Abstract
PURPOSE
Integrating ultra-sensitive PSA (uPSA) into surveillance of high-risk patients following radical prostatectomy (RP) potentially optimizes management by correctly identifying actual recurrences, promoting an early salvage strategy and minimizing overtreatment. The power of uPSA following surgery to identify eventual biochemical failures is tested.
PATIENTS AND METHODS
From 1991–2013, 247 high-risk patients with a median follow-up was 44 months after RP were identified (extraprostatic extension and/or positive margin). Surgical technique, initial PSA (iPSA), pathology and post-op PSA were analyzed. The uPSA assay threshold was 0.01 ng/mL. Conventional biochemical relapse (cBCR) was defined as PSA ≥0.2 ng/mL. Kaplan Meier and Cox multivariate analyses (MVA) compared uPSA recurrence vs. cBCR rates.
RESULTS
Sensitivity analysis identified uPSA ≥0.03 as the optimal threshold identifying recurrence. First post-op uPSA ≥0.03, Gleason grade, T-stage, iPSA, and margin status predicted cBCR. On MVA, only first post-op uPSA ≥0.03, Gleason grade, and T-stage independently predicted cBCR. First post-op uPSA ≥0.03 conferred the highest risk (HR 8.5, p<0.0001) and discerned cBCR with greater sensitivity than undetectable first conventional PSA (70% vs. 46%). Any post-op PSA ≥0.03 captured all failures missed by first post-op value (100% sensitivity) with accuracy (96% specificity). Defining failure at uPSA ≥0.03 yielded a median lead-time advantage of 18 months (mean 24 months) over the conventional PSA ≥0.2 definition.
CONCLUSION
uPSA ≥0.03 is an independent factor, identifies BCR more accurately than any traditional risk factors, and confers a significant lead-time advantage. uPSA enables critical decisions regarding timing and indication for post-op RT among high-risk patients following RP.
Keywords: PSA, ultrasensitive PSA, Radical Prostatectomy, Adjuvant Radiotherapy
INTRODUCTION
For a perspective on the scope of the problem, one should consider that each year in the US population alone, approximately 100,000 men will undergo radical prostatectomy (RP) of which about one-fourth will experience recurrence [1]. At the time of recurrence, radiation therapy (RT) remains the only potentially curative treatment available. Following surgery, some individuals are at such high risk for recurrence that a strategy of adjuvant RT is used to prevent such. Accordingly, randomized clinical trials have shown that, when compared with observation, adjuvant RT after RP does indeed reduce risk of biochemical relapse and provides an overall survival benefit among high-risk patients (those with extraprostatic disease or positive margins). However, up to half of patients receiving adjuvant RT would in fact not have failed and thus would be unnecessarily radiated. To maximize benefit and minimize overtreatment, RT should optimally be reserved for confirmed recurrences and within the earliest timeframe.
Discovered in 1979, PSA assays became incorporated into clinical use in the late 1980s at detection thresholds of 0.2 to 0.6 ng/mL [2–4]. In postoperative setting a detectable level identifies the presence of prostate cancer, but at such low disease burdens that imaging cannot reliably locate the source. Technological advances have lowered detection limits to an ‘ultrasensitive’ range as low as <0.003 ng/mL [5]. As biochemical detection limits of modern ultrasensitive PSA (uPSA) assays have decreased, questions about its appropriate use and interpretation have risen.
The AUA consensus panel evaluated 145 articles encompassing 53 definitions of post-prostatectomy BCR and concluded that BCR is best defined as a serum PSA ≥0.2 ng/mL with a second confirmatory PSA level >0.2 ng/mL [6]. While this is most widely used clinically, it is tenfold above the lower limit of detection of current assays. With improved detection thresholds the definitions of BCR warrants study.
Naturally, the success of post-operative RT depends on whether biochemical relapse is due to local vs. distant disease. Biochemical failure precedes distant metastasis by about eight years [7] so it follows that earlier detection of biochemical relapse should confer a therapeutic advantage by selecting out those patients with more probable localized failure. Large multi-institutional trials have shown that pre-salvage RT PSA level has a profound impact on the likelihood of success of salvage RT [8, 9]. A meta-analysis quantified the success of salvage RT to decrease by 2.5% with every 0.1 PSA increment [10]. Thus, detecting failure at the lowest possible PSA concentration would be valuable to establish a greater ‘lead time’ to identify recurrences early while it is most likely to be confined to the prostate bed.
Despite several studies evaluating uPSA kinetics to diagnose failure, a clear threshold and clinical usefulness is still not defined. It is uncertain at what value patients are truly destined for BCR or which values reflect clinically insignificant but anxiety-provoking uPSA fluctuations. In the present study, we evaluated a cohort of RP patients with high-risk disease who are otherwise eligible for adjuvant RT, comparing uPSA to conventional risk factors for recurrence and evaluating the utility of uPSA to diagnose BCR.
PATIENTS AND METHODS
Patient Selection
Following Institutional Review Board approval, a retrospective review was performed on the records of patients who had radical prostatectomy (RP) from 1991–2013 or who were referred for consideration of post-operative RT. There were 247 pathologically node-negative patients after RP identified with high-risk disease (pT3/4 and/or positive margins). No patient received pre-op or post-op ADT. Surgical approach, pre-operative initial PSA (iPSA), complete surgical pathology (AJCC 2002 TNM staging guidelines) and post-op PSA were assessed.
PSA Follow-up
All study patients had post-op ultrasensitive PSA (uPSA) performed. Prior to 2006 our lab ran the Beckman Coulter Access Hybritech PSA assay and from 2006 onwards used the Roche electrochemiluminescence ‘Elecsys’ immunoassay run on a Roche Modular E170. The reported lower limit of detection (analytical sensitivity) of the Hybritech assay is approximately 0.005 ng/mL and 0.014 ng/mL for the Roche assay, whereas the functional (biologic) sensitivities are approximately 0.007 and 0.030 ng/mL, respectively. For all patients the lab reported a uPSA threshold at 0.01 ng/mL. For the purpose of labeling and analysis, a so-called ‘conventional’ biochemical relapse (cBCR) was defined as PSA ≥0.2 ng/mL. Patients were censored at last follow-up or at time of adjuvant therapy (radiotherapy or androgen deprivation).
Statistical Analysis
The BCR rates were calculated by the Kaplan-Meier method and differences between groups were determined by the log-rank test. Multivariate Cox proportional hazards regression modeling was used to examine whether uPSA above a certain threshold predicted cBCR, adjusting for clinico-pathologic factors (iPSA, pathological T-stage, Gleason sum, surgical approach, and surgical margin status). Probability values <0.05 were considered statistically significant.
Sensitivity/Specificity Analysis
Sensitivity and specificity analyses were performed to compare definitions of biochemical recurrence. A true positive was defined as a detectable PSA that progressed to PSA ≥0.2 ng/mL. A true negative was defined as an undetectable PSA that never reached a value of 0.2 ng/mL. Patients with PSAs that were rising above nadir but did not yet reach a value of 0.2 within the follow-up period were excluded from this subset analysis due to insufficient follow-up time necessary to be classified as cBCR (n=139). A false positive was defined as a detectable PSA that eventually returned to undetectable levels or that subsequently decreased with time. A false negative was defined as an undetectable PSA that eventually rose to ≥0.2 ng/mL without available intervening PSA values.
RESULTS
Table 1 reports descriptive characteristics of the 247 high-risk postoperative patients. Median follow-up for the cohort was 44 months (mean 59 ± 3 months). First postoperative PSA was obtained at a median time of 3.0 months after surgery, 2nd at 7.2 months, 3rd 12.1 months, 4th at 18.6 months, and 5th at 23.9 months. The 5-year biochemical recurrence free survival (PSA ≥0.2) was 54%.
Table 1.
Cohort Descriptive Characteristics
| n | % | |
|---|---|---|
| High-Risk Pathology | ||
| T2 m+ | 112 | 45.4% |
| T3 m+ | 76 | 30.8% |
| T3 m− | 49 | 19.8% |
| T4 m+ | 8 | 3.2% |
| T4 m− | 1 | 0.4% |
| Txm+ | 0 | 0% |
| T4 m− | 0 | 0% |
| T3mx | 1 | 0.4% |
| iPSA | ||
| <10 | 175 | 70.8% |
| ≥10 to <20 | 57 | 23.1% |
| ≥20 | 13 | 5.3% |
| Unknown | 2 | 0.8% |
| Pathologic Gleason Grade | ||
| 6 | 46 | 18.6% |
| 7 | 153 | 61.9% |
| ≥8 | 48 | 19.5% |
| Pathological T-stage | ||
| T2 | 112 | 45.4% |
| T3 | 126 | 51.0% |
| T4 | 9 | 3.6% |
| Margin Status | ||
| Positive | 196 | 79.4% |
| Negative | 50 | 20.2% |
| Unknown | 1 | 0.4% |
| Surgical Type | ||
| Retropubic | 141 | 57.1% |
| Robotic | 98 | 39.7% |
| Unknown | 8 | 3.2% |
| Median Time of 1st Post-op PSA taken | 3.0 months | |
| Median # of PSAs taken | 4 (1–16) | |
| Median Follow-up | 44 months | |
| 5-year biochemical recurrence free survival (PSA ≥0.2) | 54% | |
| Median Time to BCR (PSA ≥0.2 ng/mL) | 86 months | |
Benign uPSA patterns occurred in the range from 0.01 to 0.02 ng/mL, sometimes persisting over several repeated PSA draws. Only half of patients with any postoperative of uPSA =0.01 eventually progressed to conventional biochemical recurrence. When the threshold was increased to any postoperative uPSA =0.02, about one-fourth of patients still did not experience cBCR. Once the threshold was increased to uPSA ≥0.03, nearly all patients (98%) eventually relapsed (Figure 1). Therefore, a threshold of 0.03 ng/mL was chosen to be the minimum and necessary level above potential benign patterns (assay noise, residual burnout) to identify eventual BCR.
Figure 1.
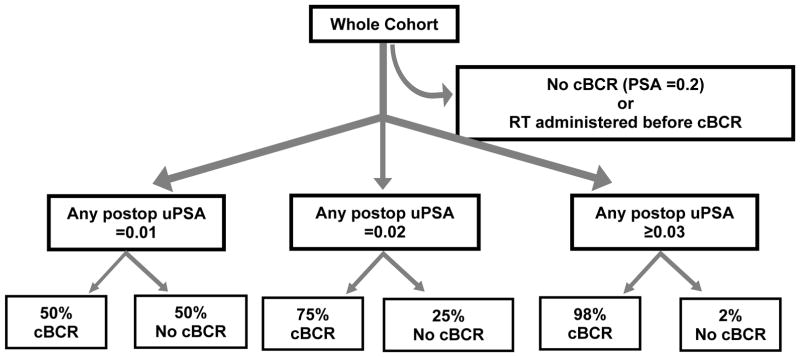
Sensitivity of post-op uPSA threshold in confirming eventual cBCR (PSA ≥0.2 ng/mL)
A first postoperative uPSA <0.03 vs. ≥0.03 was associated with 5-year cBCR-free rates of 65% vs. 10% (p<0.0001). Traditional risk factors including Gleason grade, T-stage, iPSA, and margin status also predicted for cBCR on univariate analysis (Table 2), but surgical technique did not. First postoperative uPSA ≥0.03 provided the earliest predictor of eventual cBCR. On univariate analysis, relapse occurred at a median of 5 months if first postoperative uPSA ≥0.03, compared to 31 months if Gleason ≥8, 36 months if extraprostatic disease, 31 months if iPSA ≥10, and 38 months if margin negative (Table 2).
TABLE 2.
First postoperative uPSA Compared to Traditional Risk Factors for Biochemical Relapse
| Risk Factor | Median Time to cBCR (PSA =0.2) | Univariate p-value |
|---|---|---|
| 1st Post-op uPSA ≥0.03 ng/mL vs. <0.03 ng/mL | 5 months 86 months |
<0.0001 |
|
Gleason 6 Gleason 7 Gleason 8–9 |
180 months 86 months 31 months |
<0.0001 |
| pT2 vs. pT3/4 | 152 months 36 months |
<0.0001 |
| iPSA ≥10 ng/mL vs. iPSA <10 ng/mL | 31 months 110 months |
0.0005 |
| Negative margin vs. Positive margin | 38 months 102 months |
0.013 |
| Open retropubic vs. Robotic | N/A | 0.79 |
On multivariate analysis, only first postoperative uPSA ≥0.03, Gleason grade, and T-stage independently predicted cBCR (Table 3). First postop uPSA ≥0.03 was by far the greatest risk factor for biochemical recurrence (HR 8.5, p<0.0001). Since the ‘any’ postop uPSA ≥0.03 factor identified all cBCR, it could not be used in a MVA.
TABLE 3.
Multivariate Analysis of factors associated with cBCR (PSA =0.2)
| Risk Factor | Multivariate p-value | Hazards Ratio |
|---|---|---|
| 1st Post-op PSA ≥0.03 vs. <0.03 | <0.0001 | 8.5 [5.1–14.3] |
|
Gleason 6 Gleason 7 Gleason 8–9 |
0.012 | - 3.0 [1.2–7.3] 4.4 [1.7–11.9] |
| pT2 vs. pT3/4 | 0.0022 | 2.5 [1.4–4.4] |
| iPSA ≥10 vs. <10 | 0.068 | N/A |
| positive vs. negative margin | 0.94 | N/A |
Considering the ability of first postoperative uPSA ≥0.03 to predict eventual cBCR, sensitivity/specificity analysis was employed to compare early uPSA failure to the conventional definition (PSA ≥0.2 ng/mL). Using first postoperative PSA cutoff ≥0.2 ng/mL has only 46% sensitivity to detect failures. If the relapse cutoff is lowered to first postoperative uPSA ≥0.03, sensitivity substantially increased to 70% (Table 4).
TABLE 4.
Sensitivity/Specificity Analysis of Using conventional PSA vs. uPSA to define BCR
| Undetectable 1st Post-op PSA <0.2 ng/mL [95% C.I.] | 1st Post-op uPSA <0.03 ng/mL [95% C.I.] | ANY Post-op uPSA <0.03 ng/mL [95% C.I.] | |
|---|---|---|---|
| Sensitivity (TP/TP+FN) | 46% [35–56] | 70% [59–79] | 100% [96–100] |
| Specificity (TN/TN+FP) | 100% [95–100] | 98% [88–100] | 96% [88–99] |
| PPV (TP/TP+FP) | 100% [91–100] | 98% [91–100] | 98% [93–100] |
| NPV (TN/TN+FN) | 60% [50–68] | 64% [51–75] | 100% [93–100] |
| Disease Prevalence | 56% [48–64] | 65% [56–73] | 62% [54–70] |
However, since relapse can happen many months after surgery, the negative predictive value of analyzing only the first postoperative PSA is poor with either cutoff (60% with PSA ≥0.2 and 64% with uPSA ≥0.03). Consequently, the merit of utilizing uPSA ≥0.03 to define biochemical relapse at ‘any time’ in follow-up was assessed. When the definition of biochemical relapse was expanded to include any uPSA ≥0.03, all failures missed by analyzing only the first postoperative value were captured (100% sensitivity, Table 4). Notably, lowering the threshold did not overestimate relapse, maintaining a high index of specificity (96%). Adopting any postoperative uPSA ≥0.03 to define relapse is accurate, as 98% of patients were confirmed to eventually progress to cBCR.
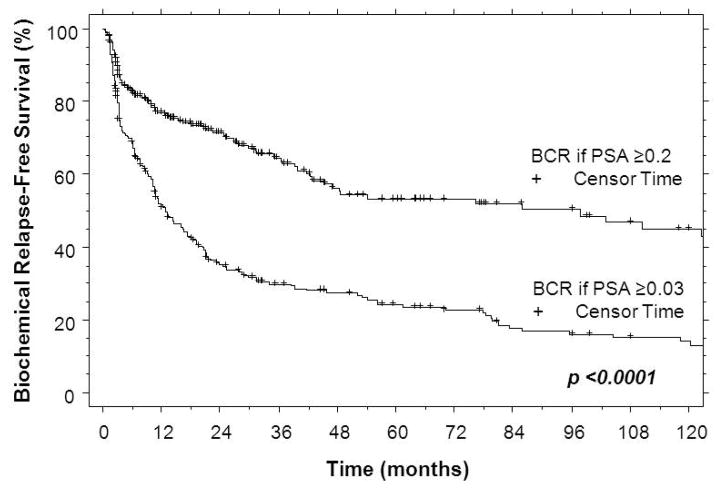
The timing of BCR can be tested using the uPSA failure definition as compared to the conventional failure definition of PSA ≥0.2 ng/mL. This comparison is shown in Figure 2 with a Kaplan-Meier comparison of PSA relapse-free survival. At 5-years, PSA relapse-free survival is 24% vs. 54% for uPSA ≥0.03 vs. PSA ≥0.2 recurrence definitions (p < 0.0001). Adopting uPSA ≥0.03 to define failure yielded a median lead-time advantage of 18 months (average lead time 24 months, range 1 to 95 months) as compared to using the conventional definition of PSA ≥0.2. There was no difference in lead-time advantage as a function of margin status (22 months with negative margin vs. 16 months with positive margin, p=0.55) but it was significantly shorter with first uPSA vs. any uPSA ≥0.03 (7 months vs. 25 months, p=0.028). This later finding is consistent with shorter tumor doubling times naturally declaring themselves earlier with first postop PSA.
Figure 2.
PSA relapse-free survival Kaplan-Meier curves demonstrating lead-time advantage with failure defined as uPSA ≥0.03 ng/mL vs. conventional failure ≥0.2 ng/mL.
With biochemical recurrence diagnosed at uPSA ≥0.03, the majority (72%) of the high-risk cohort had failed by the median follow-up of 44 months. In contrast, only 41% of the cohort met conventional (PSA ≥0.2) failure criteria. This 31% difference in BCR rate corresponds to nearly half (43%) of eventual failures otherwise missed by the conventional recurrence definition. Adopting the uPSA ≥0.03 definition of failure not only identifies actual relapses earlier, but suggests a strategy of PSA monitoring at intervals significantly shorter than 6 months, even out to five years post-op, in order derive benefit from the lead time that uPSA offers.
DISCUSSION
In the present study, we analyzed the use of postoperative uPSA to detect recurrence among patients at high-risk after radical prostatectomy who would otherwise be eligible for adjuvant RT. Our data yielded three primary conclusions when using a uPSA relapse criterion of ≥0.03 ng/mL at any time following RP: 1) it reliably identified all eventual relapses with high sensitivity (100%) and specificity (96%), 2) it was independent when compared to any of the conventional factors (HR 8.5, p<0.0001), and 3) it provided a median 18 months lead-time advantage over the conventional relapse criteria. These findings translate into a clinical management pathway for high-risk prostatectomy patients. Specifically, initial and continual monitoring with uPSA out to at least 5 years and at intervals not longer than 6 months between tests so that eventual failures can be identified earlier and postop RT can be initiated with a greater likelihood of success.
A few studies have used uPSA to risk-stratify or model failures, for example patients with undetectable uPSA at 2 to 3 years after prostatectomy are classified as less likely to fail [11,12]. The most advanced uPSA assays detect values at the pg/mL level (1 picogram, pg = 0.001 nanogram, ng) but come at the cost of false positives. The capacity to measure such low values may not be necessary or valuable, as it is known that serum PSA levels ≤30 pg/mL can be produced by nonmalignant sources of PSA [13], from benign cells left at the bladder neck, urethral margin, or periurethral glands [14–19]. Furthermore, the reported analytical sensitivity of a lab assay may differ from its ‘real-life’ diagnostic sensitivity, which is confounded by specimen sampling, processing, and interfering factors in human serum [20]. Residual cancer cells at positive surgical margins or micro-metastatic disease usually produce measurable amounts of PSA, although very rarely some high-grade and undifferentiated tumors may not produce PSA at all [21–23]. Depending on what ultrasensitive value is considered to be ‘detectable,’ the pattern over time is critical to contextualize any single measurement. These issues have contributed to the challenge of routine clinical implementation of uPSA.
Although the optimal use of uPSA has not yet been categorically established, use of the conventional assay and cutoff (PSA ≥0.2 ng/mL) is flawed. Patients with an ‘undetectable’ PSA (<0.2 ng/mL) may actually be failing, as one study showed that 23% ultimately relapsed biochemically after five years [7]. With the uPSA assay, this gradual process of BCR can be recognized much sooner. Our data showed that about half of relapses missed by conventional PSA relapse criteria would be anticipated with uPSA. The majority of our patients had uPSA failures within the first three years (82%) but a substantial number (18%) relapsed much later and therefore a closer PSA surveillance probably should extend to a minimum of 5 years.
The clinical relevance of uPSA-based early identification of recurrence lies in its direct relationship to the success of post-op radiotherapy (RT). Radiotherapy has the potential to eradicate microscopic residual disease and cure local recurrence. Currently, patient selection for adjuvant radiotherapy (ART) is based upon high-risk pathologic features (extracapsular extension, seminal vesicle invasion and/or positive surgical margins). Three randomized trials showed improved biochemical relapse-free survival with ART when compared to observation: the EORTC trial [24], the SWOG trial [25] and the ARO trial [26]. The SWOG showed that improved BCR-free survival translated into overall survival and distant metastatic-free survival advantages [27]. While these trials demonstrated the unequivocal benefit of post-op RT as compared with observation, these studies did not answer the fundamental question of ART vs. early SRT, but merely that ART is better than delayed SRT, ADT alone, or neither. Three randomized trials are underway to answer the question of ART vs. early SRT: Radiotherapy and Androgen Deprivation in Combination After Local Surgery (RADICALS, MRC-UK) [28], Radiotherapy Adjunct vs. Early Salvage (RAVES, TROG) [29], and Groupe d’Etude des Tumeurs Uro-Genital (GETUG-17) [30]. These trials will evaluate how immediate post-op RT for high-risk pathology in the setting of an undetectable PSA compares with early salvage RT at the first indication of PSA recurrence (set as >0.1 ng/mL [28] or PSA >0.2 ng/mL [29, 30]).
It is well established from numerous retrospective series and a recent pooled analysis [9] that SRT is more effective when initiated at lower PSA values. A meta-analysis quantified the success of salvage RT to decrease by 2.5% with every 0.1 PSA increment [10]. Even patients with high-risk pathology benefit from post-op RT at lower PSA values, as shown by two separate matched-pair analysis studies [31–32], as well as within the EORTC and SWOG ART trials. Collectively, all of this evidence confirms that improved outcomes are achieved when post-op RT is delivered at lower PSA entry levels.
We confirm that our data is generally representative by the fact that relapse rates in our cohort using the conventional relapse definition (PSA ≥0.2) was 54% at 5 years, exactly consistent with the rates reported in the observation arm from the three adjuvant trials of high-risk patients, which ranged from 44% to 54% [24–26]. Another indicator is shown by the stability of disease prevalence, which was 62% with failure defined at uPSA ≥0.03 and compares with the 56% prevalence using the PSA ≥0.2 failure definition.
In addition to its retrospective nature, the primary limitations of the present study includes sampling effects which are introduced by irregular and possibly too infrequent PSA time intervals, the very dependence of a PSA-based measure to define recurrence, and insufficient follow-up to determine whether lead time advantage would translate into improved distant metastatic and survival outcomes.
While the ‘ideal’ definition of BCR is a testable hypothesis, there can be little doubt that the utility of uPSA assays will be entirely lost if recurrence remains defined as a post-op PSA >0.2 ng/mL. Ultimately, whether a lead-time and earlier initiation of salvage RT translates to improved metastasis-free and overall survival needs to be tested with a randomized prospective trial.
References
- 1.Hull G, Rabbani F, Abbas F, et al. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002;167:528–534. doi: 10.1016/S0022-5347(01)69079-7. [DOI] [PubMed] [Google Scholar]
- 2.Wang T, Kawaguchi TP. Preliminary evaluation of measurement of serum prostate-specific antigen level in detection of prostate cancer. Ann Clin Lab Sci. 1986;16:461–466. [PubMed] [Google Scholar]
- 3.Graves H, Wehner N, Stamey TA. Ultrasensitive radioimmunoassay of prostate-specific antigen. Clin Chem. 1992;38:735–742. [PubMed] [Google Scholar]
- 4.Stamey T, Graves HC, Wehner N, et al. Early detection of residual prostate cancer after radical prostatectomy by an ultrasensitive assay for prostate specific antigen. J Urol. 1993;149:787–792. doi: 10.1016/s0022-5347(17)36208-0. [DOI] [PubMed] [Google Scholar]
- 5.Arai Y, Okubo K, Aoki Y, et al. Ultrasensitive Assay of Prostate-Specific Antigen for Early Detection of Residual Cancer after Radical Prostatectomy. Int J Urol. 1998;5:550–555. doi: 10.1111/j.1442-2042.1998.tb00411.x. [DOI] [PubMed] [Google Scholar]
- 6.Cookson M, Aus G, Bunett AL, et al. Variation in the Definition of Biochemical Recurrence in Patients Treated for Localized Prostate Cancer: The American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel Report and Recommendations for a Standard in the Reporting of Surgical Outcomes. J Urol. 2007;177:540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 7.Pound C, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 8.Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage Radiotherapy for Recurrent Prostate Cancer After Radical Prostatectomy. JAMA. 2004;291(11):1325–1332. doi: 10.1001/jama.291.11.1325. [DOI] [PubMed] [Google Scholar]
- 9.Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–41. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King CR. The timing of salvage radiotherapy after radical prostatectomy: a systematic review. Int J Radiat Oncol Biol Phys. 2012;84:104–11. doi: 10.1016/j.ijrobp.2011.10.069. [DOI] [PubMed] [Google Scholar]
- 11.Chang SL, Freedland SJ, Terris MK, et al. Freedom from a detectable ultrasensitive prostate-specific antigen at two years after radical prostatectomy predicts a favorable clinical outcome: analysis of the SEARCH database. Urology. 2010;75(2):439–44. doi: 10.1016/j.urology.2009.06.089. [DOI] [PubMed] [Google Scholar]
- 12.Malik RD, Goldberg JD, Hochman T, et al. Three-year postoperative ultrasensitive prostate-specific antigen following open radical retropubic prostatectomy is a predictor for delayed biochemical recurrence. Eur Urol. 2011;60(3):548–53. doi: 10.1016/j.eururo.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 13.Ellis WJ, Vessella RL, Noteboom JL, et al. Early detection of recurrent prostate cancer with an ultrasensitive chemiluminescent prostate-specific antigen assay. Urology. 1997;50(4):573–579. doi: 10.1016/S0090-4295(97)00251-3. [DOI] [PubMed] [Google Scholar]
- 14.Kamoshida S, Tsutsumi Y. Extraprostatic localization of prostatic acid phosphatase and prostate-specific antigen: distribution in cloacogenic glandular epithelium and sex-dependent expression in human anal gland. Hum Pathol. 1990;21:1108–11. doi: 10.1016/0046-8177(90)90146-v. [DOI] [PubMed] [Google Scholar]
- 15.Lepor H, Chan S, Melamed J. The role of bladder neck biopsy in men undergoing radical retropubic prostatectomy with preservation of the bladder neck. J Urol. 1998;160:2435–2439. doi: 10.1097/00005392-199812020-00013. [DOI] [PubMed] [Google Scholar]
- 16.Ponthieu A, Delgrande J, Ivaldi A. Peroperative biopsy of the sub-apical urethra during prostatectomy for cancer. Prog Urol. 1996;6:250–255. [PubMed] [Google Scholar]
- 17.Shah R, Bassily N, Wei J, et al. Benign prostatic glands at surgical margins of radical prostatectomy specimens: frequency and associated risk factors. Urology. 2000;56:721–725. doi: 10.1016/s0090-4295(00)00775-5. [DOI] [PubMed] [Google Scholar]
- 18.Yu H, Diamandis EP. Measurement of serum prostate specific antigen levels in women and in prostatectomized men with an ultrasensitive immunoassay technique. J Urol. 1995;153:1004–1008. [PubMed] [Google Scholar]
- 19.Fowler JJ, Brooks J, Pandey P, et al. Variable histology of anastomotic biopsies with detectable prostate specific antigen after radical prostatectomy. J Urol. 1995;153:1011–1014. [PubMed] [Google Scholar]
- 20.Junker R, Brandt B, Semjonow A, et al. The biologic lower detection limit of six ultrasensitive PSA assays. Anticancer Res. 1999;19:2625–2628. [PubMed] [Google Scholar]
- 21.Goldstein N. Immunophenotypic characterization of 225 prostate adenocarcinomas with intermediate or high Gleason scores. Am J Clin Pathol. 2002;117:471–477. doi: 10.1309/G6PR-Y774-X738-FG2K. [DOI] [PubMed] [Google Scholar]
- 22.Sheridan T, Herawi M, Epstein JI. The role of P501S and PSA in the diagnosis of metastatic adenocarcinoma of the prostate. Am J Surg Pathol. 2007;31:1351–1355. doi: 10.1097/PAS.0b013e3180536678. [DOI] [PubMed] [Google Scholar]
- 23.Iwakiri J, Granbois K, Wehner N, et al. An analysis of urinary prostate specific antigen before and after radical prostatectomy: evidence for secretion of prostate specific antigen by the periurethral glands. J Urol. 1993;149:783–786. doi: 10.1016/s0022-5347(17)36207-9. [DOI] [PubMed] [Google Scholar]
- 24.Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: A randomized controlled trial (EORTC trial 22911) Lancet. 2005;366:572–578. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 25.Thompson I, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: A randomized trial. JAMA. 2006;296:2329–2335. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 26.Wiegel T, Bottke D, Steiner U, et al. Phase III Postoperative Adjuvant Radiotherapy After Radical Prostatectomy Compared With Radical Prostatectomy Alone in pT3 Prostate Cancer With Postoperative Undetectable Prostate-Specific Antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924–2930. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 27.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant Radiotherapy for Pathological T3N0M0 Prostate Cancer Significantly Reduces Risk of Metastases and Improves Survival: Long-Term Followup of a Randomized Clinical Trial. J Urol. 2009;181:956–962. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker C, Sydes MR, Catton C, et al. Radiotherapy and androgen deprivation in combination after local surgery (RADICALS): a new Medical Research Council/National Cancer Institute of Canada phase III trial of adjuvant treatment after radical prostatectomy. BJU Int. 2007;99:1376–1379. doi: 10.1111/j.1464-410X.2007.06844.x. [DOI] [PubMed] [Google Scholar]
- 29.Trans-Tasman Radiation Oncology Group. (Trog 08-03) RAVES trial: Radiotherapy—Adjuvant versus early salvage [Google Scholar]
- 30.Richaud P, Sargos P, Henriques de Figueiredo B, et al. Postoperative radiotherapy of prostate cancer (GETUG-17) Cancer Radiother. 2010;14:500–503. doi: 10.1016/j.canrad.2010.07.224. [DOI] [PubMed] [Google Scholar]
- 31.Ost P, De Troyer B, Fonteyne V, et al. A Matched Control Analysis of Adjuvant and Salvage High-Dose Postoperative Intensity-Modulated Radiotherapy for Prostate Cancer. Int J Radiat Oncol Biol Phys. 2011;80(5):1316–1322. doi: 10.1016/j.ijrobp.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 32.Trabulsi EJ, Valicenti RK, Hanlon AL, et al. A multi-institutional matched-control analysis of adjuvant and salvage postoperative radiation therapy for pT3-4N0 prostate cancer. Urology. 2008;72(6):1298–302. doi: 10.1016/j.urology.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]