Abstract
As new methods for producing and isolating human glial progenitor cells (hGPCs) have been developed, the disorders of myelin have become especially compelling targets for cell-based therapy. Yet as animal modeling of glial progenitor cell-based therapies has progressed, it has become clear that transplanted hGPCs not only engraft and expand within murine hosts, but dynamically outcompete the resident progenitors so as to ultimately dominate the host brain. The engrafted human progenitor cells proceed to generate parenchymal astrocytes, and when faced with a hypomyelinated environment, oligodendrocytes as well. As a result, the recipient brains may become inexorably humanized with regards to their resident glial populations, yielding human glial chimeric mouse brains. These brains provide us a fundamentally new tool by which to assess the species-specific attributes of glia in modulating human cognition and information processing. In addition, the cellular humanization of these brains permits their use in studying glial infectious and inflammatory disorders unique to humans, and the effects of those disorders on the glial contributions to cognition. Perhaps most intriguingly, by pairing our ability to construct human glial chimeras with the production of patient-specific hGPCs derived from pluripotential stem cells, we may now establish mice in which a substantial proportion of resident glia are both human and disease-derived. These mice in particular may provide us new opportunities for studying the human-specific contributions of glia to human psychopathology, as well as to higher cognition. As such, the assessment of human glial chimeric mice may provide us new insight into the species-specific contributions of glia to human cognitive evolution, as well as to the pathogenesis of human neurological and neuropsychiatric disease.
Keywords: glial progenitor, oligodendrocytic progenitor, neural stem cell, cell transplant, mouse models
Over the past two decades, a number of groups have serially explored the use of glial progenitor cells as engraftable cellular therapeutics for the treatment of glial disorders, in particular those characterized by oligodendrocyte loss and consequent demyelination (reviewed in (Goldman et al. 2012)). The intended therapeutic targets of this approach are broad, and include pediatric disorders of myelin formation and maintenance, such as the hereditary leukodystrophies and lysosomal storage disorders(Goldman 2011), as well as the adult-onset disorders of myelin integrity, such as multiple sclerosis and the age-related white matter diseases of vascular insufficiency(Ben-Hur and Goldman 2008). With these therapeutic purposes in mind, a variety of increasingly refined methods have been established for isolating human glial progenitor cells (hGPCs), and for modeling their behavior and instructing their fates, both in vitro and in vivo, the latter via transplantation-based strategies.
Like their rodent counterparts (Raff et al. 1983), human GPCs may generate both astrocytes and oligodendrocytes (Windrem et al. 2004), and in some contexts neurons as well (Belachew et al. 2003; Nunes et al. 2003); yet despite their multilineage competence, they have been most often referred to as oligodendrocyte progenitor cells, and also as NG2 cells, the latter designation based on their expression of the NG2 chondroitin sulfate proteoglycan (Nishiyama et al. 2009; Nishiyama et al. 1996). Human GPCs – the term we will use throughout this report – were first isolated from the adult brain using fluorescence activated sorting, based initially on oligodendroglial transcription from the CNP promoter(Roy et al. 1999), and later on the expression of lineage-restricted surface epitopes (Windrem et al. 2002). These studies revealed that hGPCs could be reliably and efficiently isolated from human brain tissue based on positive selection for glial gangliosides recognized by the A2B5 antibody, with concurrent negative selection by depletion of PSA-NCAM-defined neuroblasts (Nunes et al. 2003). Later studies established that the cells may be isolated on the basis of the CD140a-defined ectodomain of the PDGFα receptor, which includes all of the potentially oligoneogenic cells of the human brain (Sim et al. 2011), while progressively more oligodendrocyte-biased phenotypes may be selected on the basis of concurrent expression of the CD9 tetraspanin, or of the oligodendrocytic sulfatide recognized by the O4 antibody (Douvaras et al. 2014; Piao et al. 2015). Whether derived from adult or fetal human brain, each of these phenotypes has now proven efficient, in a variety of laboratories and model systems, in remyelinating structurally-demyelinated or hypomyelinated tissue.
Human GPCs may be transplanted to restore lost myelin
Beginning with pioneering studies by Gumpel and Lachapelle, and independently by Blakemore and colleagues, over 25 years ago (Blakemore et al. 1990; Gumpel et al. 1987; Lachapelle et al. 1983), the ability of transplanted oligodendroglia to effect myelin repair has been studied in a number of model systems, using a number of cellular sources (reviewed in (Ben-Hur and Goldman 2008; Franklin and ffrench-Constant 2008)). Efforts focusing on the specific use of glial progenitor cells rather than their derived oligodendrocytes, and emphasizing the use of human GPCs in particular, are more recent(Windrem et al. 2004; Windrem et al. 2002; Windrem et al. 2008). These studies have been performed in several models of both congenital and acquired dysmyelination, but we will discuss data obtained in only one of these models - arguably the most informative among them for assessing the myelinogenic competence of candidate GPCs - the shiverer mouse. Shiverer (MBP shi/shi) is a congenitally hypomyelinated mouse deficient in myelin basic protein (Popko et al. 1987; Readhead et al. 1987). Using immunodeficient shiverer mice as hosts (rag2−/− x MBP shi/shi) (Windrem et al. 2004), we developed a multi-site delivery procedure by which donor GPCs could be introduced into the major presumptive white matter tracts of newborn mice, allowing the broad dispersal of human donor cells throughout the recipient CNS (Windrem et al. 2008). This approach resulted in widespread donor cell engraftment throughout the brain and spinal cord, with infiltration of the forebrain, brainstem and cerebellum, and ultimately the spinal cord and roots (Figure 1). The donor hGPCs exhibited highly efficient oligodendrocyte differentiation and functional myelination in these hypomyelinated mice, with the dense and efficient myelination of congenitally unmyelinated white matter tracts throughout the CNS. This in turn was associated with the substantially prolonged survival of these mice, with frank rescue and phenotypic recovery of a large minority(Windrem et al. 2008). Indeed, whereas untreated shiverers invariably die by 20–21 weeks of age, a fraction of neonatally-engrafted mice achieved normal lifespans of over 2 years, suggesting the potential power of this approach towards remyelinating dysmyelinated brain tissue (Figure 1).
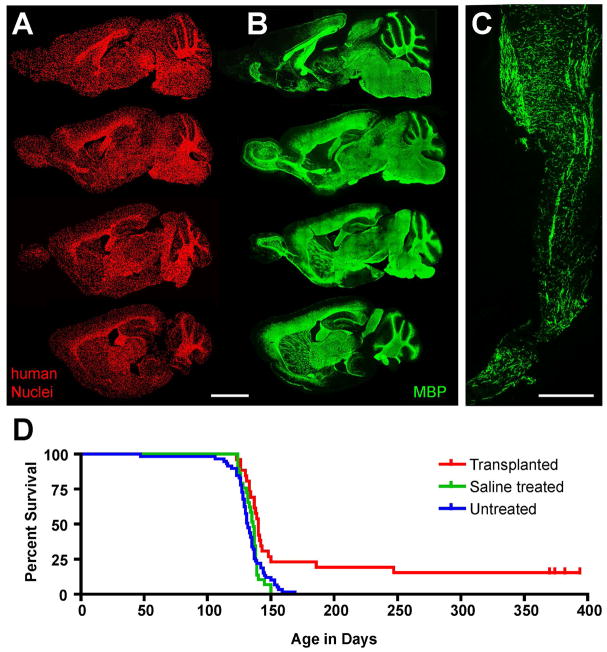
Figure 1. Human glial progenitors can colonize and fully myelinate the hypomyelinated mouse CNS.
A–B. Sagittal images of an immmunodeficient shiverer mouse brain (MBP shi/shi x rag2−/−), sacrificed at 1 year after neonatal transplant of hGPCs. Each image in A and B represents a montage of 50 to 100 individual 10x photomicrographs; each series begins 750 μm lateral to the midline and continues at 600 μm intervals. A. Human donor cells, immunolabeled in 14 μm cryosections using an anti-human nuclear antibody (hN; red). B Myelin basic protein (MBP; green) in sections adjacent or nearly so to their matched sections in A. C. Donor-derived MBP (green) in the spinal cord at 1 year, at the level of the conus medullaris. D shows a Kaplan-Meier plot of the effect of neonatal hGPC engraftment upon the survival of these mice. Shiverer x rag2−/− mice were either engrafted at birth with hGPCs (n=26, red), injected with saline (n = 29, green), or not treated (n=59, blue). Whereas all control mice died between 18 and 21 weeks of age, a fraction of engrafted mice lived substantially longer than any controls; some were frankly rescued, with concomitant recovery of neurological phenotype as well. The experiment was terminated at 13 months, and rescued mice were sacrificed for observation through 2 years of age. Scale: 200 μm.
A, B and D from (Windrem et al. 2008).
In the course of these studies, we noticed two unexpected outcomes. First, that hGPCs mature in a highly context-dependent fashion, such that those donor cells that engraft presumptive white matter develop as myelinogenic oligodendrocytes and fibrous astrocytes, while those cells invading cortical and subcortical gray matter that differentiate do so as astrocytes(Windrem et al. 2004). These observations recall earlier studies of context-dependent differentiation by murine neural stem cells (NSCs) transplanted into shiverers, which typically differentiated as oligodendroglia in the white matter, and as astrocytes in the gray matter (Mitome et al. 2001; Yandava et al. 1999). Just as in those studies, the phenotypic malleability of hGPCs by the local environment is profound, so much so that the distribution as well as the differentiation of hGPCs differs depending upon whether the cells are transplanted into normally-myelinated or congenitally-hypomyelinated recipients: in myelin wild-types, transplanted hGPCs infiltrate in a relatively uniform fashion in both the gray and white matter, ultimately differentiating as astrocytes or remaining as resident glial progenitors. In contrast, hGPCs transplanted into shiverer mice first preferentially expand in the callosal and capsular white matter, giving rise therein to new oligodendrocytes as well as astrocytes and daughter hGPCs (Figure 2).
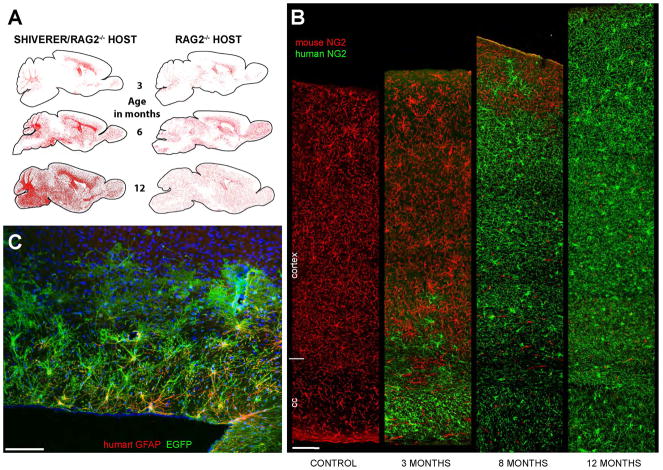
Figure 2. Human GPCs out-compete and ultimately replace resident mouse GPCs.
A. Human glial progenitor cells (hGPCs), neonatally transplanted into either congenitally hypomyelinated shiverer x rag2−/− (left column) or normally myelinated rag2−/− (right column) mouse brain, disperse and expand broadly throughout the brain as a function of age. hGPCs reach higher density in white matter than gray matter of the hypomyelinated shiverer (left), in contrast to their relatively uniform distribution in normally myelinated brain (right). Red dots indicate individual human donor GPCs, as labeled by human nuclear antigen (left and right columns). B. Progressive replacement of host mouse GPCs, by donor hGPCs, as identified by species-specific antibodies against NG2, compared to an unengrafted control mouse (left-most column). By one year, hGPCs have replaced mouse GPCs throughout the entire depth of the cortex. C. EGFP-tagged human astrocytes in the corpus callosum, cortex, and fimbria in a 2-year old rag2−/− mouse. The hGPCs were labeled with an EGFP-expressing lentivirus (green) in vitro, prior to transplantation. Dorsal is toward the top and rostral to the left in this sagittal section. (EGFP, green; human-specific GFAP, red). Scale: 100 μm.
A and B, from (Windrem et al. 2014).
Second, and even more stunning, the implanted human cells not only integrate into the recipient brains, but over time predominate; they first displace and then ultimately replace the resident glial populations of their hosts. In both shiverers and myelin wild-type recipients, colonization of the cortical and subcortical gray by migrating hGPCs is followed first by their selective expansion, and then by the inexorable displacement and in situ death of resident murine glial progenitors (Windrem et al. 2014). As a result of this apparent competition between resident mouse GPCs and the invading human GPCs, by a year of age the donor hGPCs are typically distributed in a relatively uniform manner throughout the forebrain white and gray matter, while mouse GPCs are scarce, and often absent from the now human glial-dominant forebrains.
Human donor glial progenitors dominate the competition
It bears emphasizing that the competitive advantage of human over murine glial progenitors is evident in wild-type as well as in shiverer mice; in both recipient environments, the human glial progenitors typically out-competed their murine counterparts. The selective expansion of the human glial population in the mouse appears at least in part to be a product of the more sustained proliferation of the transplanted human OPCs, which appear to retain cell-autonomous regulation of expansion, and expand in numbers by over 40-fold in the corpus callosum alone over the first year of life. Yet the competitive advantage of human GPCs on the mouse environment is clearly based on more than preferential expansion, since the murine GPCs are completely replaced by their human counterparts over time. Rather, the process of hGPC colonization seems overtly competitive. Human GPCs typically expand outwards from their periventricular and callosal points of introduction, in advancing waves that seem to repulse resident murine progenitors, which then die, both in situ and upon retreat to the cortical surface (Figure 2). The human GPCs ultimately attain a relatively uniform distribution, achieving apparent contact inhibition in a manner analogous to that reported developmentally by Bergles and colleagues (Hughes et al. 2013). While the molecular basis for the competitive dominance of the human GPCs is unknown, several recent studies have identified differential expression of both MYC and hedgehog-dependent pathways as contributing to clonal dominance during early ontogeny (Amoyel and Bach 2014; Amoyel et al. 2014; Claveria et al. 2013). Further assessment of differential gene expression by mouse and human GPCs in vivo may permit the discovery of similar regulators of competition that favor the relative dominance of human over mouse GPCs when the two are in direct competition, a decidedly unnatural situation that might nonetheless provide us important insights into the differential expansion of favored glial cell populations in the developing human brain, and the signals that determine which among competing populations is ultimately dominant.
Regardless of its molecular basis, the domination of the host brains by human GPCs leads to the slow but inexorable glial humanization of these brains, as mature astrocytes undergo presumably normal turnover in adulthood, with astrocytic replacement from now-humanized resident progenitor pools. This process results in the substantial astrocytic humanization of these rodent brains, first by fibrous astrocytes of the white matter, and then by protoplasmic astroglia of both cortical and subcortical gray matter (Windrem et al. 2008) (Figure 2). In both shiverer and myelin wild-type hosts, the proportion of human astrocytes thus increases monotonically as a function of time, and is matched by a corresponding decrease in the proportion of mouse astrocytes. Over time, these brains thus become chimeric for human astrocytes, as well as for human glial progenitors, and in shiverer mice, this process includes replacement of host oligodendrocytes by their human counterparts as well. As a result, in one-year old shiverer mice neonatally engrafted with human GPCs, essentially all resident glial progenitor cells, all oligodendrocytes, and large proportions of all astrocytes are of human origin (Figure 2).
Interestingly, the slow astrocytic colonization of hGPC-engrafted an chimeric brains provided a revelation of its own, in that it suggests that adult human astrocytes may differ from their murine counterparts in their origin, as well as in their functional repertoires. Human glial chimeras preterminally tagged with the mitotic marker BrdU revealed that while new human astrocytes are continuously recruited to these brains from engrafted human GPCs, astrocytic production from murine progenitors is essentially nil; Windrem et al identified no mouse GFAP+ cells that incorporated BrdU, at either 4 or 8 months of age. This observation suggests a fundamental distinction in the origin of new astroglia in the brains of adult rodents and humans; whereas in mice resident astrocytes have been reported as the principal source of new astrocytes in adulthood (Ge et al. 2012), with GPCs serving principally as oligodendrocyte progenitors (Bu et al. 2004; Kang et al. 2010; Nishiyama et al. 2009; Tripathi et al. 2010), human glial progenitors are notably bipotential for both oligodendrocytes and astrocytes in the adult brain (Nunes et al. 2003; Sim et al. 2011; Sim et al. 2009). Such species-specific differences in the in vivo differentiation and fate potential of GPCs are not self-evident, and their identification – and by inference our broadened understanding and ability to model astrocytic turnover in the adult human brain - is a product of our now being able to study human glia in vivo, in real-time.
Human glia maintain uniquely hominid features in chimeras
Human astrocytes are larger and more complex than those of rodents; human cortical astrocytes can exhibit over triple the diameter, and 10-fold the number of terminal processes, as rodent astrocytes (Oberheim et al. 2006; Oberheim et al. 2009). Even more intriguing, human astrocytes have both structural features and functional competencies unique to hominids, and exhibit a range of astrocytic pleomorphism without precedent in infraprimate mammals. On that basis, we have postulated that the functional roles of glia have expanded during evolution, and especially so with the appearance of hominids (Oberheim et al. 2006; Oberheim et al. 2009). These evolutionary changes are of particular interest because astrocytes have been shown to play vital roles in information processing within the CNS (Araque et al. 1999; Kang et al. 1998). Astrocytes are required for synaptogenesis and maintenance of synaptic density (Ullian et al. 2001), and a number of specific astrocytic modulators of synaptic plasticity have been identified, including the glypicans (Allen et al. 2012) and TNFα (Stellwagen and Malenka 2006), among others. Importantly, these ligands may be differentially expressed by human astroglia, potentially offering advantages to human astroglia in the regulation of synaptic plasticity, relative to infraprimate glia (Han et al. 2013; Oberheim et al. 2009).
On the basis of these observations, we asked if the greater structural complexity of human astrocytes relative to those of rodents might be accompanied by functional differences. In particular, we asked whether human glial chimeric mouse brains, with their substantial colonization by human astroglia and their progenitors, might manifest functional distinctions from wild-type mice, and if so whether these functional differences might reflect aspects of human cognitive evolution (Han et al. 2013). This possibility was anticipated by the observation that human astrocytes propagate Ca2+ waves significantly more rapidly than rodents (Oberheim et al. 2009). To define the human-selective contributions of astrocytic complexity to network function, we therefore assessed the behavior of human glial chimeras to both matched unengrafted and allografted mice. To that end, hGPCs, pre-biased in vitro to astrocytic phenotype, were transplanted into neonatal immunodeficient mice, thereby establishing human glial chimeras with especially large complements of human astrocytes as well as GPCs. By 7–10 months of age, the majority of all forebrain OPCs and astrocytes in these mice were typically of human origin (Figure 2). The engrafted human glia appeared to mature in a cell-autonomous fashion, in that the diameter, domain size and morphology of human astrocytes in the chimeric mouse brain each approximated that of astrocytes in the normal adult human brain.
Human GPC chimeric mice as systems for assessing human glial function
Since human glia appeared to maintain the cell-autonomous size, complexity and domain architecture of human astrocytes in the mouse environment, we asked if human glial chimeric brains exhibited increased synaptic plasticity. To that end, Han and colleagues(Han et al. 2013) compared the threshold for inducing hippocampal long-term potentiation (LTP) in chimeric mice with that of littermate controls. We found that high frequency stimulation significantly potentiated the field EPSP slopes of neurons in the chimeric hippocampi, and indeed did so to a larger degree and for a substantially longer period, than in unengrafted littermate controls. Thus, human glial chimeras manifested substantially facilitated LTP (Figure 3). Importantly, such stable, long-lasting changes in synaptic function are thought to be involved in learning and memory, of which LTP is typically considered an in vitro surrogate. Since hippocampal LTP was enhanced in chimeric mice, we next asked whether the human glial chimeras might have cognitive advantages over their unengrafted or mouse GPC-allografted counterparts. We found that the human glial chimeras indeed performed better than control mice across a variety of learning tasks, that included auditory fear conditioning, novel object and place recognition, and Barnes maze navigation (Figure 3). In all of these tests - but not in any test of social interactivity or primary perception - the human glial chimeras performed better and acquired new causal associations more quickly than did murine-allografted or untransplanted controls (Han et al. 2013). In short, they learned more rapidly, and at least along the axes of the tests performed, could be defined - if colloquially so - as smarter. As such, these glial chimeras may provide us a viable - if provocative - model by which to evaluate the species-specific contributions of human glia to human cognition.
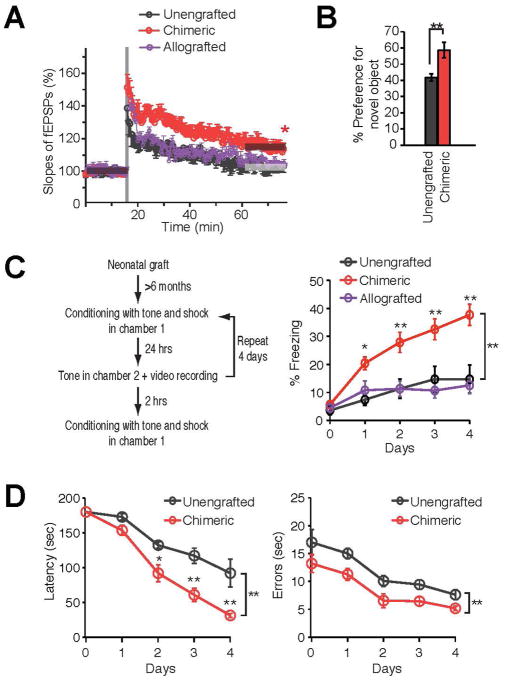
Figure 3. Human glial chimeras manifest enhanced long-term potentiation and learn more rapidly.
A. Induction of LTP by two trains of high-frequency stimulation (each train consisted of 100 pulses at 100 Hz, with 30 s between bursts) in human chimeric mice, but not in unengrafted littermates or mice allografted with conspecific mouse GPCs (n=7 mice/group, *p<0.05; t-test compares fEPSP slopes before and 60 min after stimulation, for each group). B. Object-Location Memory Task (OLT) in chimeric mice and their unengrafted rag1 null littermate controls demonstrated a learning advantage in chimeric mice, via enhanced recognition of and preference for the novel displaced object (n=7, **p<0.01, one-way ANOVA). C. Auditory fear conditioning assessed in a cohort of human glial chimeric, mouse allografted, and unengrafted rag2−/− control mice. The human glial chimeric mice exhibit prolonged freezing behavior in test chamber 2 during exposure to the tonal conditioned stimulus, when compared to their unengrafted mice and allografted controls (n=5–20, *p<.05, **p<0.01, 2-way repeated-measures ANOVA with Bonferroni t-tests). D. Barnes maze testing in chimeric and unengrafted immunodeficient littermate controls. Chimeric mice demonstrated a significant learning advantage, as reflected in a shorted latency and fewer errors in solving the maze (n=6, *p<0.05, **p,0.01, 2-way ANOVA with Bonferroni t-tests).
From (Han et al. 2013).
hiPSC-derived glial chimeras as models of human genetic and inflammatory CNS disease
The studies described thus far used hGPCs derived from human brain tissue, of both adult and fetal origin. Yet a number of groups have reported protocols by which GPCs, and their derived astrocytes and oligodendrocytes, might be alternatively derived from pluripotential stem cells (Hu et al. 2009; Izrael et al. 2007; Wang et al. 2013). These protocols have improved over the past 2 years in both efficiency and speed, so that bipotential oligodendrocyte-astrocyte progenitors may be produced in quantity from pluripotential cells in less than three months, while myelinating oligodendroglia may be produced within four (Douvaras et al. 2014; Piao et al. 2015; Stacpoole et al. 2013). The cells produced by these protocols are at least as efficient at myelinogenesis in vivo as their tissue-derived counterparts, myelinating most axons within the hypomyelinated shiverer forebrain within several months after neonatal transplant (Figure 4), and rescuing at least a fraction of neonatally-engrafted shiverer mice from otherwise certain death (Wang et al. 2013). Yet while these hGPC differentiation protocols were developed so as to scale up production of hGPCs to clinically-useful levels, the ramifications of this capability on our understanding of the role of glia in brain function may prove profound. In particular, our ability to generate glia from human embryonic stem cells and induced pluripotential cells allows us to establish human glial chimeras using glia produced from individual patients, on a disease-specific basis, and to use those chimeric mice to assess the specific pathology of the disease-derived glia, as well as their broader contributions to disease pathogenesis. By way of early example, Fossatti and colleagues produced hGPCs from iPSCs derived from patients with primary progressive multiple sclerosis (PPMS), prepared and separated the oligodendrocyte-restricted daughter cells from these cultures, and then transplanted the immature oligodendroglia into shiverer mice, in which the cells differentiated as myelinogenic cells that ensheathed resident axons (Douvaras et al. 2014; Fossati and Douvaras 2014). While the analysis was too early to identify any stigmata of PPMS, the authors were able to report success in the establishment of mice chimerized with PPMS-derived oligodendroglia. Although it remains unclear whether such mice will prove informative in the study of MS, it seems likely that mice chimerized with hGPCs derived from patients suffering from a broad variety of hereditary myelin disorders will ultimately prove informative in defining the respective roles of those glia in disease pathogenesis.
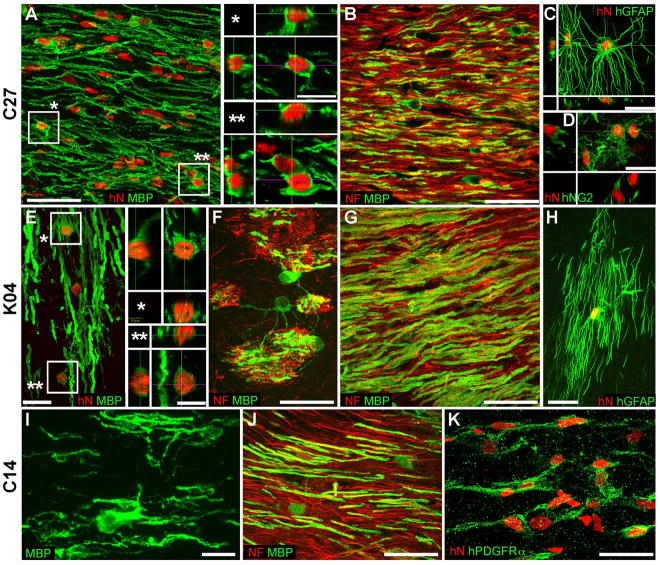
Figure 4. Neonatal engraftment with hiPSC GPCs produces patient-specific human glial chimeras.
Confocal images of the callosal and capsular white matter of shiverer mice neonatally engrafted with hiPSC OPCs produced from hiPSCs derived from 3 independent patient-derived lines, designated C27 (A–D); K04 (E–H); and C14 (I–K). A, G, and J show abundant, donor-derived myelin basic protein expression (MBP, green) by C27, K04, and C14 hiPSC hGPCs (hNA, red), respectively. Representative z stacks of individual hNA+ oligodendroglia are shown as asterisks in A and E. By 19 weeks, hiPSC oligodendroglia derived from all 3 lines (B, C27; E–F, K04; J, C14) robustly myelinated axons (neurofilament, red). hiPSC-derived oligodendroglial morphologies are exemplified in panels F (K04) and I (C14); F in particular shows multi-axon myelination by single oligodendroglia in the striatum. hiPSC hGPCs also generated astroglia (C, C27; H, K04), which exhibited the complex fibrous morphologies typical of human astrocytes (human-specific GFAP, green). Many cells also remained as progenitors, immunostaining for NG2 (D, C27) or PDGFαR (K, C14). Scale: 50 μm (A–C, G, J); 20 μm (C–F, H, K); and 10 μm (I, insets to A and E).
From (Wang et al. 2013).
Human glial chimeras as hosts for infections unique to the human brain
Human glial chimeras may have applications beyond that of modeling the species-specific role of human glia in neurological function and dysfunction. In particular, these mice may permit us to better assess the biology of human-selective infectious and inflammatory diseases of the CNS. For instance, a number of pathogenic viruses of the human brain are gliotrophic, and some are specifically human in their species-selectivity. Viruses such as the JCV polyomavirus, the cause of progressive multifocal leukoencephalopathy (PML), and human herpesvirtus-6, another prominent viral encephalitis of immunocompromised individuals, have never been amenable to experimental study in vivo, since these are human glial-specific pathogens(Haley and Atwood 2014). On that basis, we asked whether human glial chimerization might permit these animals to be infected with human gliotrophic viruses in vivo, and if so whether these infected human glial chimeras might develop pathology replicating that of humans. By neonatally engrafting immunodeficient myelin-deficient rag2−/− x shi/shi mice with hGPCs, we established mice with a fully humanized white matter, which were then infected by intracerebral injection of JCV (Kondo et al. 2014). Within several weeks thereafter, the JCV-infected mice manifested oligodendrocytic loss with demyelination and astroglial proliferation, replicating thereby the cardinal features of human PML (Figure 5).
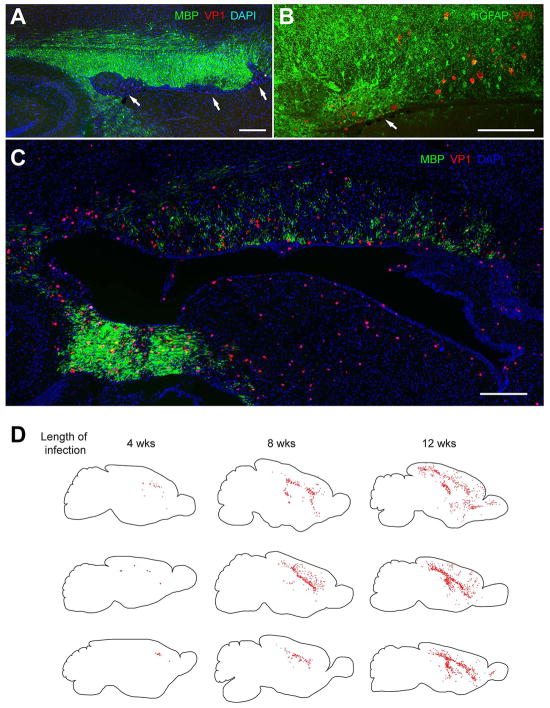
Figure 5. Human glial chimeric mice can develop a prototypic human viral encephalitis.
JCV infection and spread in vivo was tracked by immunostaining human glial-engrafted shiverer brains for the late viral VP1 antigen, as a function of time after infection. In these chimeric shiverers, essentially all myelinating oligodendrocytes, as well as most GPCs and large numbers of astrocytes, are human. A–B, By 4 weeks after infection by the polyomavirus JCV, focal regions of demyelination (A, arrows) and infection-associated astrogliosis (B, arrow) are noted in the forebrain white matter of human glial chimeric mice, typically abutting the callosal wall of the lateral ventricle. C, By 11 weeks after infection, diffuse hypomyelination of the corpus callosa and capsules of infected chimeric mice was noted. D, Sagittal sections of 3 different infected chimeras at each of 3 time-points are shown; individual VP1+ cells are dot-mapped onto the schematic. VP1+ human cells became progressively more widespread with time, with JCV infection progressing from the site of viral injection to include much of the forebrain white matter by 12 weeks post-infection, with marked cortical spread by that point as well. Scale: 200 μm (A, C); 100 μm (B).
From (Kondo et al. 2014).
We further noted that in the JCV-infected human glial chimeras, that hGPCs and astrocytes were infected more rapidly than were oligodendrocytes, and viral replication was noted primarily in astrocytes and hGPCs rather than in oligodendrocytes, which instead exhibited viral T antigen-associated apoptotic death. By establishing human glial chimeras in wild-type rather than hypomyelinated hosts - which produced mice colonized with human astrocytes and GPCs but not oligodendrocytes - we then established that human astroglia were sufficient for JCV spread, and that astrocytes, rather than oligodendrocytes, were the principal targets for, and reservoir of, infection in vivo (Kondo et al. 2014).
This study established the utility of glial chimeras for studying the biology of human gliotrophic viruses in vivo, and promises to be the first of many using human glial-transplanted animals to investigate human-specific brain infections in vivo. Perhaps most importantly though, the work also revealed the limits of our knowledge of these human gliotropic encephalitides, and hence the great promise afforded by human glial chimeras for both mechanistic discovery and therapeutic modeling of these previously enigmatic and still-lethal disorders.
The future
The availability of human glial chimeric mice allow us to now assess the natural history, network contributions, and pathophysiology of human glia in vivo, in the context of the normal adult brain (Goldman and Windrem 2009; Windrem et al. 2008). This model lends itself to the study of a number of questions never before approachable, for want of an appropriate in vivo model of human glial function. First, we have only begun to consider the species-specific influences of human glia upon neural network function. Astrocytes clearly play a central role in synaptic efficiency and plasticity in mammals, and human astrocytes, with their greater fiber complexity than that of infraprimate mammals, coordinate the excitability of many more synapses within their individual geographic domains (Oberheim et al. 2006; Oberheim et al. 2009) (Araque et al. 1999; Kang et al. 1998). Hominid evolution has thus been attended by progressive astrocytic complexity, which may in turn have contributed to the increased rate and efficiency of information processing in the primate brain. As such, do the unique capabilities of the human brain thereby reflect the evolution of human-specific astrocytic or glial progenitor functions? Is glial specialization the basis for human cognitive evolution? The high degree of human glial chimerization of these brains should permit us to address these questions, by rigorously evaluating the in vivo contributions of both human astrocytes and their progenitor cells to neural network activity, and hence their respective roles in human cognition.
Just as human glial chimeras may prove useful models by which to assess the role of glia in human cognitive processing, our ability to construct those mice with glia generated from human embryonic stem cells and induced pluripotential cells allows us to now establish human glial chimeras mice with glia derived from single patients (Figure 4), and from defined disease genotypes and phenotypes. That capability promises to significantly advance our understanding of the contributions of glia to the pathogenesis of a wide variety of neurological disorders, many of which have hitherto simply been assumed to be entirely neuronal in nature. For instance, the derivation of human GPCs from embryonic stem cells or induced pluripotential cells carrying the polyglutamine repeat expansion of Huntington disease (HD), and the establishment of human glial chimeras using those huntingtin mutant hGPCs, may permit us to define the specific contributions of glia to the neuropathology and clinical phenotype of HD. Similarly, our understanding and therapeutic modeling of disorders such as amyotrophic lateral sclerosis (ALS), already reported to derive in part from astrocytic pathology (Di Giorgio et al. 2007; Meyer et al. 2014; Yamanaka et al. 2008), may be substantially refined by comparing the in vivo phenotypes of mice engrafted with hGPCs generated from patients with different ALS-associated mutations.
Taking this approach further, we may anticipate the construction of human glial chimeras using hGPCs produced from iPSCs of patients with hereditary neuropsychiatric conditions, and the use of these mice to define and isolate the contribution of glia to these most enigmatic of brain disorders. Indeed, the phylogenetic appearance of one of the major neuropsychiatric disorders of humans, schizophrenia, seems roughly concurrent with the evolution of morphological complexity by human astroglia (Horrobin 1998; Oberheim et al. 2009) More broadly, this approach may be of particular value in assessing the role of glia in those neurological disorders that are fundamentally unique to humans, including not only schizophrenia, but also bipolar disease and autism spectrum disorders, whose phylogenetic appearances parallel that of human astrocytic evolution. By way of example, human glial chimeric mice established with hGPCs derived from schizophrenic patient-derived iPSCs may pose a useful, and potentially intriguing, model for assessing the specific contributions of human astroglia to schizophrenic behavioral and cognitive pathology. In broader terms, such disease-specific iPSC-derived human glial chimeras may permit us to better define the role of glial dysfunction in a broad swath of neurodegenerative and neuropsychiatric disorders, in which the relative contributions of glial pathology remain unclear, and for which the corrective targeting of glial dysfunction may prove a highly promising therapeutic opportunity.
Main points.
Human glial progenitor cells (hGPCs) injected into newborn mice replace host glia, yielding glial chimeras
Human glial chimeric mice are faster learners, suggesting a glial role in cognitive evolution
Patient-derived iPSC hGPC chimeras can model glial contributions to neurological disease
Acknowledgments
Supported by the Mathers Charitable Foundation, NINDS, NIMH, the National Multiple Sclerosis Society, the Adelson Medical Research Foundation, the New York Stem Cell Research Program (NYSTEM), and the Novo Nordisk Foundation.
Footnotes
Commercial interest: Drs. Goldman and Windrem have a patent on chimeric mouse models, US patent no. US7524491B2; the patent is owned by the University of Rochester, and the authors receive no income from it.
Citations
- Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, Barres BA. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012;486:410–4. doi: 10.1038/nature11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoyel M, Bach EA. Cell competition: how to eliminate your neighbours. Development. 2014;141:988–1000. doi: 10.1242/dev.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoyel M, Simons BD, Bach EA. Neutral competition of stem cells is skewed by proliferative changes downstream of Hh and Hpo. EMBO J. 2014 doi: 10.15252/embj.201387500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–15. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. Journal of Cell Biology. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hur T, Goldman SA. Prospects of cell therapy for disorders of myelin. Ann N Y Acad Sci. 2008;1142:218–49. doi: 10.1196/annals.1444.014. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Crang AJ, Franklin RJ. Transplantation of glial cell cultures into areas of demyelination in the adult CNS. Prog Brain Res. 1990;82:225–32. doi: 10.1016/s0079-6123(08)62608-4. [DOI] [PubMed] [Google Scholar]
- Bu J, Banki A, Wu Q, Nishiyama A. Increased NG2(+) glial cell proliferation and oligodendrocyte generation in the hypomyelinating mutant shiverer. Glia. 2004;48:51–63. doi: 10.1002/glia.20055. [DOI] [PubMed] [Google Scholar]
- Claveria C, Giovinazzo G, Sierra R, Torres M. Myc-driven endogenous cell competition in the early mammalian embryo. Nature. 2013;500:39–44. doi: 10.1038/nature12389. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–14. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douvaras P, Wang J, Zimmer M, Hanchuk S, O’Bara MA, Sadiq S, Sim FJ, Goldman J, Fossati V. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Reports. 2014;3:250–9. doi: 10.1016/j.stemcr.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati V, Douvaras P. Generating induced pluripotent stem cells for multiple sclerosis therapy. Regen Med. 2014;9:709–11. doi: 10.2217/rme.14.63. [DOI] [PubMed] [Google Scholar]
- Franklin RJM, ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Ge WP, Miyawaki A, Gage FH, Jan YN, Jan LY. Local generation of glia is a major astrocyte source in postnatal cortex. Nature. 2012;484:376–80. doi: 10.1038/nature10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA. Progenitor cell-based treatment of the pediatric myelin disorders. Arch Neurol. 2011;68:848–56. doi: 10.1001/archneurol.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Nedergaard M, Windrem MS. Glial progenitor cell-based treatment and modeling of neurological disease. Science. 2012;338:491–5. doi: 10.1126/science.1218071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Windrem MS. Non-human animals with human glial chimeric brains patent. 7 524,491 B2. US. 2009
- Gumpel M, Lachapelle F, Gansmuller A, Baulac M, Baron van Evercooren A, Baumann N. Transplantation of human embryonic oligodendrocytes into shiverer brain. Ann N Y Acad Sci. 1987;495:71–85. doi: 10.1111/j.1749-6632.1987.tb23666.x. [DOI] [PubMed] [Google Scholar]
- Haley SA, Atwood WJ. An animal model for progressive multifocal leukoencephalopathy. J Clin Invest. 2014;124:5103–6. doi: 10.1172/JCI79186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–53. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrobin DF. Schizophrenia: the illness that made us human. Med Hypotheses. 1998;50:269–88. doi: 10.1016/s0306-9877(98)90000-7. [DOI] [PubMed] [Google Scholar]
- Hu BY, Du ZW, Zhang SC. Differentiation of human oligodendrocytes from pluripotent stem cells. Nat Protoc. 2009;4:1614–22. doi: 10.1038/nprot.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16:668–76. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izrael M, Zhang P, Kaufman R, Shinder V, Ella R, Amit M, Itskovitz-Eldor J, Chebath J, Revel M. Human oligodendrocytes derived from embryonic stem cells: Effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol Cell Neuroscience. 2007;34:310–323. doi: 10.1016/j.mcn.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–92. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–81. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Windrem MS, Zou L, Chandler-Militello D, Schanz SJ, Auvergne RM, Betstadt SJ, Harrington AR, Johnson M, Kazarov A, et al. Human glial chimeric mice reveal astrocytic dependence of JC virus infection. J Clin Invest. 2014;124:5323–36. doi: 10.1172/JCI76629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachapelle F, Gumpel M, Baulac M, Jacque C, Duc P, Baumann N. Transplantation of CNS fragments into the brain of shiverer mutant mice: extensive myelination by implanted oligodendrocytes. I. Immunohistochemical studies. Dev Neurosci. 1983;6:325–34. doi: 10.1159/000112359. [DOI] [PubMed] [Google Scholar]
- Meyer K, Ferraiuolo L, Miranda CJ, Likhite S, McElroy S, Renusch S, Ditsworth D, Lagier-Tourenne C, Smith RA, Ravits J, et al. Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proc Natl Acad Sci U S A. 2014;111:829–32. doi: 10.1073/pnas.1314085111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitome M, Low HP, van Den Pol A, Nunnari JJ, Wolf MK, Billings-Gagliardi S, Schwartz WJ. Towards the reconstruction of central nervous system white matter using neural precursor cells. Brain. 2001;124:2147–61. doi: 10.1093/brain/124.11.2147. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nature Reviews Neuroscience. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, Jiang L, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nature Medicine. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- Oberheim N, Wang X, Goldman S, Nedergaard SA. Astrocytic complexity distinguishes the human brain. Trends in Neurosciences. 2006;29:1–10. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–87. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao J, Major T, Auyeung G, Policarpio E, Menon J, Droms L, Gutin P, Uryu K, Tchieu J, Soulet D, et al. Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitors Remyelinate the Brain and Rescue Behavioral Deficits following Radiation. Cell Stem Cell. 2015;16:198–210. doi: 10.1016/j.stem.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popko B, Puckett C, Lai E, Shine HD, Readhead C, Takahashi N, Hunt SW, 3rd, Sidman RL, Hood L. Myelin deficient mice: expression of myelin basic protein and generation of mice with varying levels of myelin. Cell. 1987;48:713–21. doi: 10.1016/0092-8674(87)90249-2. [DOI] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–6. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Readhead C, Popko B, Takahashi N, Shine HD, Saavedra RA, Sidman RL, Hood L. Expression of a myelin basic protein gene in transgenic shiverer mice: correction of the dysmyelinating phenotype. Cell. 1987;48:703–12. doi: 10.1016/0092-8674(87)90248-0. [DOI] [PubMed] [Google Scholar]
- Roy NS, Wang S, Harrison-Restelli C, Benraiss A, Fraser RA, Gravel M, Braun PE, Goldman SA. Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J Neurosci. 1999;19:9986–95. doi: 10.1523/JNEUROSCI.19-22-09986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim FJ, McClain CR, Schanz SJ, Protack TL, Windrem MS, Goldman SA. CD140a identifies a population of highly myelinogenic, migration-competent and efficiently engrafting human oligodendrocyte progenitor cells. Nat Biotechnol. 2011;29:934–41. doi: 10.1038/nbt.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim FJ, Windrem MS, Goldman SA. Fate determination of adult human glial progenitor cells. Neuron Glia Biol. 2009;5:45–55. doi: 10.1017/S1740925X09990317. [DOI] [PubMed] [Google Scholar]
- Stacpoole SR, Spitzer S, Bilican B, Compston A, Karadottir R, Chandran S, Franklin RJ. High yields of oligodendrocyte lineage cells from human embryonic stem cells at physiological oxygen tensions for evaluation of translational biology. Stem Cell Reports. 2013;1:437–50. doi: 10.1016/j.stemcr.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–9. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Tripathi RB, Rivers LE, Young KM, Jamen F, Richardson WD. NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J Neurosci. 2010;30:16383–90. doi: 10.1523/JNEUROSCI.3411-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–61. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M, et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12:252–64. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, Nunes MC, Rashbaum WK, Schwartz TH, Goodman RA, McKhann G, Roy NS, Goldman SA. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nature Medicine. 2004;10:93–97. doi: 10.1038/nm974. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Roy NS, Wang J, Nunes M, Benraiss A, Goodman R, McKhann GM, Goldman SA. Progenitor cells derived from the adult human subcortical white matter disperse and differentiate as oligodendrocytes within demyelinated lesions of the rat brain. J Neurosci Res. 2002;69:966–975. doi: 10.1002/jnr.10397. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Schanz SJ, Guo M, Tian GF, Washco V, Stanwood N, Rasband M, Roy NS, Nedergaard M, Havton LA, et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–65. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, Schanz SJ, Morrow C, Munir J, Chandler-Militello D, Wang S, Goldman SA. A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brains are chimeric for human glia. J Neurosci. 2014;34:16153–61. doi: 10.1523/JNEUROSCI.1510-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–3. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yandava BD, Billinghurst LL, Snyder EY. “Global” cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain. Proc Natl Acad Sci U S A. 1999;96:7029–34. doi: 10.1073/pnas.96.12.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]