Abstract
The design, synthesis and biological evaluation (anticancer and antimalarial activity) of bis-β-carbolines, based on the structure of the naturally occurring alkaloid neokauluamine, is described.
Keywords: Manzamine alkaloids, structure-activity relationships (SAR), β-carboline heterocycle
Graphical abstract
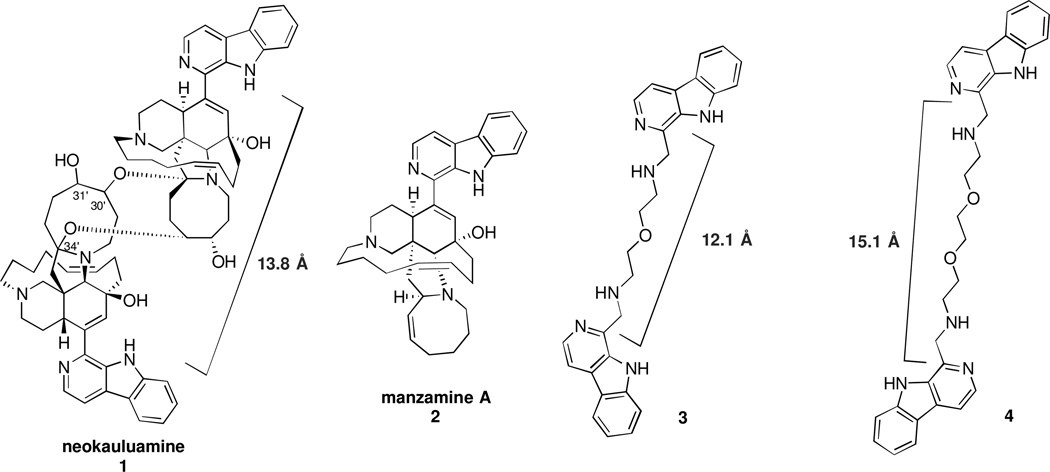
The structurally complex dimeric manzamine alkaloid neokauluamine 1 (Figure), isolated by Hamann and coworkers, exhibits cytotoxicity against human lung and colon carcinoma cells, and is also significantly more active against parasitemia (P. berghei) in mice than either chloroquine or artemesinin.1 Extensive precedent exists for the biological activity of β-carboline containing natural products, including manzamine A, 2.2 Notably, Coldham has recently reported that structurally simplified analogs of 2 containing the β-carboline moiety retain significant biological activity,3 highlighting the importance of the β-carboline moiety for biological potency.
Figure.
Structures of Neokauluamine 1, Manzamine A 2, and Bis-β-Carboline Dimers 3 and 4
The dimeric structure of 1 can be viewed as a complex scaffold for the orientation of two β-carboline units with a ca. 13.8 Å distance. The enormous importance of polyvalent interactions in biology,4 and our recent work with bisaminoquinolines that function as potent inhibitors of autophagy,5 suggested that designed β-carboline dimers with similar distances between the two β-carboline moieties could display important biological activity.6 We describe herein the design, synthesis and initial biological evaluation of such dimers that demonstrate potent anticancer and antimalarial activity.
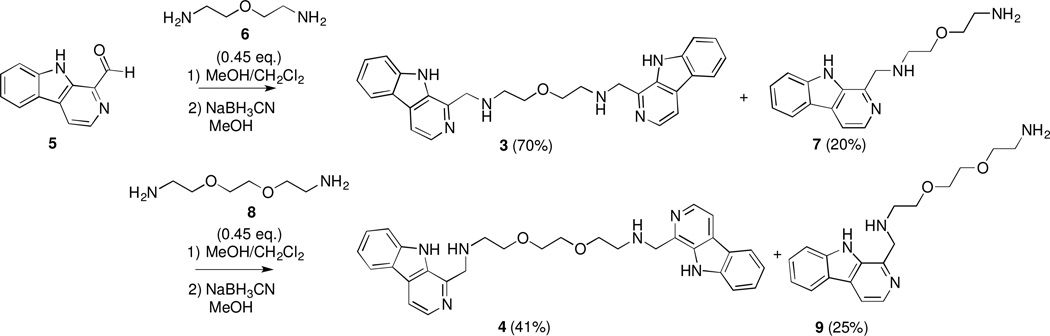
While the published structure of neokauluamine, determined through NMR analysis, did not assign the stereochemistry at C-30’, C-31’, and C-34’, molecular modeling at the level of MMFF (SPARTAN v. 10.0, Wavefunction, Inc.) of each of the possible diastereomers revealed that the distance between the two β-carboline moieties is ca. 13.8 Å for each of the possible diastereomers. We reasoned that replacement of the dimeric manzamine core structure with simple linkers could be used to establish comparable distances between the β-carboline heterocycles. The appropriate linker lengths were established using ChemBio3 Pro 13.0 to calculate the lengths of the extended conformations of two commercially available diamines 6 and 8, the structures of which are shown in the Figure. The β-carboline dimers were prepared by reductive amination7 of linkers 6 and 8 with 1-formyl-β-carboline 5,8 as outlined in the Scheme. Partial conversion to dimers, 39 and 410, respectively, led to the concomitant formation of monomeric β-carbolines 711 and 912, which serve as important control compounds to test the importance of the dimeric structures for biological activity.
Scheme.
Synthesis of Monomeric and Dimeric β-Carboline Analogues
Cytotoxicity assays were performed against two cancer cell lines, H1299 (lung) and A375 (melanoma), for which sensitivity to β-carbolines containing structures has been established by Coldham and others,3 as well as IMR90 (normal lung fibroblast). The results are summarized in Table 1. We find that the dimeric β-carbolines 3 and 4, which are comparable in potency to both neokauluamine 1 and manzamine A 2, are ca. 10× more potent than the monomeric β-carbolines 7 and 9. A significant difference was also observed in the selectivity of these dimeric compounds for cancer vs. non-cancer cells. As indicated in Table 1, the selectivity index (SI) was ca. 8× greater for the dimeric compounds vs. the monomeric ligands against both cell lines.
Table 1.
Cytotoxicity (IC50) and selectivity index (SI) for manzamine A, neokauluamine, and monomeric and dimeric β-carboline analogues against H1299 (Human non-small cell lung carcinoma cell line), A375 (human malignant melanoma) and IMR90 (human Caucasian fetal lung fibroblast).
| H1299 IC50 (µM) |
A375 IC50 (µM) |
IMR90 IC50 (µM) |
SI IMR90/ H1299 |
SI IMR90/ A375 |
|
|---|---|---|---|---|---|
| neokauluamine 1 | 1.7 | - | - | ||
| manzamine A 2 | 1.9 | 4.9 | 79 | 41.2 | 16 |
| 3 | 1.6 | 3 | 200.6 | 123.5 | 67 |
| 4 | 1.8 | 2.2 | 127.7 | 69.1 | 58 |
| 7 | 12.6 | 14.1 | 117.5 | 9.4 | 8 |
| 9 | 14.5 | 24.1 | 181.3 | 12.5 | 8 |
We have further found that dimeric compounds 3 and 4 that we have prepared are comparable in their antimalarial potency to manzamine A 2 against Mycobacterium tuberculosis (H37Rv) and significantly more active than the corresponding monomeric ligands 7 and 9. Similar differences in antibacterial activity were observed between dimeric and monomeric β-carbolines against S. aureus, MRS, E. coli and M intracellulare. The only system that we examined for which this trend did not hold was P. aeruginosa.
In summary, inspired by the unique structure and interesting biological activities of neokauluamine, we have prepared simple dimeric β-carbolines that are significantly more potent against both cancer and malarial cell lines than the corresponding monomeric ligands. Our results are consistent with the importance of multivalency in biological systems. Further studies to understand the bases for these differences and the specificity of the relevant interactions are currently underway in our laboratory and our results will be reported in due course.
Table 2.
Antituberculosis and antimicrobial activities of the monomeric and dimeric β-carbolines
| H37Rv | S. aureus | MRS | E. coli | P. aeruginosa | M. intracellulare | |
|---|---|---|---|---|---|---|
| MIC [µg/ml] | IC50 (µM) | |||||
| 213 | 1.5 | 0.9 | 1.3 | - | - | 0.6 |
| 3 | 1.3 | 1.4 | 1.4 | 4.86 | >20 | 3.15 |
| 4 | 2.3 | 2.02 | 3.74 | 11.28 | >20 | 9.1 |
| 7 | 24.0 | >20 | >20 | >20 | >20 | >20 |
| 9 | 17.8 | >20 | >20 | >20 | >20 | >20 |
Acknowledgments
We (J.D.W and M.M.) gratefully acknowledge the generous financial support of the NIH (P01-CA114046). We would also like to thank the Royal Thai Government for fellowship support (J.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.El Sayed KA, Kelly M, Kara UAK, Ang KKH, Katsuyama I, Dunbar DC, Khan AA, Hamann MT. J. Am. Chem. Soc. 2001;123:1804. doi: 10.1021/ja002073o. [DOI] [PubMed] [Google Scholar]
- 2.Cao R, Peng W, Wang Z, Xu A. Curr. Med. Chem. 2007;14:479. doi: 10.2174/092986707779940998. [DOI] [PubMed] [Google Scholar]
- 3.Boursereau Y, Coldham I. Bioorg. Med. Chem. Lett. 2004;14:5841. doi: 10.1016/j.bmcl.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 4.Vance D, Shah M, Joshi A, Kane RS. Biotechnol. Bioeng. 2008;101:429. doi: 10.1002/bit.22056. [DOI] [PubMed] [Google Scholar]
- 5.McAfee Q, Zhang Z, Samanta A, Levi SM, Ma X-H, Piao S, Lynch JP, Uehara T, Sepulveda AR, Davis LE, Winkler JD, Amaravadi RK. Proc. Natl. Acad. Sci. 2012;109:8253. doi: 10.1073/pnas.1118193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbaras D, Kaiser M, Brun R, Gademann K. Bioorg. Med. Chem. Lett. 2008;18:4413. doi: 10.1016/j.bmcl.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 7.General procedure for the synthesis of monomeric and dimeric β-carbolines. A mixture of 1-formyl-β-carboline 5 (1 eq.) and diamine (0.45 eq.) in a mixture of CH3OH:CH2Cl2 (2:1; 0.1 M) was stirred at ambient temperature for 16 h. The solvent was evaporated under reduced pressure to give the crude Schiff base, which was used directly in the next step without further purification. To a solution of the Schiff base in anhydrous CH3OH (0.1 M) was added NaBH3CN (5 eq.) at 0 °C. The mixture was warmed to ambient temperature with stirring for 16 h and then concentrated under reduced pressure. The residue was dissolved in CH2Cl2 and washed with aqueous Na2CO3. The organic layer was then dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude product was purified by flash chromatography on silica gel (NH4OH:CH3OH:CH2Cl2, 1:10:90) to give the corresponding mono- and dimeric-β-carbolines.
- 8.Skouta R, Hayano M, Shimada K, Stockwell BR. Bioorg. Med. Chem. Lett. 2012;22:5707. doi: 10.1016/j.bmcl.2012.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.1H NMR (500 MHz, CDCl3) of 3: 2.86 (tt,, J = 5 Hz, 4 H), 3.55 (t, J = 5 Hz, 4 H), 4.40 (s, 4 H), 7.21-7.26 (m, 2 H), 7.43-7.51 (m, 4 H), 7.83 (d, J = 5 Hz, 2 H), 8.09 (d, J = 8 Hz, 2 H), 8.34 (d, J = 5 Hz, 2 H). 13C NMR (126 MHz, CDCl3): 48.7,, 54.4, 70.1, 111.6, 113.6 119.5, 121.3, 121.6, 128.1, 128.9, 134.9, 137.8, 140.2, 143.2. FTIR (CHCl3, film, cm−1): 3223, 2893, 1627, 1432, 1325, 1123. HRMS: [M+H]+ calc. 465.2403, found 465.2393.
- 10.1H NMR of 4 (500 MHz, CDCl3): 2.88 (t, J = 5 Hz, 4H), 3.66 - 3.71 (m, 8 H), 4.36 (s, 4 H), 7.22 - 7.26 (m, 2 H), 7.48 - 7.53 (m, 4 H), 7.81 (d, J = 5 Hz, 2 H), 8.09 (d, J = 8 Hz, 2 H), 8.30 (d, J = 5 Hz, 2 H). 13C NMR (126 MHz, CDCl3): 48.7, 54.7, 70.2, 70.3, 111.7, 113.5, 119.5, 121.4, 121.6, 128.0, 128.9, 135.0, 138.0, 140.2, 143.4. FTIR (CHCl3, film, cm−1): 3151, 2887, 1626, 1431, 1241, 1124. HRMS: [M+H] calc. 509.2665, found 509.2662. [M+Na]+ calc. 531.2484, found, 531.2483.
- 11.1H NMR of 7 (500 MHz, CDCl3): 2.84 - 2.93 (m, 4 H), 3.49 (t, J = 5 Hz, 2 H), 3.59 (t, J = 5 Hz, 2 H), 4.42 (s, 2 H), 7.25 (m, 1H), 7.49-7.56 (m, 2H), 7.85 (d, J = 5 Hz, 1H), 8.11 (d, J = 8 Hz, 1H), 8.33 (d, J = 6 Hz, 1H). 13C NMR (126 MHz, CDCl3): 41.5,, 48.8,, 54.4,, 70.0,, 72.6,, 111.7, 113.6, 119.5, 121.3, 121.6, 128.1, 128.9, 134.9, 137.9, 140.3, 143.3. FTIR (CHCl3, film, cm−1): 3159, 1627, 1568, 1325, 1121. HRMS: [M+H]+ calc. 285.1715, found 285.1714.
- 12.1H NMR of 9 (500 MHz, CDCl3): 2.85 (t, J = 5 Hz, 2 H), 2.92 (t, J = 5 Hz, 2 H), 3.52 (t, J = 5 Hz, 2 H), 3.61 - 3.72 (m, 6 H), 4.43 (s, 2 H), 7.22 - 7.27 (m, 1 H), 7.49 - 7.57 (m, 2 H), 7.85 (d, J = 5 Hz, 1H), 8.12 (d, J = 8 Hz, 1 H), 8.33 (d, J = 5 Hz, 1 H). 13C NMR (126 MHz, CDCl3): 41.6, 48.7, 54.6, 70.2, 70.3, 70.5, 73.1, 111.7, 113.5, 119.5, 121.4, 121.6, 128.0, 128.8, 134.9, 137.9, 140.0, 143.5. FTIR (CHCl3, thin film, cm−1): 2920, 1626, 1567, 1456, 1122 cm−1. HRMS: [M+H] calc. 329.1978, found 329.1981. [M-H]− calc. 327.1821, found 327.1824. [M+Na]+ calc. 351.1797, found 351.1786.
- 13.Rao KV, Kasanah N, Wahyuono S, Tekwani BL, Schinazi RF, Hamann MT. J. Nat. Prod. 2004;67:1314. doi: 10.1021/np0400095. [DOI] [PMC free article] [PubMed] [Google Scholar]