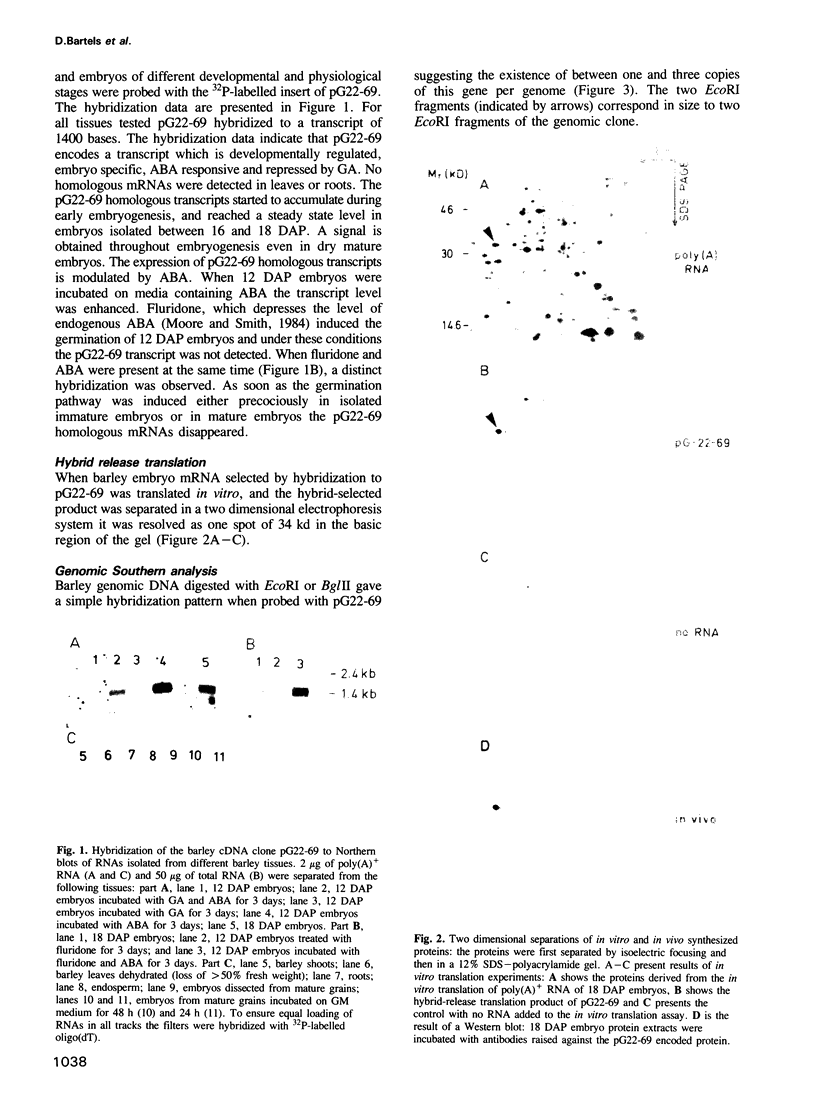
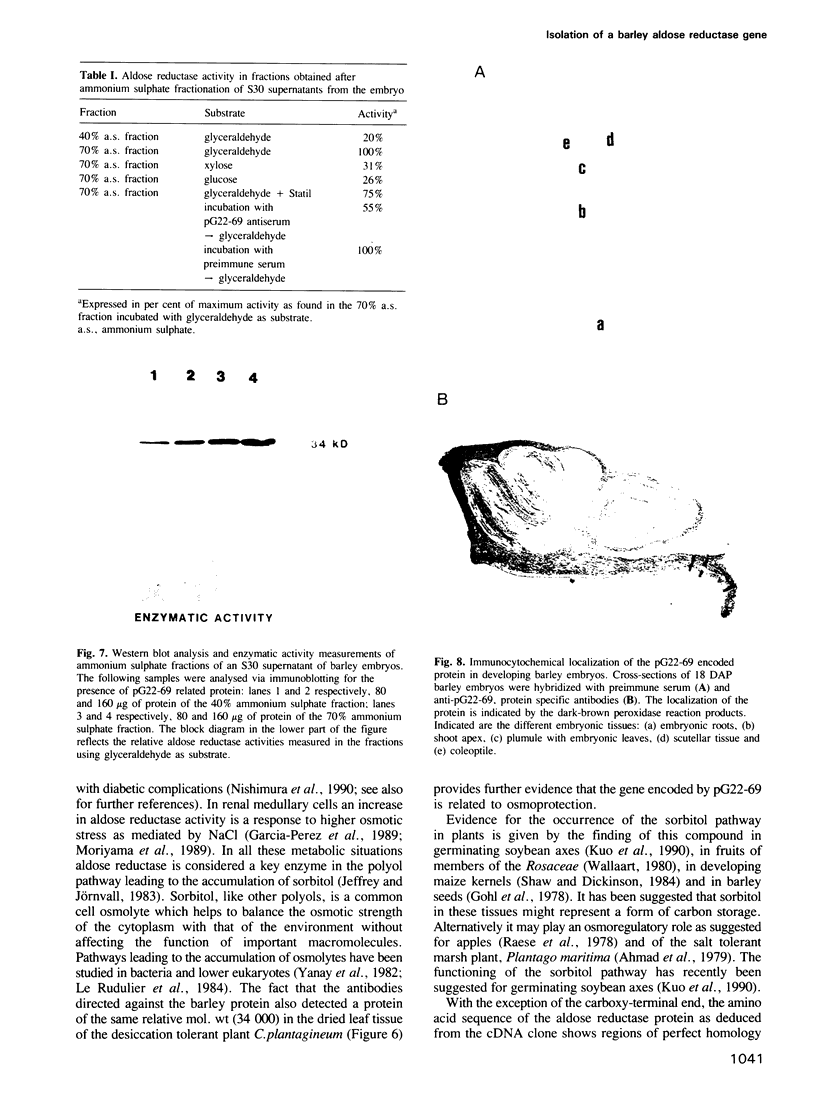
Abstract
In most higher plants a period of desiccation is the terminal event in embryogenesis. Excised barley embryos acquire desiccation tolerance at a precise developmental stage and cDNA clones have been isolated which are temporally linked with desiccation tolerance. One such clone (pG22-69) with a putative gene product of 34 kd displays high structural homology to mammalian genes encoding an NADPH dependent aldose reductase involved in the synthesis of sorbitol. This first aldose reductase gene of plants is expressed constitutively during embryo maturation and is modulated by the plant hormones abscisic acid (ABA) and gibberellic acid (GA). Immunohistochemistry showed that the protein is preferentially expressed in tissues formed at early stages in embryogenesis. Measurements of enzymatic activity indicate that pG22-69 encodes an active aldose reductase. The finding of this reductase activity and the cloning of the corresponding gene supports the existence of a metabolic pathway in plants playing a role in the synthesis of osmolytes like sorbitol. The significance of this work is that genes of related structure and functions are being used in diverse organisms to fulfil stress related biological requirements.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Marks C. B., Lazarus R., Miller J., Stafford K., Seymour J., Light D., Rastetter W., Estell D. Production of 2-Keto-L-Gulonate, an Intermediate in L-Ascorbate Synthesis, by a Genetically Modified Erwinia herbicola. Science. 1985 Oct 11;230(4722):144–149. doi: 10.1126/science.230.4722.144. [DOI] [PubMed] [Google Scholar]
- Bohren K. M., Bullock B., Wermuth B., Gabbay K. H. The aldo-keto reductase superfamily. cDNAs and deduced amino acid sequences of human aldehyde and aldose reductases. J Biol Chem. 1989 Jun 5;264(16):9547–9551. [PubMed] [Google Scholar]
- Carper D., Nishimura C., Shinohara T., Dietzchold B., Wistow G., Craft C., Kador P., Kinoshita J. H. Aldose reductase and p-crystallin belong to the same protein superfamily as aldehyde reductase. FEBS Lett. 1987 Aug 10;220(1):209–213. doi: 10.1016/0014-5793(87)80905-5. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Perez A., Martin B., Murphy H. R., Uchida S., Murer H., Cowley B. D., Jr, Handler J. S., Burg M. B. Molecular cloning of cDNA coding for kidney aldose reductase. Regulation of specific mRNA accumulation by NaCl-mediated osmotic stress. J Biol Chem. 1989 Oct 5;264(28):16815–16821. [PubMed] [Google Scholar]
- Jeffery J., Jörnvall H. Enzyme relationships in a sorbitol pathway that bypasses glycolysis and pentose phosphates in glucose metabolism. Proc Natl Acad Sci U S A. 1983 Feb;80(4):901–905. doi: 10.1073/pnas.80.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki N., Tanimoto T., Tanaka A. Characterization of aldose reductase and aldehyde reductase from rat testis. Biochim Biophys Acta. 1989 Jun 13;996(1-2):30–36. doi: 10.1016/0167-4838(89)90090-3. [DOI] [PubMed] [Google Scholar]
- Kuo T. M., Doehlert D. C., Crawford C. G. Sugar metabolism in germinating soybean seeds: evidence for the sorbitol pathway in soybean axes. Plant Physiol. 1990 Aug;93(4):1514–1520. doi: 10.1104/pp.93.4.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Moore R., Smith J. D. Growth, graviresponsiveness and abscisic-acid content of Zea mays seedlings treated with fluridone. Planta. 1984;162:342–344. [PubMed] [Google Scholar]
- Moriyama T., Garcia-Perez A., Burg M. B. Osmotic regulation of aldose reductase protein synthesis in renal medullary cells. J Biol Chem. 1989 Oct 5;264(28):16810–16814. [PubMed] [Google Scholar]
- Nelles L. P., Bamburg J. R. Rapid visualization of protein--dodecyl sulfate complexes in polyacrylamide gels. Anal Biochem. 1976 Jun;73(2):522–531. doi: 10.1016/0003-2697(76)90202-5. [DOI] [PubMed] [Google Scholar]
- Nishimura C., Matsuura Y., Kokai Y., Akera T., Carper D., Morjana N., Lyons C., Flynn T. G. Cloning and expression of human aldose reductase. J Biol Chem. 1990 Jun 15;265(17):9788–9792. [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrash J. M., Favello A. D. Isolation and characterization of cDNA clones encoding aldose reductase. Curr Eye Res. 1989 Oct;8(10):1021–1027. doi: 10.3109/02713688908997394. [DOI] [PubMed] [Google Scholar]
- Poulsom R. Inhibition of hexonate dehydrogenase and aldose reductase from bovine retina by sorbinil, statil, M79175 and valproate. Biochem Pharmacol. 1986 Sep 1;35(17):2955–2959. doi: 10.1016/0006-2952(86)90492-2. [DOI] [PubMed] [Google Scholar]
- Schade S. Z., Early S. L., Williams T. R., Kézdy F. J., Heinrikson R. L., Grimshaw C. E., Doughty C. C. Sequence analysis of bovine lens aldose reductase. J Biol Chem. 1990 Mar 5;265(7):3628–3635. [PubMed] [Google Scholar]
- Schmelzer E., Kruger-Lebus S., Hahlbrock K. Temporal and Spatial Patterns of Gene Expression around Sites of Attempted Fungal Infection in Parsley Leaves. Plant Cell. 1989 Oct;1(10):993–1001. doi: 10.1105/tpc.1.10.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J., John M., Wieneke U., Krüssmann H. D., Schell J. Expression of the nodulation gene nodA in Rhizobium meliloti and localization of the gene product in the cytosol. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9581–9585. doi: 10.1073/pnas.83.24.9581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. R., Dickinson D. B. Studies of sugars and sorbitol in developing corn kernels. Plant Physiol. 1984 May;75(1):207–211. doi: 10.1104/pp.75.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriver K., Mundy J. Gene expression in response to abscisic acid and osmotic stress. Plant Cell. 1990 Jun;2(6):503–512. doi: 10.1105/tpc.2.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Tomarev S. I., Zinovieva R. D., Dolgilevich S. M., Luchin S. V., Krayev A. S., Skryabin K. G., Gause G. G., Jr A novel type of crystallin in the frog eye lens. 35-kDa polypeptide is not homologous to any of the major classes of lens crystallins. FEBS Lett. 1984 Jun 11;171(2):297–302. doi: 10.1016/0014-5793(84)80508-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Fujii Y., Nakayama K., Ohkubo H., Kuramitsu S., Kagamiyama H., Nakanishi S., Hayaishi O. Structural similarity of bovine lung prostaglandin F synthase to lens epsilon-crystallin of the European common frog. Proc Natl Acad Sci U S A. 1988 Jan;85(1):11–15. doi: 10.1073/pnas.85.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- de Jong W. W., Hendriks W., Mulders J. W., Bloemendal H. Evolution of eye lens crystallins: the stress connection. Trends Biochem Sci. 1989 Sep;14(9):365–368. doi: 10.1016/0968-0004(89)90009-1. [DOI] [PubMed] [Google Scholar]