (NC) and paranasal sinus (PNS) are rare tumors that represent 3-5% of all head and neck cancers (1). Tumors of the maxillary sinus are twice as frequent as those of the nasal cavity and ethmoid sinus (2). Squamous cell carcinoma (SCC) is the most common histopathology seen in the NC and PNS. Multimodal approach including surgery and radiotherapy (RT) is the recommended treatment method. However, the location of the tumor, its proximity to critical structures such as optic system, brain, cranial nerves and salivary glands limits radical surgery or high dose RT options. The current therapeutic strategies are based on retrospective studies, since NC and PNS cancers are rarely observed tumors.
Robotic stereotactic radiosurgery (SRS) uses precisely aimed beams of ionizing radiation coming from different directions to meet at a specific point while sparing the normal tissues close to the target. Therefore, it allows us to deliver ablative radiation doses to the tumor with high accuracy. However, there are only a few data related with the role of SRS in PNS and NC cancers (3). Therefore, we retrospectively evaluated our treatment results with SRS in patients with NC and PNS tumors.
Materials and Methods
The medical charts of 27 patients with the PNS or NC tumors treated in our department using robotic SRS between August 2007 and October 2008 were evaluated. Median age was 53 years (range, 27-84 years). Eleven patients were female and sixteen were male. All patients had biopsy proven diagnosis of malignancy. SRS was delivered for the purpose of primary treatment or reirradiation. Robotic SRS was delivered with cyberknife (Accuray Incorporated, Sunnyvale, CA, USA). Informed consent was obtained prior to treatment from all patients.
Most common histopathology was SCC (44%). The disease involved the maxillary sinus in 15 patients (55%). Only one patient who had SRS as a boost treatment had lymph node positivity (N2c disease). SRS was applied to 6 patients (22%) for reirradiation, while the others received it as a primary treatment. Seven patients had SRS as a boost dose to external beam RT. The patient characteristics are shown in Table I. Fourteen patients received chemotherapy.
Table I.
The characteristics of the patients.
| Characteristics | Number of patients |
|---|---|
| Age (median) | 53 (range, 27-84 years) |
| Gender (male/female) | 16/11 |
| Primary site | |
| Maxillary sinus | 15 |
| Ethmoid sinus | 6 |
| Nasal cavity | 3 |
| Frontal sinus | 2 |
| Sphenoidal sinus | 1 |
| Pathology | |
| SCC | 12 |
| MMT | 5 |
| ACC | 4 |
| MM | 4 |
| Others | 2 |
| Type of SRS | |
| Primary (SRS only) | 6 |
| Primary (boost) | 7 |
| Adjuvant (SRS only) | 8 |
| Reirradiation | 6 |
| Chemotherapy | 14 |
| Previous radiotherapy dose (median-Gy) | 50 (range, 44-60 Gy) |
Abbreviations: SCC = Squamous cell carcinoma; MMT = Malignant mesenchymal tumor; ACC = Adenoid cystic carcinoma; MM = Malignant melanoma; SRS = Stereotactic radiosurgery.
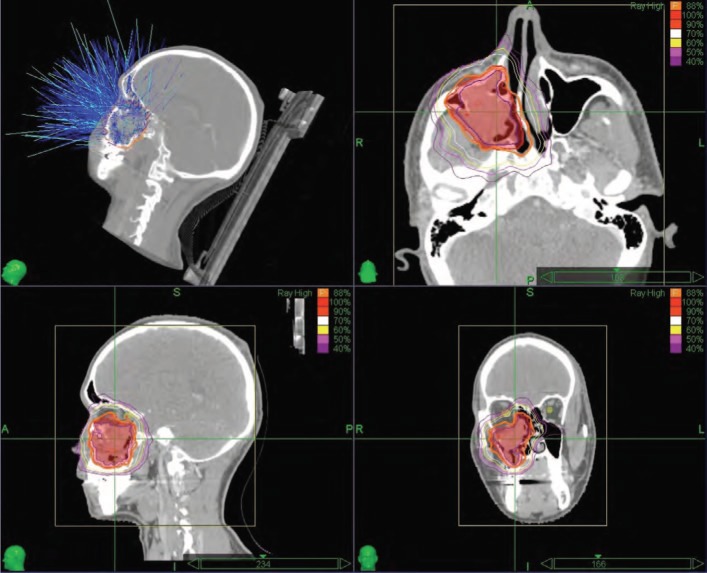
All patients underwent a pre-treatment contrast enhanced computerized tomography (CT) with a slice thickness of 1.25 mm. CT images were fused with T1 and T2 weighted fat suppression magnetic resonance imaging (MRI) sequences and the gross tumor volume (GTV) was delineated. Clinical target volume (CTV) was outlined by adding 5.0 mm to GTV. An extra 1.0 mm was added to CTV for planning target volume (PTV). Radiation doses were prescribed at the margin (95% volume border of the PTV). Treatment plan was generated with Multiplan (Accuray Incorporated, Sunnyvale, CA, USA) inverse planning system (Figure 1). All individual plans were evaluated and approved by two radiation oncologist (GO and MC).
Figure 1:
Treatment plan and dose distribution of a patient with maxillary sinus carcinoma (Isodose curves; Magenta: 40%, Pink: 50%, Yellow: 60%, White: 70%, Orange: 90%, Red: 100%, Prescription isodose was dark orange line: 88%).
The median dose to the tumor was 31 Gy (range, 15-37.5 Gy) in median 5 fractions (range, 3-5 fractions). The dose was normalized to 75% isodose line (range, 65-88%). The median homogeneity and conformity indices were 1.33 (1.23-1.54) and 1.43 (1.18-2.01), respectively (Table II).
Table II.
SRS characteristics and dose volume data for 27 patients.
| Characteristic | Median |
|---|---|
| Dose (Gy) | 31 (range, 15-37.5) |
| Fraction number | 5 (range, 3-5) |
| Prescribed isodose (%) | 75 (range, 65-88) |
| Conformity index | 1.43 (range, 1.18-2.01) |
| Homogeneity index | 1.33 (range, 1.23-1.54) |
All patients were evaluated for tumor growth and clinical outcome every 3 months in the first 2 years and then annually. The lesions were evaluated via MRI. Local control was defined as the absence of progressive disease after robotic SRS on MRI. Tumor regression was defined as 20% decrease in tumor volume on the basis of the last available MRI. Survival time was calculated starting from last day of SRS to the last follow up visit or time of death. Overall survival was computed using the Kaplan-Meier method. All statistical analysis was performed using the SPSS 15.0 software.
Results
The median follow-up after SRS was 21.4 months (range, 3-59 months). We found that 76% of patients were free of local relapse (Table III and Figure 2). Local progression was observed in 3 patients and 3 patients developed distant metastases (lung metastasis). One- and two- year survival estimates for the entire group were 95.2% (SEM = 0.046) and 77.1% (SEM = 0.102), respectively. One- and two-year survival estimates for patients with non-melanoma histology were 94.1% (SEM = 0.057) and 80.5% (SEM = 0.102), respectively. The patients with SCC had one- and two-year survival estimates of 100% and 76.2% (SEM = 0.148), respectively.
Table III.
Therapeutic response to SRS.
| SRS response | Number of patients (%) |
|---|---|
| Stable disease | 5 (19.2) |
| Complete response | 11 (42.3) |
| Partial response | 4 (14.8) |
| Progressive disease | 6 (23) |
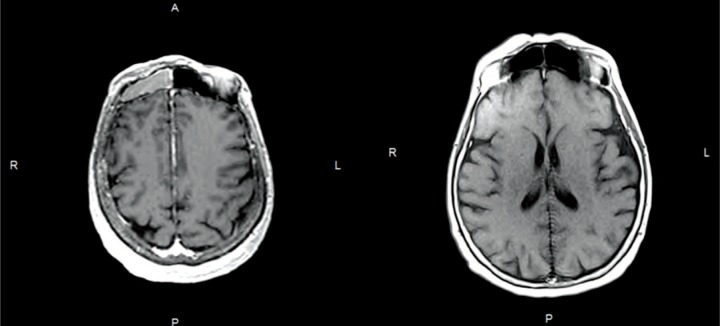
Figure 2:
(A) Malignant melanoma located at the right frontal sinus at the treatment MRI of the patient. (B) The control MRI of the patient 2 years after treatment. The lesion showed complete response after robotic SRS.
SRS related late complications were observed in 7 patients (brain necrosis in 2 patients, optic neuropathy in 2 patients, osteoradionecrosis in 2 patients and trismus in 1 patient). One patient developed osteoradionecrosis 3 months after tooth extraction from the mandibula. The clinical characteristics of patients with serious toxicities were summarized in Table IV. Patients with brain necrosis were asymptomatic. The patients with bone necrosis received hyperbaric oxygen treatment and long-term antibiotic therapy. The responses of those treatments were only stabilization without regression.
Table IV.
Clinical characteristics of patients with SRS related late complications.
| Complication | Date of complication (months) | Pathology | Age | Tumor site | Total dose (Gy) | Fraction number | SRS intent | Primary surgery | Primary radiotherapy dose (Gy) | Dose to the organ at risk (maximum) |
|---|---|---|---|---|---|---|---|---|---|---|
| Brain necrosis | 4 months | Adenoid cystic carcinoma | 50 | Maxillary sinus | 37.5 | 5 | Primary (SRS only) | – | – | Temporal lobe dose, 37.5 Gy |
| Optic neuropathy | 14 months | Adenoid cystic carcinoma | 48 | Maxillary sinus | 30 | 5 | Reirradiation | + | 62.9 + 50 | Optic chiasma dose, 27 Gy Left optic nerve dose, 8.2 Gy Right optic nerve dose, 7.6 Gy |
| Osteoradionecrosis (maxilla) | 15 months | Malignant melanoma | 77 | Ethmoid sinus | 31 | 3 | Adjuvant | + | – | Base of maxilla dose, 30 Gy |
| Osteoradionecrosis (maxilla) | 4 months | Squamous cell cancer | 48 | Ethmoid sinus | 32.5 | 5 | Adjuvant | + | – | Maxilla dose, 32.5 Gy |
| Trismus | 4 months | Adenoid cystic carcinoma | 70 | Maxillary sinus | 32.5 | 5 | Reirradiation | + | 50 | Temporomandibular joint dose, 17 Gy |
| Brain necrosis | 10 months | Malignant melanoma | 59 | Sphenoid sinus | 35 | 5 | Primary (SRS only) | – | – | Temporal lobe dose, 46 Gy |
| Optic neuropathy | 6 months | Malignant mesenchymal tumor | 48 | Maxillary sinus | 37.5 | 5 | Adjuvant | + | – | Optic chiasma dose, 31 Gy Left optic nerve dose, 9.8 Gy Right optic nerve dose, 10.4 Gy |
Discussion
Due to its advanced stage at presentation and close anatomic relationship with orbit, optic chiasma and brain, the management of PNS cancers is still challenging. This study represents our therapeutic results in PNS cancers treated with robotic SRS at a single institution. To the best of our knowledge, current study is the first evaluating robotic SRS in the management of PNS and NC malignancies.
Blanco et al. reported the treatment results of 106 patients with PNS cancer treated by preoperative (n = 28), postoperative (n = 41), or primary RT (n = 37) (4). The mean dose to the primary tumor was 60.9 ± 8.2 Gy, 55.7 ± 9.6 Gy and 61.7 ± 8.9 Gy, respectively, for patients undergoing postoperative, preoperative or definitive RT. Their five-year LC and OS rates were 58% and 27%, respectively for the whole group. Addition of surgery to RT improved the DFS, however loco-regional recurrence was the main failure pattern despite of aggressive local treatments. Duthoy et al. delivered median 70 Gy (range, 60-70 Gy) with intensity modulation radiation therapy (IMRT) after surgery for 39 patients (5).
Median follow up was 31 months. They reported 68% 4-year LC and 59% 4-year OS rates. They compared their results with conventional RT and concluded that postoperative IMRT for sinonasal carcinoma resulted in good LC, with a low acute toxicity and no RT-induced blindness. Chen et al. evaluated the treatment results of patients with PNS and they concluded that there is a significant improvement in toxicity rates in the treatment of PNS tumors over the years (6). Duprez et al. reported their late toxicity, LC and survival results after IMRT for sinonasal tumors (7). They delivered 70 Gy in 35 fractions to 130 patients. Median follow up was 52 months. They did not observe any radiation induced blindness in 86 patients who were available for late toxicity assessment. The grade of late ocular toxicity was Grade 3 (n = 11), Grade 2 (n = 31), Grade 3 (n = 33) and Grade 0 (n = 11). Brain necrosis and osteoradionecrosis occurred in 6 and 1 patients, respectively. Five-year LC and OS rates were 59% and 52%, respectively. Minimized ocular toxicity and increased disease control rates suggest IMRT as a standard treatment for PNS tumors.
These findings suggest the possibility of increased LC and survival with increased radiation doses and improved radiation techniques. Robotic SRS provides the chance to deliver ablative doses to the tumor with minimum doses to normal structures compared to other techniques. Due to heterogeneity of our cohort, it is difficult to make proper comparisons with the literature. It is also obvious that there are only few studies with robotic SRS in patients with NC or PNS tumors. To the best of our knowledge, there is one study with robotic SRS reported by Iwata et al. (3). They reported their treatment results in 51 patients with locally recurrent PNS and nasal carcinoma. In their study, previous RT dose was median 60 Gy. They delivered median 35 Gy (range, 20-41.5 Gy) in 3-5 fractions. After a median follow up of 21 months, 1 year survival rate was 67%. Their Grade 3 or higher toxicity rate was 23%. In our study 6 patients received Cyberknife treatment for the purpose of reirradiation. The patients have non-melanoma histopathology and their previous RT dose ranged from 50 to 60 Gy. We applied doses in between 28.5-35 Gy. Three patients showed disease progression. One patient with progressive disease had optic neuropathy and one patient with complete response had trismus during the follow up. Our reirradiated patient number is small to make a direct comparison. However, it seems that stereotactic reirradiation might be a feasible and an effective option for the treatment of recurrent nasal and PNS tumors.
Lee et al. reported their long-term results of 26 medically inoperable patients who received robotic SRS as a boost treatment for head and neck cancer (8). They had 8 patients (30.8%) with nasal cavity and PNS tumors. The median EBRT dose before SRS was 50.4 Gy. Median SRS boost dose of 21 Gy (range, 10-25 Gy) was delivered in median 5 (range, 2-5) fractions. The complete response rate was 80.8% in the whole group. Grade > 3 late toxicities developed in 9 patients. They concluded that boost treatment achieves high local control with high toxicity. In our study we have 7 patients who received robotic SRS as a boost treatment. The response rate and the toxicity profile of our cohort are similar to their series.
The major limitation of current study is the retrospective nature of our cohort. Furthermore, our study includes heterogeneous group of patients, and the follow up period is short. However, it is noteworthy that NC and PNS tumors are rare tumors and current literature about SRS for this group is limited. In the absence of similar retrospective data, our cohort showed that robotic SRS seems to be a feasible treatment strategy for patient with PNS tumors. However, further prospective studies with longer follow up period should be performed.
Acknowledgement
This study is supported by Hacettepe University Research Grant Project: 1-05 A 101 009.
Abbreviations
- PNS:
Paranasal Sinus
- NC:
Nasal Cavity
- SRS:
Stereotactic Radiosurgery
- MRI:
Magnetic Resonance Imaging
- SEM:
Standard Error of Mean
- RT:
Radiotherapy
- SCC:
Squamous Cell Carcinoma
- CT:
Computerized Tomography
- GTV:
Gross Tumor Volume
- CTV:
Clinical Target Volume
- PTV:
Planning Target Volume
Footnotes
Conflict of Interest: We have no conflict of interest.
References
- 1.Parsons JT, Mendenhall WM, Stringer SP, et al. Nasal cavity and paranasal sinuses. In: Principles and practice of radiation oncology, Third Edition, Perez CA, Brady LW. (Eds.), Philadelphia, PA: Lippincott-Raven; (1997), pp. 941–959. [Google Scholar]
- 2.Beitler JJ, Wadsworth JT, Hudgins PA, Ang KK. Sinonasal cancer. In: Clinical radiation oncology, Third Edition, Gunderson LL, Tepper JE. (Eds.), Philadelphia, PA: Elsevier Saunders; (2012), pp. 665–690. [Google Scholar]
- 3.Iwata H, Tatewaki K, Inoue M, Yokota N, Sato K, Shibamoto Y. Salvage stereotactic reirradiation using the cyberknife for the local recurrence of nasal or paranasal carcinoma. Radiother Oncol 104, 355–360 (2012). DOI: 10.1016/j.radonc.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Blanco AI, Chao KS, Ozyigit G, Adli M, Thorstad WL, Simpson JR, Spector GJ, Haughey B, Perez CA. Carcinoma of paranasal sinuses: long-term outcomes with radiotherapy. Int J Radiat Oncol Biol Phys 59, 51–58 (2004). DOI: 10.1016/j.ijrobp.2003.09.101. [DOI] [PubMed] [Google Scholar]
- 5.Duthoy W, Boterberg T, Claus F, Ost P, Vakaet L, Bral S, Duprez F, Van Landuyt M, Vermeersch H, De Neve W. Postoperative intensity-modulated radiotherapy in sinonasal carcinoma: clinical results in 39 patients. Cancer 104, 71–82 (2005). DOI: 10.1002/cncr.21100. [DOI] [PubMed] [Google Scholar]
- 6.Chen AM, Daly ME, Bucci MK, Xia P, Akazawa C, Quivey JM, Weinberg V, Garcia J, Lee NY, Kaplan MJ, El-Sayed I, Eisele DW, Fu KK, Phillips TL. Carcinomas of the paranasal sinuses and nasal cavity treated with radiotherapy at a single institution over five decades: are we making improvement? Int J Radiat Oncol Biol Phys 69, 141–147 (2007). DOI: 10.1016/j.ijrobp.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 7.Duprez F, Madani I, Morbée L, Bonte K, Deron P, Domján V, Boterberg T, De Gersem W, De Neve W. IMRT for sinonasal tumors minimizes severe late ocular toxicity and preserves disease control and survival. Int J Radiat Oncol Biol Phys 83, 252–259 (2012). DOI: 10.1016/j.jgo.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Lee DS, Kim YS, Cheon JS, Song JH, Son SH, Jang JS, Kang YN, Kang JH, Jung SL, Yoo IeR, Jang HS. Long-term outcome and toxicity of hypofractionated stereotactic body radiotherapy as a boost treatment for head and neck cancer: the importance of boost volume assessment. Radiat Oncol 7, 85 (2012). DOI: 10.1186/1748-717X-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]