Abstract
Objective
To assess thyroid function, the presence of thyroid antibodies, as well as the presence of goiter and/or nodules in subjects without a prior diagnosis of thyroid disorders, in a region with mild to moderate iodine deficiency.
Design and methods
This cross-sectional study is based on data coming from first and third visits of participants in the SardiNIA survey. We performed two different analyses. In one we assessed the prevalence of unknown thyroid dysfunctions among 6,252 subjects who had a medical examination and blood collection for assays of thyrotropin, free thyroxine, and antibodies against thyroperoxidase (AbTPO) and against thyroglobulin (AbTG). In a second analysis, we evaluated the frequency of undiagnosed goiter and nodules among 3,377 subjects who had a thyroid ultrasound scan. Subjects were excluded if they had a previous history of thyroid disorders or presence of goiter and/or nodules, or thyroid surgery, or if they were taking drugs that could impair thyroid function.
Results
We found a low prevalence of overt thyroid dysfunction (hyperthyroidism 0.4%, hypothyroidism 0.7%). The rates of subclinical hypothyroidism and hyperthyroidism were 4.7% and 2.4%, respectively. Almost 16% of individuals were positive for at least one antibody, and 5.2% for both AbTG and AbTPO. Nodules were detected in 17.4% of the subjects, and the prevalence of goiter was 22.1%.
Conclusions
Undiagnosed biochemical thyroid dysfunctions, unknown nodules and goiter were common in subjects living in a mild to moderate iodine deficient area. In this community, thyroid disorders often go undetected and screening could be reasonable in subjects at higher risk.
Keywords: Epidemiology, undiagnosed thyroid disease, unknown thyroid nodules, mild to moderate iodine deficiency area
Introduction
Thyroid disorders are one of the most frequent pathologies found in the general population, but identifying thyroid disease can be clinically challenging because subclinical thyroid dysfunction and autoimmune thyroiditis are often asymptomatic and usually diagnosed biochemically. Abnormal thyroid function has important public health consequences. Suppressed TSH levels have been associated with an increased risk of atrial fibrillation, premature atrial beats, stroke, and all cause mortality (1–3). In addition, suppressed TSH level have been associated with decreased bone density (4). Moreover, overt hypothyroidism contributes to elevated serum low-density lipoprotein cholesterol levels and is a risk factor for coronary heart disease, heart failure and atherosclerosis (5, 6). Some studies also suggest that this may also be true in patients with subclinical hypothyroidism (7). Most epidemiological surveys have reported biochemical aspects of thyroid disorders, although with different results. The difficulties for determinations and their comparison arise from the variable definitions of disease state, the heterogeneity of the populations studied, the range of normality of biochemical parameters, and the sensitivity of thyroid function tests used. For example, the introduction of assays for serum thyroid stimulating hormone (TSH) sensitive enough to distinguish between normal and low concentrations allowed the identification of subjects with subclinical hyperthyroidism. Additional variables include genetic (8, 9) and environmental factors, such as iodine intake (10, 11). Indeed, almost one-third of the world’s population lives in areas of iodine deficiency (12), and the prevalence of goiter can be as high as 80%. The introduction of ultrasonography in epidemiological studies has increased the diagnostic power to assess the presence of goiter and/or nodules in comparison with studies in which goiter was assessed by physical examination (13). In the present study, a large cohort provided a unique opportunity to conduct a cross-sectional study of abnormal thyroid function and morphology. We assess both the prevalence of abnormal biochemical thyroid disease and the frequency of nodules and goiter in subjects without known thyroid abnormalities.
Materials and methods
The SardiNIA study investigates more than 300 genotypic and phenotypic aging-related traits in a longitudinal survey. The main features of this project have been described detailed elsewhere (14). All residents from 4 towns (Lanusei, Arzana, Ilbono, and Elini) in a valley in Sardinia (Italy) were invited to participate. Since November 2001, participants had visit and blood tests about every three years, generating three complete surveys, and some new subjects were progressively enrolled to complete family units.
We performed two analyses. In one we analysed the frequency of biochemical thyroid disorders, including all subjects who had a medical examination at the first time in the first or third visit waves. [We did not include individuals from the second survey because TSH was the only parameter checked at that visit.] We excluded all subjects with self-reported thyroid disease (i.e., Hashimoto’s thyroiditis, hyperthyroidism or hypothyroidism) and those taking drugs impairing thyroid function (levothyroxine, thyrostatics, amiodarone, and carbolithium). The final cohort numbered 6,252 (female 54,8%) aged 14–102. A second analysis was performed to observe the prevalence of undiagnosed thyroid nodules and goiter. We assessed all subjects who performed thyroid ultrasound, which was carried out during the third visit wave. Subjects who reported thyroid nodules, goiter, or thyroid surgery were excluded, yielding a final sample of 3,377 (female 52.4%). The iodine status at the time of evaluation was not assessed. However, the urinary iodine excretion of subjects living in this area comes from a study by Martino et al., showing a mild to moderate iodine deficiency (15).
Biochemical and hormone assays
Blood venous samples were drawn between 7 and 8 a.m. after an overnight fast. Serum samples were assayed for TSH, free thyroxine (FT4), and antibodies against thyroperoxidase (AbTPO) and against thyroglobulin (AbTG) using an automated chemiluminescence assay system (Immulite 2000, Siemens, Germany). The method is a two-site, solid-phase chemiluminescent immunometric assay. Normal values are TSH, 0.4–4.0 μIU/ml; FT4, 0.89–1.76 ng/dl; AbTPO, < 35 IU/ml; AbTG, < 40 IU/ml.
Overt hypothyroidism was defined as a serum TSH level above the upper limit of the reference range and serum FT4 level below the lower limit of reference range. Overt hyperthyroidism was defined by serum TSH levels below the lower limit of reference range and serum FT4 level above the upper limit of reference range. We defined subclinical thyroid dysfunction as serum FT4 levels in the normal reference range together with high serum TSH (subclinical hypothyroidism) or low serum TSH (subclinical hyperthyroidism).
Thyroid ultrasound
Conventional thyroid ultrasound was performed with a real-time instrument (ATL 3500 HDI machine with a linear transducer 5–12 MHz). Subjects were supine, with neck hyperextended. Nodule was defined as the presence of any distinct lesion within the thyroid gland detected with ultrasound, regardless the echoic pattern. Subjects with nodules were divided into two categories: “solitary nodule” if only one nodule was detected, and “two or more nodules” (otherwise defined “multiple”) if the ultrasound scan revealed the presence of at least two nodules. Thyroid volume was calculated according to the ellipsoid model (16). Goiter was defined as a thyroid volume exceeding 13 mL in women and 18,1 mL in men (17), regardless the presence of nodules.
Each participant signed an informed consent form. All study methods were conducted according to the principles expressed in the Declaration of Helsinki and were approved by the governing Ethics Committee, ASL4.
Statistical analysis
Results were expressed as median and interquartile range. Statistical differences in frequencies were tested by the exact Fisher test in Stata 12.0 for Macintosh. Significance was set at P < 0.05.
Results
Biochemical thyroid dysfunctions
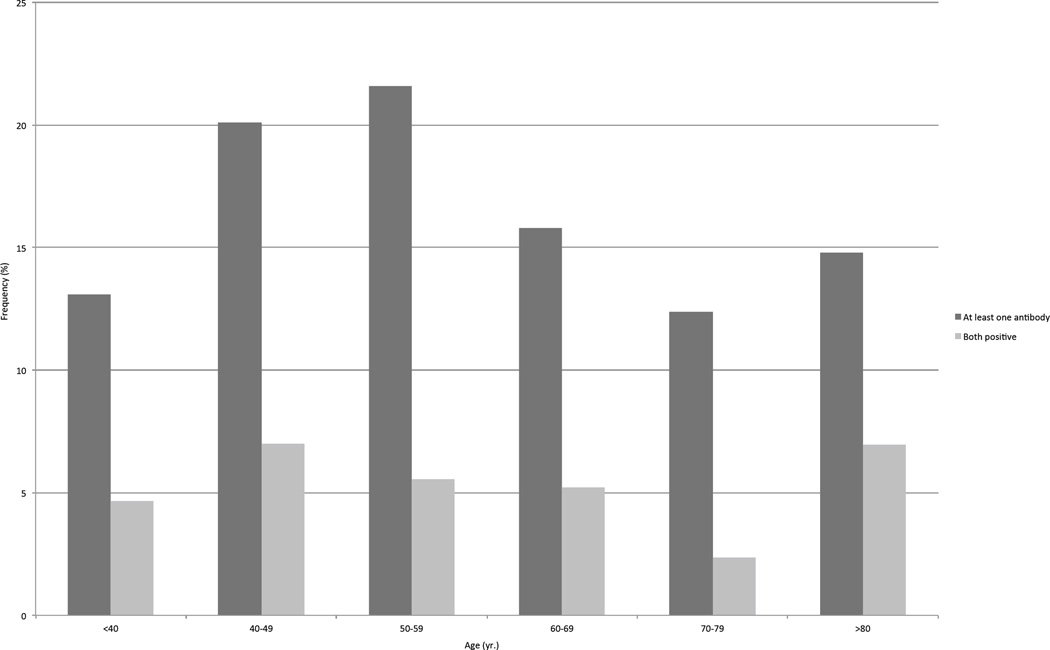
The baseline characteristics of the subjects are shown in Table 1. Overt hypothyroidism and hyperthyroidism were diagnosed in 42 (0.7%) and 23 (0.4%) participants, respectively. Subclinical hypothyroidism was found in 293 (4.7%) individuals, whereas subclinical hyperthyroidism was detected in 152 (2.4%) subjects. The prevalence of thyroid dysfunctions progressively increased with age, to reach a maximum of 13.9% in subjects older than 80 (Table 2). The distribution of AbTPO and AbTG across age groups was shown in Figure 1. Table 3 displayed the frequency of AbTPO and AbTG across thyroid dys functions. The frequency of thyroid antibodies was higher in woman than in men (p<0.001), as when considering the presence of at least one antibody (20.9% vs. 9.9%), as when detected both together (6.9% vs. 3.1%), as shown in Table 4. Among the whole sample studied, 1307 (20.9%) individuals had at least one biochemical thyroid disorder: 313 had hyper- or hypo-function of the gland without detectable thyroid antibodies, 797 showed only the positivity of at least one antibody, and 197 had a thyroid dysfunction associated with at least one circulating antibody.
Table 1.
Characteristics of subjects in the analyses.
| Biochemical analysis | Ultrasound analysis | |
|---|---|---|
| Subjects (n) | 6252 | 3377 |
| Sex (M/F) | 2826/3426 | 1608/1769 |
| Age (yr.) | 41.7 (28.8 – 57) | 45.8 (34.4 – 59.4) |
| TSH (µIU/ml) | 1.61 (1.05 – 2.32) | 2.37 (1.12 – 2.37) |
| FT4 (ng/dl) | 1.27 (1.14 – 1.4) | 1.39 (1.12 – 1.39) |
n, absolute number. Data are expressed as median (interquartile range).
Abbreviations: thyrotropin, TSH; free thyroxine, FT4.
Table 2.
Distribution of thyroid status across different age groups.
| Thyroid status | Age (yr.) | ||||||
|---|---|---|---|---|---|---|---|
| < 40 | 40–49 | 50–59 | 60–69 | 70–79 | ≥ 80 | ||
| Overt hypothyroidism |
n % |
10 0.3 |
11 1.0 |
9 1.0 |
7 0.9 |
4 0.9 |
1 0.9 |
| Subclinical hypothyroidism |
n % |
161 5.5 |
51 4.6 |
30 3.3 |
26 3.5 |
16 3.8 |
9 7.8 |
| Euthyroidism | n % |
2721 92.9 |
1027 92.2 |
845 91.9 |
671 89.6 |
379 89.0 |
99 86.1 |
| Subclinical hyperthyroidism |
n % |
27 0.9 |
22 2.0 |
33 3.6 |
41 5.5 |
24 5.6 |
5 4.3 |
| Overt hyperthyroidism |
n % |
9 0.3 |
3 0.3 |
3 0.3 |
4 0.5 |
3 0.7 |
1 0.9 |
| Total | n % |
2928 100 |
1114 100 |
920 100 |
749 100 |
426 100 |
115 100 |
| Any dysfunction* | n % |
207 7.0 |
87 7.9 |
75 8.2 |
78 10.4 |
47 11.0 |
16 13.9 |
Presence of any biochemical thyroid dysfunction (subclinical and overt).
Figure 1.
Frequency of antibodies against thyroglobulin and thyroperoxidase across different age groups.
Table 3.
Frequency of antibodies against thyroperoxidase and against thyroglobulin across thyroid status.
| Thyroid status | Only AbTPO | Only AbTG | AbTPO and/or AbTG |
AbTPO and AbTG |
No antibodies | |
|---|---|---|---|---|---|---|
| Overt hypothyroidism |
n % |
29/42 69.1 |
13/42 31.0 |
31/42 73.8 |
11/42 26.2 |
11/42 26.2 |
| Subclinical hypothyroidism |
n % |
117/293 39.9 |
81/293 27.7 |
129/293 44.0 |
69/293 23.5 |
164/293 56.0 |
| Euthyroidism | n % |
533/5742 9.3 |
489/5742 8.5 |
797/5742 13.9 |
225/5742 3.9 |
4945/5742 86.1 |
| Subclinical hyperthyroidism |
n % |
14/152 9.2 |
21/152 13.8 |
26/152 17.1 |
9/152 5.9 |
126/152 82.9 |
| Overt Hyperthyroidism |
n % |
8/23 34.8 |
11/23 47.8 |
11/23 47.8 |
8/23 34.8 |
12/23 52.2 |
| Total | n % |
701/6252 11.2 |
615/6252 9.8 |
994/6252 15.9 |
322/6252 5.2 |
5258/6252 84.1 |
Abbreviations: antibodies against thyroperoxidase, AbTPO; antibodies against thyroglobulin, AbTG
Table 4.
Frequency of thyroid disorders stratified by sex.
| Biochemical analysis | Female (n= 3426) |
Male (n=2826) |
Total (n=6252) |
P value¶ |
|---|---|---|---|---|
| Thyroid dysfunction | 335 (9.8%) | 175 (6.2%) | 510 (8.2%) | < 0.001 |
| Overt hypothyroidism | 30 (0.9%) | 12 (0.4%) | 42 (0.7%) | 0.016# |
| Subclinical hypothyroidism | 198 (5.8%) | 95 (3.4%) | 293 (4.7%) | < 0.001§ |
| Subclinical hyperthyroidism | 92 (2.7%) | 60 (2.1%) | 152 (2.4%) | ns |
| Overt hyperthyroidism | 15 (0.4%) | 8 (0.3%) | 23 (0.4%) | ns |
| Antibodies | ||||
| Only AbTPO | 494 (14.4%) | 207 (7.3%) | 701 (11.2%) | < 0.001 |
| Only AbTG | 456 (13.3%) | 159 (5.6%) | 615 (9.8%) | < 0.001 |
| AbTPO and/or AbTG | 715 (20.9%) | 279 (9.9%) | 994 (15.9%) | < 0.001 |
| AbTPO and AbTG | 235 (6.9%) | 87 (3.1%) | 332 (5.2%) | < 0.001 |
| Any biochemical thyroid disorders* | 895 (26.1%) | 412 (14.6%) | 1307 (20.9%) | < 0.001 |
Data show the number of patients in each group, with percentages in parentheses.
Abbreviation: antibodies against thyroperoxidase, AbTPO; antibodies against thyroglobulin, AbTG.
Female vs. men (Fisher's exact test).
Overt hypothyroidism vs. euthyroid
Subclinical hypothyroidism vs. euthyroid
Presence of thyroid dysfunction and/or presence of at least one thyroid antibody
Thyroid nodules and goiter
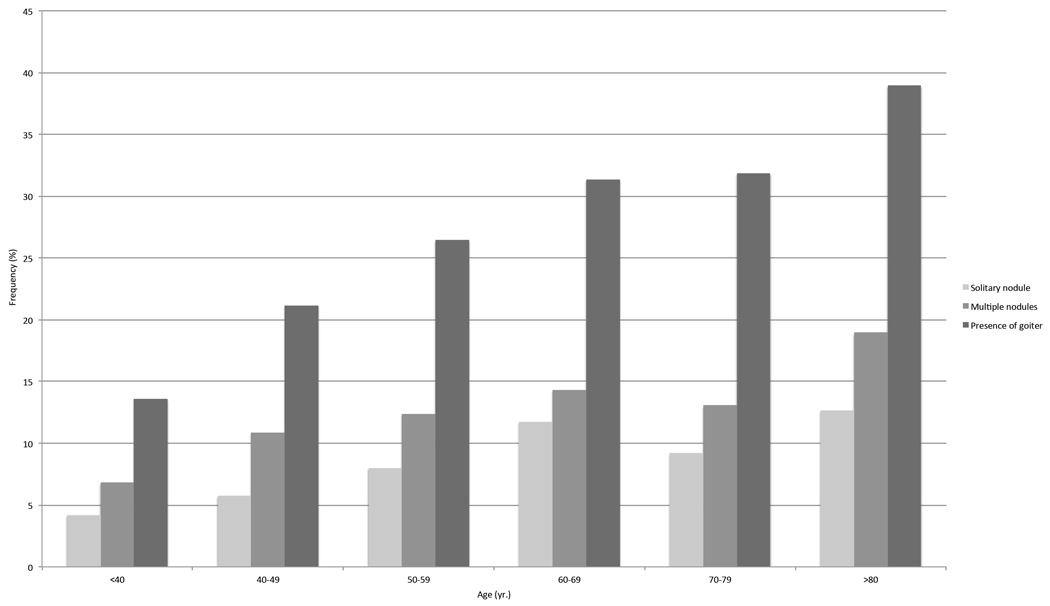
The second analysis included 3,377 subjects who performed thyroid ultrasound during the third survey. Among those, 588 (17.4%) showed the presence of nodularity: 232 had a solitary nodule and 356 had at least two nodules. The presence of undiagnosed thyroid nodules progressively increased with aging, with the highest prevalence in subjects aged 80 or older (31.6%, Figure 2). The presence of nodules (solitary or multiple) was associated with thyroid antibodies (AbTPO and/or AbTG) in 19% of subjects. The overall prevalence of goiter was 22.1% and progressively increased with aging, as reported in Figure 2. It was observed in 748 subjects, among which 99 (13.2%) had a solitary nodule, 106 (14.2%) had multiple nodules, and 543 (72.6%) did not show nodularity. Female had an higher frequency of nodularity than male (p<0.001, Table 5), as for solitary nodule (8.0 % vs. 5.6%) as for multiple (11.2% vs. 9.8%), while the prevalence of goiter was increased in male (26.1% vs. 18.5%, p<0.001), as reported in Table 5.
Figure 2.
Prevalence of nodules and goiter across age groups.
Table 5.
Frequency of goiter and nodules stratified by sex.
| Ultrasound analysis | Female (n=1769) | Male (n=1608) | Total (n=3377) | P value¶ |
|---|---|---|---|---|
| Goiter | 328 (18.5%) | 420 (26.1%) | 748 (22.1%) | < 0.001 |
| Nodules | 341 (19.2%) | 247 (15.4%) | 588 (17.4%) | 0.002 |
| Solitary nodule | 142 (8.0%) | 90 (5.6%) | 232 (6.9%) | 0.002 |
| Two or more nodules | 199 (11.2%) | 157 (9.8%) | 356 (10.5%) | ns |
Data show the number of patients in each group, with percentages in parentheses
Female vs. men (Fisher's exact test).
Discussion
Although thyroid dysfunction has multiple implications for public health, the magnitude of the problem is not completely known, nor is the relationship to other thyroid alterations well delineated. Our large population-based study in an area with mild to moderate iodine deficiency provides comprehensive data on the prevalence of unknown thyroid dysfunctions, autoimmune thyroid disorders, and unknown thyroid nodularity and goiter.
Overt hyperthyroidism was found in 0.4% (23/6252) of the sample. This finding is somewhat higher than those described in Norway (18) and in in the Colorado Study (19), but similar to those reported by Volzke in Germany (20). The prevalence of unknown subclinical hyperthyroidism was 2.4%. These findings were again lower than reported in some studies (17), but higher than others (20–23). Differences between studies may reflect different age ranges of cohorts and other environmental factors. Particularly important is the local iodine level. For example, a higher prevalence of hyperthyroidism due to an increased frequency of toxic nodular goiter has been documented in an iodine deficient community (17), and subclinical hyperthyroidism was also more frequent in an iodine deficient compared to an iodine replete area (17, 20, 22). Our survey showed a prevalence of hyperthyroidism which is somewhere between the prevalence reported in iodine deficient and iodine repleted areas, and consistent with the mild to moderate iodine deficiency in this area. However, we cannot differentiate the type of hyperthyroidism because we did not assess antibodies against TSH-receptor.
The prevalence of overt hypothyroidism was 0.7% (42/6252), with a higher frequency in females (71.4%) than in males (28.6%). This prevalence is similar to that reported by Volzke (20) but higher than reported by Aghini-Lombardi et al. in an another survey in Italy (17) and by Lucas et al. in Spain (21). Moreover, we found unknown subclinical hypothyroidism in 4.7% of subjects, which is substantially higher than that previously reported.
In this study the prevalence of thyroid antibodies (AbTPO, AbTG or both) was 15.9% which is higher than reported in previous studies. Whether iodine intake influences the development of thyroid autoimmunity remains controversial, however. Indeed, both low and high iodine intake has been associated with an increased tendency toward thyroid autoimmune abnormalities (24–26). According to some authors, the development of goiter due to iodine deficiency might overexpose the immune system to thyroid antigens, leading to immune reactions (27, 28). Assuming that iodine intake might interfere with the thyroid autoimmune process, only genetically predisposed subjects would present the disease (29). In keeping with this hypothesis, our data are consistent with a very high incidence of autoimmune diseases such as chronic autoimmune thyroiditis, type 1 diabetes mellitus, and multiple sclerosis in the Sardinian population (30). The prevalence of thyroid dysfunctions linked to the presence of AbTPO and/or AbTG ranged from 73.8% in subjects with overt hypothyroidism to 17.1% in those with subclinical hyperthyroidism (Table 3). Moreover, 13.9% of euthyroid individuals had circulating thyroid antibodies. These data are in line with previous findings that overt and subclinical hypothyroidism generally are autoimmune, whereas subclinical hyperthyroidism is usually related to multinodular goiter, with relatively frequent detection of serum thyroid antibodies, especially in goitrous females (17, 27, 31, 32) and in older subjects, as confirmed in our analysis. The second analysis included 3377 patients who performed thyroid ultrasound. We found 588 subjects (17.4%) with unknown thyroid nodules, 232 with solitary nodules, and 356 with at least two nodules. These findings are comparable to those reported by other authors (20). The prevalence of thyroid nodules increased with age up to 80 and beyond (31.6%).
Age and sex distributions were similar to those observed in previous reports in iodine moderate-deficient area, with a progressive increased prevalence of goiter in older subjects, though the prevalence of goiter was rather lower than reported by Martino et al. in the same geographic area (15). Different methods of estimation may account for this difference.
One exclusion criterion was self-reported patient data for previous diagnosis of thyroid disease and/or medications. Hence, all the data should be analysed with some reservations about possible undercounting, but the results of our analysis are consistent previous reports.
A major finding of our study was a 20.9% (1307/6252) prevalence of undiagnosed biochemical abnormalities of the thyroid gland. In this group, 313 subjects (23.9%) had thyroid dysfunction, the positivity of thyroid antibodies was found in 797 subjects (61%), whereas thyroid dysfunction together with circulating antibodies were found in 197 participants (15.1%). Our study indicates that the prevalence of thyroid dysfunction and/or abnormal thyroid anatomy are rather frequent in an unselected population living in this mild to moderate iodine deficient region. Although the demographic characteristics of the population studied may not be completely generalizable, in this study we provide additional epidemiological data on the undiagnosed thyroid disorders confirming the magnitude of thyroid dysfunction also in this area.
The large number of patients with abnormal thyroid parameters and/or thyroid nodules, coupled with the well-established gradual progression of thyroid dysfunction, indicate potential benefit from testing for abnormal thyroid function, at least in these geographic areas.
Acknowledgments
Funding
This work was supported by the National Institute on Aging (contract NO1-AG-1-2109).
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, Wilson P, Benjamin EJ, D'Agostino RB. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. New England Journal of Medicine. 1994;331:1249–1252. doi: 10.1056/NEJM199411103311901. [DOI] [PubMed] [Google Scholar]
- 2.Sheu JJ, Kang JH, Lin HC, Lin HC. Hyperthyroidism and risk of ischemic stroke in young adults: a 5-year follow-up study. Stroke. 2010;41:961–966. doi: 10.1161/STROKEAHA.109.577742. [DOI] [PubMed] [Google Scholar]
- 3.Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet. 2001;358:861–865. doi: 10.1016/S0140-6736(01)06067-6. [DOI] [PubMed] [Google Scholar]
- 4.Ross DS, Neer RM, Ridgway EC, Daniels GH. Subclinical hyperthyroidism and reduced bone density as a possible result of prolonged suppression of the pituitary-thyroid axis with L-thyroxine. American Journal of Medicine. 1987;82:1167–1170. doi: 10.1016/0002-9343(87)90219-1. [DOI] [PubMed] [Google Scholar]
- 5.McQuade C, Skugor M, Brennan DM, Hoar B, Stevenson C, Hoogwerf BJ. Hypothyroidism and moderate subclinical hypothyroidism are associated with increased all-cause mortality independent of coronary heart disease risk factors: a PreCIS database study. Thyroid. 2011;21:837–843. doi: 10.1089/thy.2010.0298. [DOI] [PubMed] [Google Scholar]
- 6.Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. Journal of Clinical Endocrinology & Metabolism. 2003;88:2438–2444. doi: 10.1210/jc.2003-030398. [DOI] [PubMed] [Google Scholar]
- 7.Rodondi N, Aujesky D, Vittinghoff E, Cornuz J, Bauer DC. Subclinical hypothyroidism and the risk of coronary heart disease: a meta-analysis. American Journal of Medicine. 2006;119:541–551. doi: 10.1016/j.amjmed.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Teumer A, Rawal R, Homuth G, Ernst F, Heier M, Evert M, Dombrowski F, Völker U, Nauck M, Radke D, Ittermann T, Biffar R, Döring A, Gieger C, Klopp N, Wichmann HE, Wallaschofski H, Meisinger C, Völzke H. Genome-wide association study identifies four genetic loci associated with thyroid volume and goiter risk. American Journal of Human Genetics. 2011;88:664–673. doi: 10.1016/j.ajhg.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomer Y. Genetic susceptibility to autoimmune thyroid disease: past, present, and future. Thyroid. 2010;20:715–725. doi: 10.1089/thy.2010.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S, Pedersen IB, Carlé A. Iodine intake as a determinant of thyroid disorders in populations. Best Practice & Research Clinical Endocrinology & Metabolism. 2010;24:13–27. doi: 10.1016/j.beem.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Knudsen N, Laurberg P, Perrild H, Bülow I, Ovesen L, Jørgensen T. Risk factors for goiter and thyroid nodules. Thyroid. 2002;12:879–888. doi: 10.1089/105072502761016502. [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann MB. Iodine deficiency. Endocrine Reviews. 2009;30:376–408. doi: 10.1210/er.2009-0011. [DOI] [PubMed] [Google Scholar]
- 13.Vanderpump MP. The epidemiology of thyroid disease. British Medical Bulletin. 2011;99:39–51. doi: 10.1093/bmb/ldr030. [DOI] [PubMed] [Google Scholar]
- 14.Pilia G, Chen WM, Scuteri A, Orrú M, Albai G, Dei M, Lai S, Usala G, Lai M, Loi P, Mameli C, Vacca L, Deiana M, Olla N, Masala M, Cao A, Najjar SS, Terracciano A, Nedorezov T, Sharov A, Zonderman AB, Abecasis GR, Costa P, Lakatta E, Schlessinger D. Heritability of cardiovascular and personality traits in 6,148 Sardinians. PLoS Genetics. 2006;2:e132. doi: 10.1371/journal.pgen.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martino E, Loviselli A, Velluzzi F, Murtas ML, Carta M, Lampis M, Murru R, Mastinu A, Arba ML, Sica V, Taberlet A, Grasso L, Maccherini D, Antonangeli L, Aghini-Lombardi F. Endemic goiter and thyroid function in central-southern Sardinia. Report on an extensive epidemiological survey. Journal of Endocrinological Investigation. 1994;17:653–657. doi: 10.1007/BF03349681. [DOI] [PubMed] [Google Scholar]
- 16.Brunn J, Blocjk U, Ruf J, Bos I, Kunze WP, Scriba PC. Volumetrie der schilddrusenlappen mittels real-time-sonographie. Deutsche Medizinische Wochenschrift. 1993;287:1206–1207. doi: 10.1055/s-2008-1070506. [DOI] [PubMed] [Google Scholar]
- 17.Aghini-Lombardi F, Antonangeli L, Martino E, Vitti P, Maccherini D, Leoli F, Rago T, Grasso L, Valeriano R, Balestrieri A, Pinchera A. The spectrum of thyroid disorders in an iodine-deficient community: the Pescopagano survey. Journal of Clinical Endocrinology & Metabolism. 1999;84:561–566. doi: 10.1210/jcem.84.2.5508. [DOI] [PubMed] [Google Scholar]
- 18.Bjoro T, Holmen J, Krüger O, Midthjell K, Hunstad K, Schreiner T, Sandnes L, Brochmann H. Prevalence of thyroid disease, thyroid dysfunction and thyroid peroxidase antibodies in a large, unselected population. The Health Study of Nord-Trondelag (HUNT) European Journal of Endocrinology. 2000;143:639–647. doi: 10.1530/eje.0.1430639. [DOI] [PubMed] [Google Scholar]
- 19.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Archives of Internal Medicine. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 20.Völzke H, Lüdemann J, Robinson DM, Spieker KW, Schwahn C, Kramer A, John U, Meng W. The prevalence of undiagnosed thyroid disorders in a previously iodine-deficient area. Thyroid. 2003;13:803–810. doi: 10.1089/105072503768499680. [DOI] [PubMed] [Google Scholar]
- 21.Lucas A, Julián MT, Cantón A, Castell C, Casamitjana R, Martínez-Cáceres EM, Granada ML. Undiagnosed thyroid dysfunction, thyroid antibodies, and iodine excretion in a Mediterranean population. Endocrine. 2010;38:391–396. doi: 10.1007/s12020-010-9397-2. [DOI] [PubMed] [Google Scholar]
- 22.Knudsen N, Bülow I, Laurberg P, Ovesen L, Perrild H, Jørgensen T. Low socio-economic status and familial occurrence of goitre are associated with a high prevalence of goitre. European Journal of Epidemiology. 2003;18:175–181. doi: 10.1023/a:1023001400945. [DOI] [PubMed] [Google Scholar]
- 23.O'Leary PC, Feddema PH, Michelangeli VP, Leedman PJ, Chew GT, Knuiman M, Kaye J, Walsh JP. Investigations of thyroid hormones and antibodies based on a community health survey: the Busselton thyroid study. Clinical Endocrinology (Oxf) 2006;64:97–104. doi: 10.1111/j.1365-2265.2005.02424.x. [DOI] [PubMed] [Google Scholar]
- 24.Konno N, Yuri K, Taguchi H, Miura K, Taguchi S, Hagiwara K, Murakami S. Screening for thyroid diseases in an iodine sufficient area with sensitive thyrotrophin assays, and serum thyroid autoantibody and urinary iodide determinations. Clinical Endocrinology (Oxf) 1993;38:273–281. doi: 10.1111/j.1365-2265.1993.tb01006.x. [DOI] [PubMed] [Google Scholar]
- 25.Alsayed A, Gad AM, Abdel-Baset H, Abdel-Fattah A, Ahmed A, Azab A. Excess urinary iodine is associated with autoimmune subclinical hypothyroidism among Egyptian women. Endocrine Journal. 2008;55:601–605. doi: 10.1507/endocrj.k07e-165. [DOI] [PubMed] [Google Scholar]
- 26.Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S, Pedersen IB, Carlé A. Iodine intake as a determinant of thyroid disorders in populations. Best Practice & Research Clinical Endocrinology & Metabolism. 2010;24:13–27. doi: 10.1016/j.beem.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Fenzi GF, Giani C, Ceccarelli P, Bartalena L, Macchia E, Aghini-Lombardi F, Vitti P, Lari R, Ceccarelli C, Baschieri L, et al. Role of autoimmune and familial factors in goiter prevalence. Studies performed in a moderately endemic area. Journal of Endocrinological Investigation. 1986;9:161–164. doi: 10.1007/BF03348088. [DOI] [PubMed] [Google Scholar]
- 28.Costa A, de Filippis V, Balsamo A, Ravarino N, Testori O, Torchio B, Valmaggia P, Zoppetti G. Serum autoantibodies and thyroid lymphocytic infiltration in endemic goitre. Clinical & Experimental Immunology. 1984;56:143–148. [PMC free article] [PubMed] [Google Scholar]
- 29.Tomer Y, Davies TF. Searching for the autoimmune thyroid disease susceptibility genes: from gene mapping to gene function. Endocrine Reviews. 2003;24:694–717. doi: 10.1210/er.2002-0030. [DOI] [PubMed] [Google Scholar]
- 30.Sardu C, Cocco E, Mereu A, Massa R, Cuccu A, Marrosu MG, Contu P. Population based study of 12 autoimmune diseases in Sardinia, Italy: prevalence and comorbidity. PLoS One. 2012;7:e32487. doi: 10.1371/journal.pone.0032487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mariotti S, Sansoni P, Barbesino G, Caturegli P, Monti D, Cossarizza A, Giacomelli T, Passeri G, Fagiolo U, Pinchera A, et al. Thyroid and other organ-specific autoantibodies in healthy centenarians. Lancet. 1992;339:1506–1508. doi: 10.1016/0140-6736(92)91265-a. [DOI] [PubMed] [Google Scholar]
- 32.Laurberg P, Pedersen KM, Hreidarsson A, Sigfusson N, Iversen E, Knudsen PR. Iodine intake and the pattern of thyroid disorders: a comparative epidemiological study of thyroid abnormalities in the elderly in Iceland and in Jutland, Denmark. Journal of Clinical Endocrinology & Metabolism. 1998;83:765–769. doi: 10.1210/jcem.83.3.4624. [DOI] [PubMed] [Google Scholar]