Abstract
Relational thinking, or the ability to represent the relations between items, is widespread in the animal kingdom. However, humans are unparalleled in their ability to engage in the higher-order relational thinking required for reasoning and other forms of abstract thought. Here we propose that the versatile reasoning skills observed in humans can be traced back to developmental and evolutionary changes in the lateral frontoparietal network (LFPN). We first identify the regions within the LFPN that are most strongly linked to relational thinking, and show that stronger communication between these regions over the course of development supports improvements in relational reasoning. We then explore differences in the LFPN between humans and other primate species that could explain species differences in the capacity for relational reasoning. We conclude that fairly small neuroanatomical changes in specific regions of the LFPN and their connections have led to big ontogenetic and phylogenetic changes in cognition.
Introduction
Theories of evolution spawned a vigorous debate in the mid 19th century over whether humans could possibly have descended from apes. This debate, in turn, provided the impetus for research in comparative neuroanatomy aimed at discovering how humans’ brains differ from those of other primates. Without the benefit of our current understanding of the functions of different brain regions, mid-19th century anatomists honed in on species differences that are not considered functionally significant today. In reviewing this line of research, eminent primate neurophysiologist Charlie Gross (Gross, 1993) concluded, tongue in cheek, that “one basic human characteristic seems to be the need to establish differences between ourselves and our closest relatives.”
In part because the conclusions of this earlier line of research were flawed, the search for brain differences between humans and other primate species fell out of favor. However, recent findings have provided insight into some of the evolutionary changes that may have rendered humans capable of abstract thought. Here we propose that a set of small changes in the lateral frontoparietal network (LFPN) enabled humans to process higher-order relations between mental representations, a form of relational thinking that is central to higher cognitive functions in humans. In this Perspective we focus primarily on relational reasoning, but we acknowledge that this is one of many cognitive abilities that rely on the LFPN.
First, we define relational thinking and discuss its centrality to human cognition. Second, we provide evidence that relational thinking relies on the LFPN. Third, we review studies identifying the role of regions within the LFPN in human reasoning, and argue that strengthening and refinement of this network over childhood and adolescence is central to the development of reasoning ability. Fourth, we review differences in the LFPN between humans and other primates that may underlie differences in the capacity for higher-order relational thinking.
Relational Thinking: A Cornerstone of Human Cognition
Relational thinking spans the gamut from basic relational binding necessary to learn associations among stimuli in our environment to higher-order relational comparisons in which we generate connections among abstract, semantic structures and categories (Gentner, 2010). First-order relations come in many forms: visuospatial (e.g., the apple is to the left of the cup), semantic (e.g., a hammer is used to hit a nail), numerical (e.g., 4 is greater than 2), temporal (e.g., water is poured into a coffee filter after the coffee grounds), etc. By contrast, a second-order relation is defined by its hierarchical nature; it is a relation between relations, i.e., one in which it is necessary to jointly consider several first-order relations (e.g., a mountain is larger than a molehill, just as a cat is larger than a mouse; Halford et al., 1998). Identifying higher-order relations requires abstracting over perceptual information, and instead focusing on roles and relations shared between the lower-order relational pairs.
Relational thinking supports various kinds of reasoning, such as analogical mapping and other forms of deductive reasoning. Examples of laboratory tasks used to measure relational reasoning are shown in 1. Problems of this sort rely on the ability to jointly consider multiple relations. Indeed, others have argued that our most abstract thought hinges on the ability to integrate multiple first-order relations into a more general higher-order relational category (Koechlin and Hyafil, 2007; Penn et al., 2008; Badre and D’Esposito, 2009).
We have argued that relational thinking is important not only for reasoning, but also for multiple other cognitive functions in humans, including decision-making, for which it is necessary to be able to compare the expected value of different choices, and episodic memory retrieval, during which a memory must be evaluated according to specific criteria (Bunge and Wendelken, 2009). Seeking to test the hypothesis that a common set of brain regions is involved in relational thinking across cognitive tasks, we performed a meta-analysis using the software “Neurosynth” (http://neurosynth.org; Yarkoni et al., 2011).
The search term “relational” yielded 46 studies in the Neurosynth database focused on memory, reasoning, decision-making, or higher-level perception. These studies involve a wide range of cognitive tasks and hail from multiple laboratories (see Neurosynth website for a complete list of studies). The forward inference map revealed voxels that are consistently reported in studies involving the term “relational” (dark blue voxels in Figure 2). This analysis yielded multiple regions, including lateral prefrontal and parietal regions as well as the hippocampus, which have been implicated in relational binding (Cohen et al., 1999). A more stringent analysis based on a reverse inference map revealed voxels that were specifically reported more often with the term “relational” than any of the other 524 search terms in the database (light blue voxels in Figure 2). In particular, this meta-analysis implicates rostrolateral prefrontal cortex (RLPFC), dorsolateral prefrontal cortex (DLPFC), and inferior parietal lobule/sulcus (IPL/IPS) in relational thinking.
Figure 2. Meta-Analysis of fMRI Activations Associated with the Term “Relational”.
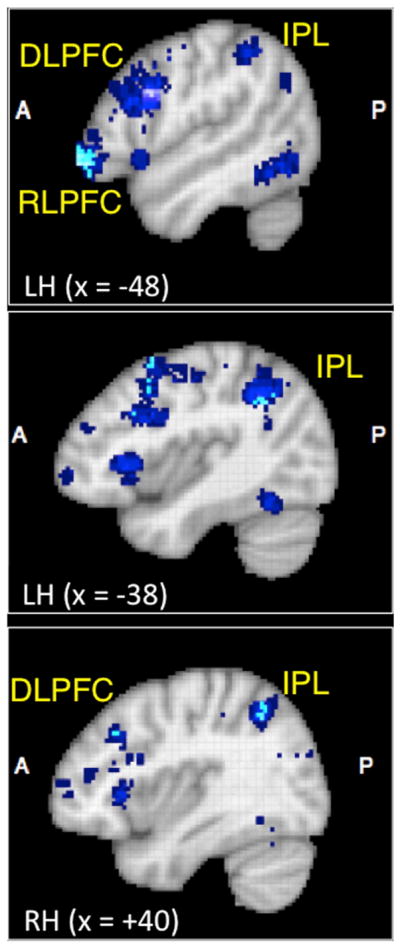
Results from Neurosynth.org (Yarkoni et al., 2011) using the search term “relational.” Items in light blue represent regions that are reported more selectively with the term relational relative to 525 other keywords (reverse inference, Z ≥ 1.96). Items in dark blue represent regions that are consistently activated with the term relational (forward inference). LH, left hemisphere; RH, right hemisphere; RLPFC, rostrolateral prefrontal cortex; DLPFC, dorsolateral prefrontal cortex; IPL, inferior parietal lobule. Additional regions identified by the forward inference search, but not shown here, include hippocampus, in-sula, and posterior cingulate cortex. See Yarkoni et al. (2011) and Neurosynth.org for further information.
Contributions of Lateral Prefrontal and Parietal Cortices to Relational Reasoning
It has long been known that damage to the prefrontal cortex leads to dysfunction of higher cognitive abilities (e.g., Luria, 1966; Stuss and Knight, 2013). Much neuropsychological research has emphasized the importance of both prefrontal and parietal cortices for high-level cognition in humans (Roca et al., 2010; Woolgar et al., 2010, 2013). Neuropsychological studies focusing specifically on relational reasoning have shown that patients with damage to prefrontal or parietal cortex have difficulty integrating multiple relations during geometric reasoning, analogical reasoning, and transitive inference tasks (Jung and Haier, 2007; Krawczyk et al., 2010b).
Beginning with Prabhakaran et al. (1997) and Christoff et al. (2001), a series of fMRI studies has implicated the LFPN in relational reasoning (see Krawczyk, 2012 for review). Over the last decade, numerous carefully controlled fMRI studies have allowed us to differentiate the contributions to relational reasoning of the three regions in the LFPN highlighted in Figure 2: the IPL, RLPFC, and DLPFC. We briefly review the hypothesized functions of each of these regions, and then delve into a few of the studies that provide support for their involvement in relational reasoning more specifically.
The IPL has been implicated in a variety of cognitive functions, including working memory, focused attention, episodic memory retrieval, etc. (see Ciaramelli et al., 2008 for a review). This region has been proposed to play a central role in the representation of relations between stimuli (Feng et al., 2014; Van Opstal and Verguts, 2013). Consistent with this hypothesis, IPL activation scales with the number of relations to be considered (Crone et al., 2009; Hampshire et al., 2011; Watson and Chatterjee, 2012), and is more active while processing specific, rather than general, relations (e.g., Figure 1B: “the green ball is heavier than the orange ball,” as compared with “the green and orange balls are associated with one another”; Wendelken and Bunge, 2010).
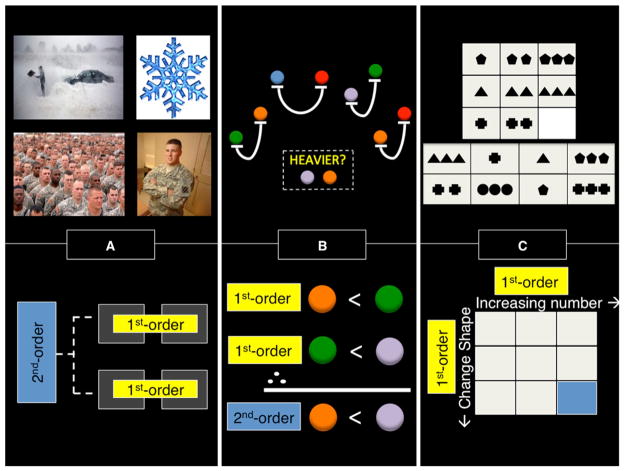
Figure 1. Relational Reasoning Tasks.
Examples of tasks used to investigate relational reasoning (top), and schematics illustrating first- and second-order relations used within each task (bottom).
(A) In order to solve the example analogy (blizzard: snowflake:: army: soldier) the reasoner must first recall the relationship between blizzard and snowflake (e.g., comprised of), and then compare this relation to one extracted from army and soldier. One’s ultimate decision is a higher-order comparison of two semantic relations abstracted from the perceptual objects.
(B) A transitive inference problem in which the participant has to integrate the weight relationships among pairs of balls. In this example, orange balls are lighter than green ones, and green balls are lighter than purple ones, so by combining these two relations one can make the conclusion that purple is heavier than orange.
(C) A geometric relational reasoning task in which one may jointly consider relations across rows (i.e., increase number of objects) and columns (i.e., change object shape) to come to the correct solution. Although this task does not require explicit representation of a second-order relation, the two first-order relations must be integrated to complete the missing piece of the array, highlighted in blue.
RLPFC has been linked to various high-level cognitive functions (see Ramnani and Owen, 2004), including prospective memory (Benoit et al., 2012), abstract thinking (Christoff et al., 2009; Badre and D’Esposito, 2009), counterfactual thinking (Donoso et al., 2014), tracking alternative outcomes during decision-making (Boorman et al., 2011), and planning (Gerlach et al., 2014). Several research groups have sought a parsimonious account of RLFPC’s function, although a consensus has not yet been reached. As discussed further below, we have proposed that RLPFC plays a fundamental role in the comparison and/or integration of several sets of mental representations (e.g., Bunge and Wendelken, 2009; Wendelken et al., 2008b).
The DLPFC has been implicated in the manipulation of items in working memory, performance monitoring, interference suppression, and response selection. DLPFC activation scales with task difficulty across a variety of paradigms, including relational reasoning tasks (Kroger et al., 2002; Wendelken et al., 2008a (see Figure 4A); Cho et al., 2010; Krawczyk et al., 2010a; Hampshire et al., 2011). Thus, DLPFC is thought to play a supporting role in the performance of many cognitively demanding tasks.
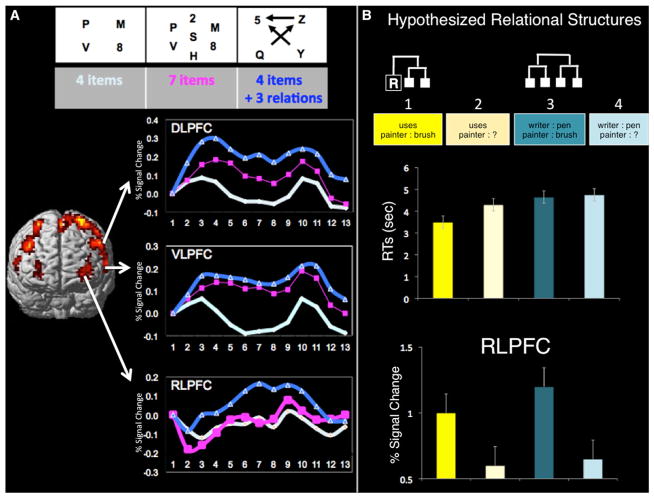
Figure 4. Functional Dissociation between RLPFC and More Posterior Lateral PFC.
(A) Both DLPFC and VLPFC follow increased working memory load. Rather than tracking task difficulty, RLPFC activates more preferentially for trials in which relational information is present (i.e., the arrows). Figure adapted with permission from Wendelken et al. (2008a).
(B) Participants were presented with four conditions in which they were given a relational term or had to extract a relation from a pair of related words, and in which they had to compare or complete the analogical expression. Relational structures are shown for the two conditions that are hypothesized to necessitate them. White boxes represent objects (e.g., painter), whereas boxes with an R inside represent relational terms (e.g., uses). RLPFC activation is greater when participants are asked to make comparisons (i.e., problem types 1 and 3), rather than complete an analogy (i.e., problem types 2 and 4), even though task difficulty is greater for trials in which participants have to extract a relation. Data from Wendelken et al. (2008b).
Although RLPFC, IPL, and DLPFC have been implicated in numerous cognitive functions, we focus here on their contributions to relational reasoning. Figure 3 illustrates a study in which we constructed two comparable relational matching tasks to be performed by the same set of individuals. These tasks required participants to consider semantic relations (e.g., swans and boats both belong in the water) or visuospatial relations (e.g., both of these stimuli are comprised of straight lines). Across both the semantic and visuospatial tasks, RLPFC and IPL were engaged more strongly when participants were asked to perform one second-order relational comparison than two first-order relational comparisons, even though the stimuli were identical for both first- and second-order relational trials (Wendelken et al., 2012).
Figure 3. Overlapping Activation in RLPFC for Visuospatial and Semantic Relational Reasoning.
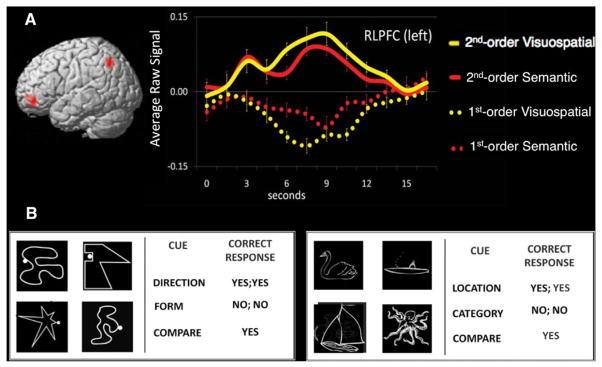
(A) Time series analysis from Wendelken et al. (2012). Event-related time courses were extracted from selected regions by averaging across trial-specific time series for each condition. For the visuospatial condition, participants had to decide whether two objects had straight or curvy lines, or had to decide whether dots placed on the objects were both on the left or right side. For the semantic condition, participants had to answer whether the picture was an animal or vehicle, or whether the picture was of something that resides in water or on land. Comparison trials meant deciding whether a higher-order relationship existed between both pairs. Despite the visual display being exactly the same for first- and second-order relational decisions, and the fact that participants needed to make decisions on both pairs for all trials, BOLD signal in the RLPFC increased only for those conditions where multiple relations must be compared in order to successfully make a judgment.
(B) Examples of stimuli used for nonverbal shapes (left) or semantic objects (right). See main text for description of the judgments made. Figure adapted with permission from Wendelken et al. (2012).
RLPFC Is Not Sensitive to Task Difficulty, Per Se, but Rather to Relational Demands
In general, tasks that require second-order relational thinking are more difficult than those that require only first-order relational thinking. Thus, it is reasonable to wonder whether RLPFC activation simply scales with task difficulty. We have conducted two studies that provide evidence to the contrary. Figure 4A features a working memory study in which participants were asked to maintain in working memory either 4 items, 7 items, or 4 items + 3 relations, indicated by unidirectional arrows between individual items (e.g., Q comes before Z). Whereas DLPFC activation scaled with task difficulty, RLPFC was more active on trials involving 4 items + 3 relations than 7 items, but did not distinguish between a working memory load of 7 and 4 (Wendelken et al., 2008a). Figure 4B features a propositional analogy study (Wendelken et al., 2008b) with four different conditions, in which the easiest problems (e.g., evaluating whether the term “uses” describes the relationship between a writer and a pen, i.e., problem type 1) engaged RLPFC more strongly than the most difficult problems (e.g., completing an analogy, such as “painter is to brush as writer is to…?”, i.e., problem type 4). Comparison problems, such as those exemplified in Figure 4B problem types 1 and 3, lend themselves more naturally to evaluation of a relation between relations than do completion problems (i.e., problem types 2 and 4). In Figure 4B, we show the hypothesized relational structures that people could create for these comparison problems. For completion problems, by contrast, participants could represent higher-order relations, but need not do so. Indeed, these problems could instead be solved by controlled semantic retrieval, wherein the initial relational term places constraints on the retrieval of an associate of item C. The results featured in Figure 4B illustrate the point that, although higher-order relational thinking is certainly cognitively demanding, task difficulty in and of itself cannot account for the engagement of RLPFC on relational reasoning tasks.
The LFPN and Reasoning Ability
In the prior section, we focused on the functions of specific regions within the LFPN. However, neuroscientific research in animals demonstrates that prefrontal and parietal cortices are tightly connected anatomically, and work closely together in the service of cognition (Fuster, 2008; Passingham and Wise, 2012). In humans, we can measure the structural integrity of white matter tracts that connect regions within the LFPN through the use of diffusion-weighted imaging techniques like diffusion tensor imaging (DTI). Additionally, we can measure functional connectivity within the LFPN by calculating temporal correlations in BOLD signal fluctuations between regions during periods when participants are either at rest or engaged in a task.
Functional connectivity analyses based on our fMRI studies of relational reasoning reveal that RLPFC, DLPFC, and IPL are more strongly coupled when participants integrate higher-order versus lower-order relational information (Wendelken et al., 2012; task shown in Figure 3), and that the strength of coupling between RLPFC and brain regions involved in either visuospatial or semantic processing depends on the type of relations participants are considering (Wendelken et al., 2012; task shown in Figure 3). Thus, a change in the type of relational thinking required leads to a change in the network properties of the LFPN.
Another way to demonstrate the link between the LFPN and relational thinking is to show a relationship between strength of network connectivity and cognitive task performance. Below we review evidence that strength of the LFPN contributes to developmental changes and individual differences in high-level cognition.
Developmental Changes and Individual Differences in the LFPN
Reasoning ability improves throughout childhood and well into adolescence in humans (Fry and Hale, 2000; McArdle et al., 2002), albeit with large individual differences. Figure 5A illustrates both the large age-related changes and high degree of interindividual variability, showing within- and between-subject changes with age in performance on a standard matrix reasoning test similar to the one featured in Figure 1C (Wechsler Abbreviated Scale of Intelligence; Wechsler, 1999).
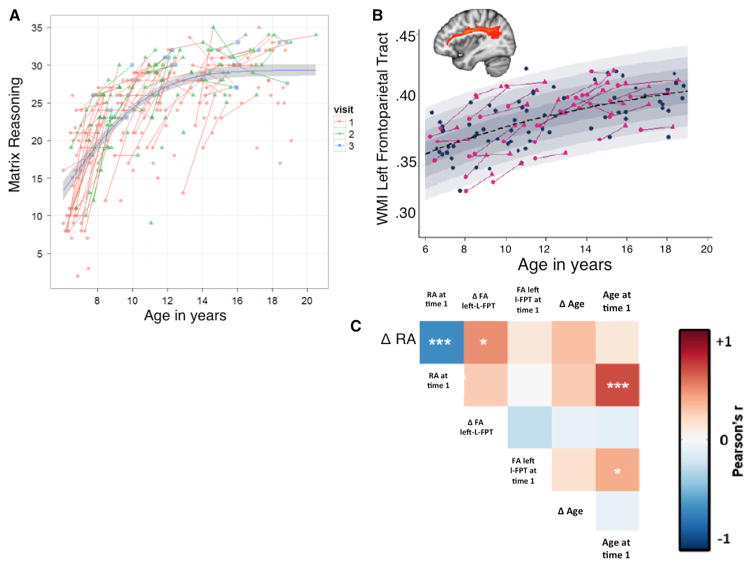
Figure 5. Development of Reasoning Ability.
(A) Developmental pattern of reasoning ability in 165 typically developing children and adolescents. Red, green, and blue dots indicate performance on the participant’s first, second, or third behavioral testing session, respectively. Red lines show changes in performance from the first to the second visit, and green lines show changes from the second to the third visit. The average delay between assessments was approximately 1.5 years.
(B) Increases in white matter integrity of a left frontoparietal tract connecting L RLPFC and L IPL increases nonlinearly across age. The dashed bar represents the mean, and each gray bar represents 0.5 SDs.
(C) Partial correlations plot demonstrating that participants’ changes in reasoning ability across sessions 1.5 years apart are positively correlated with a change in fractional anisotropy in this left frontoparietal tract. Figures reprinted with permission from Whitaker (2012).
Structural Brain Development
Structural MRI and diffusion-weighted imaging techniques have been used to measure gray and white matter maturation in the developing human brain (see Dennis and Thompson, 2013 for review). A reduction in cortical thickness with age was observed, such that thinning proceeded more quickly in sensory and motor cortex than in association cortex (Giedd and Rapoport, 2010). Longitudinal MRI research reveals that parietal cortex undergoes cortical thinning throughout childhood and early adolescence, whereas PFC undergoes these changes throughout adolescence and into early adulthood (Gogtay et al., 2004). At a cellular level, this cortical thinning is thought to reflect high levels of synaptic pruning—and also possibly a shift in the observed boundary between gray and white matter due to the myelination of fibers entering the cortical mantle (Zielinski et al., 2014). White matter maturation has been measured more specifically with DTI. Increased directionality of water diffusion in the brain measured over childhood and adolescence is thought to reflect myelination of long-range fibers (Lebel and Beaulieu, 2011). Longitudinal DTI research reveals structural changes well into adulthood in many white matter tracts (Lebel et al., 2008), and we have observed strong age-related changes throughout the entire white matter skeleton from ages 6–18 (Ferrer et al., 2013).
Developmental cognitive neuroscientists have begun to link specific anatomical changes to specific aspects of cognitive development, including reasoning, although this endeavor is complicated by the many anatomical and cognitive changes that take place at once in the developing child. Indeed, we have found that reasoning ability is strongly related to cortical thinning in prefrontal and parietal cortices, among other regions, but after controlling for the strong influence of age on both reasoning ability and cortical thinning across ages 6–18, we find no residual relationship between these variables (Wendelken et al., 2011). We set out to test whether the development of reasoning can be linked to strengthened communication between RLPFC and IPL. To this end, we used probabilistic tractography to identify white matter tracts extending between these regions in the left and right hemispheres (Whitaker, 2012). Indeed, white matter integrity in these frontoparietal tracts is strongly correlated with reasoning ability, although in cross-sectional analyses these relationships are no stronger than for a global measure of white matter integrity across the brain (Ferrer et al., 2013). The nonspecificity of this result is consistent with other work showing a relationship between fluid intelligence and white matter integrity of all major white matter tracts (Chiang et al., 2009; Tamnes et al., 2010; Haász et al., 2013). Longitudinal analyses, on the other hand, hint at the possibility that the development of the left RLPFC-IPL tract is a particularly strong predictor of changes in reasoning ability (Figure 5B; Whitaker, 2012). As shown in Figure 5C, the relationship between change in reasoning over the course of approximately 1.5 years and concomitant change in white matter integrity in the left RLPFC-IPL tract—but not the right RLPFC-IPL tract or global white matter—survived when partialling out initial values and effects of age. Consistent with the inference that these DTI data reflect individual differences in myelination, which should translate into differences in the speed of communication between brain regions, the relationship between white matter integrity and reasoning was mediated by processing speed (Ferrer et al., 2013).
LFPN Activation in Reasoning Tasks
fMRI studies of reasoning involving several age groups have demonstrated reliable age differences in PFC and parietal activation over development (Eslinger et al., 2009; Dumontheil et al., 2010). Specifically, using a first-order reasoning task, Eslinger et al. observed decreasing engagement of lateral PFC with increasing age (Eslinger et al., 2009). Dumontheil et al. observed an increase in RLPFC activation for second-order versus first-order reasoning problems from younger adolescents to older adolescents (Dumontheil et al., 2010). Results from our research complement and extend these results. We have found that even 7- to 10-year-olds strongly engaged LFPN regions while performing a relational matching task (Figure 6A), but that they activated these regions similarly for second- and first-order relations, such that only a small region within left DLPFC met significance for the direct contrast between these conditions (Figure 6B; Wendelken et al., 2011). By contrast, 11- to 14-year-olds engaged left and right DLPFC as well as dorsomedial PFC more strongly for second-order than first-order relations, and 15- to 18-year-olds engaged left RLPFC and bilateral IPL for this contrast. Indeed, only the older adolescents exhibited a pattern similar to that observed previously for adults with this paradigm (Bunge et al., 2009).
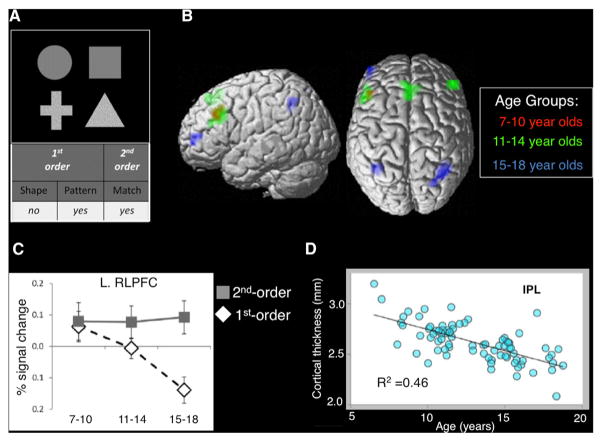
Figure 6. Age-Related Changes in the Brain that Support Reasoning Development.
(A) Task illustration from Wendelken et al. (2011). Each trial consisted of a yes/no judgment based on a stimulus array that contained four patterned shapes. On Shape and Pattern trials participants determined whether there was a shape or pattern match in either pair, respectively. On Match trials, participants decided whether the bottom pair of stimuli matched along the same dimension (i.e., Shape or Pattern) as the top pair of stimuli. Shape or Pattern trials require first-order relational processing, whereas Match trials require second-order relational integration.
(B) Whole-brain activation for the second-order > first-order contrast for 7- to 10-year-olds (red), 11-to 14-year-olds (green), and 15- to 18-year-olds (blue). Note: the lack of RLPFC and IPL for the younger-aged groups is due to the fact that these regions were equivalently engaged for first- and second-order relational trials.
(C) Across childhood and adolescence, left RLPFC became less engaged for first-order relational trials.
(D) Cortical thickness diminishes across age in the IPL. Functional specificity displayed in L RLPFC across age was negatively related to cortical thickness in IPL, such that the thinner the IPL cortex was, the more functional specificity observed in L RLPFC (data not shown). Figures adapted with permission from Wendelken et al. (2011).
Plotting left RLPFC activation as a function of age, we found no change in activation for second-order trials, but a decrease in activation on first-order trials (Figure 6C). Through structural equation modeling, we were able to show that this age-related decrease in RLPFC activation for first-order relations can be accounted for in part by cortical thinning in IPL (Figure 6D; Wendelken et al., 2011). We have hypothesized that cortical reorganization within the IPL leads to greater efficiency in processing first-order relations, thereby reducing relational processing demands within RLPFC. We hypothesize that increased communication between these brain regions over development supports their functional specialization.
LFPN Functional Connectivity
In childhood, functional connectivity appears to be dominated by connections among neighboring regions; during adolescence, there appears to be a shift toward greater long-range functional connectivity (Fair et al., 2008) that is likely driven by the increased speed of conduction associated with myelination (Khundrakpam et al., 2013; Hwang and Luna, 2013). However, it is not the case that all long-range connection strengths increase with age; rather, there is some evidence that they become more selective. In particular, we have found that temporal coupling between RLPFC and IPL increases over childhood and adolescence, whereas connectivity between RLPFC and the superior parietal lobule decreases.
Individual differences studies indicate that better reasoning ability is associated with stronger functional connectivity, in particular within the LFPN. Of greatest relevance to the present review, a study focusing on the neurodevelopment of second-order relational reasoning recently showed that frontoparietal connections strengthened with age, and were more tightly coupled than shorter-range frontoinsular connections for second- relative to first-order relational problems (Bazargani et al., 2014). More broadly, various studies have shown that tighter resting-state functional connectivity among LFPN regions is associated with better relational reasoning, as measured by nonverbal IQ scores (Langeslag et al., 2013) or full-scale IQ scores (Song et al., 2008; Li and Tian, 2014). Taken together, these studies suggest that tighter coupling within the LFPN is associated with better cognitive performance on various tasks, including relational reasoning.
Relational Reasoning in Humans as Compared with Other Primates
Having identified the key brain network that supports relational reasoning in humans, and having described how the development of this region coincides with our ability to perform relational reasoning, we next consider the possibility that small changes to the LFPN over evolution may have supported the emergence of higher-order relational reasoning in humans. To this end, we will first review comparative behavioral studies comparing human and nonhuman primates on relational reasoning tasks, and then discuss differences in neuroanatomy between humans and other primates.
Comparative Behavioral Differences on Relational Reasoning Tasks
In their 2008 article “Darwin’s Mistake,” Penn, Holyoak, and Povinelli argue that humans alone represent higher-order relational structures. To illustrate this idea, they describe a relational match-to-sample task that has been used in various species, in which participants are shown a pair of stimulus displays that are either identical or different (i.e., AA or AB), followed by two pairs of stimulus displays (i.e., CC and CD, where each letter stands for specific, nonoverlapping stimulus object arrays). The participant must decide which pair matches the first pair. For these tasks, the problem is to make a matching judgment among stimuli that—although they differ featurally—share a common relation (e.g., both pairs exhibit identical stimuli within pairs). Although great apes (Flemming et al., 2008; Haun and Call, 2009) and Old World monkeys (Flemming et al., 2013) are capable of solving these types of problems, Penn et al. claim that humans alone use higher-order abstract categories of “same” and “different” to guide their judgment, whereas other nonhuman primates may rely on perceptual variability of items within each pair to guide their decision.
Evidence in favor of the idea that nonhuman primates rely on perceptual similarity to solve relational-match-to-sample tasks comes from several studies (Fagot et al., 2001; see also Fagot and Thompson, 2011; Flemming et al., 2013). In one such study, baboons and humans performed a task in which the number of items in the stimulus display varied between 2 and 16 (Fagot et al., 2001). As the set size increases, identical and nonidentical stimulus sets become more and more perceptually dissimilar (i.e., AAAAA versus ABCDE, as compared with AA versus AB, where each letter represents a different object), thereby rendering the task of relational matching easier for a participant relying on a perceptual strategy. Indeed, set size influenced task accuracy for baboons, but not humans: baboons exhibited chance performance (50%) when asked to make difference judgments for set sizes below four, whereas humans never performed below 86%, regardless of the set size. This finding suggests that humans and nonhuman primates use different strategies when performing the relational match task.
In addition to strategy differences between species, humans are much faster than nonhuman primates when it comes to performing relational reasoning tasks. Humans can grasp novel relational tasks very quickly with verbal instructions (Cole et al., 2011), but even when humans and baboons are both taught a relational matching task through trial and error, it takes baboons over ten times as many trials to learn a basic form of the task—i.e., AAAA matches BBBB, not CDEF (400 versus 35 trials on average to reach criterion of 80% accuracy; Flemming et al., 2013). These results do not necessarily prove that nonhuman primates are unable to reason using higher-order thinking, but if it is possible to train nonhumans to produce human-like performance on tasks associated with higher-order relational thinking, it is certainly not something that comes naturally to them.
Cortical Expansion Differences Are Found in Regions Supporting Relational Reasoning
Our neuroanatomy comparison between human and nonhuman primates will first begin with gross anatomical differences in LFPN regions across species. Although humans and chimpanzees begin their neurodevelopment in a similar fashion, relative to chimpanzee neural development, the human neocortex develops at a faster rate even by the 16th week in gestation and continues to grow at a relatively faster rate after birth (Sakai et al., 2012). The end result is that the average human brain is triple the size of the chimpanzee brain (Boddy et al., 2012). Such an increase is partly due to humans having greater cortical thickness, but this increase pales in comparison to our relatively much greater cortical surface area (see Geschwind and Rakic, 2013 for a review). Far from this cortical expansion being equally likely for all brain regions, humans’ cortical expansion occurs more in association areas (i.e., prefrontal, parietal, and temporal cortex). In turn, we have slightly smaller surface area relative to chimpanzees in primary motor and sensory areas (Buckner and Krienen, 2013). Buckner and Krienen suggest that these different patterns in cortical expansion across evolution may differentially promote higher-order cognition supported by association cortex, possibly at the expense of greater motor and sensory processing. Interestingly, regions showing the greatest expansion in surface area across development overlap with those found to have the greatest increase relative to nonhuman primates (Figure 7; Fjell et al., 2013).
Figure 7. Cortical Expansion Differences between Primate Species.
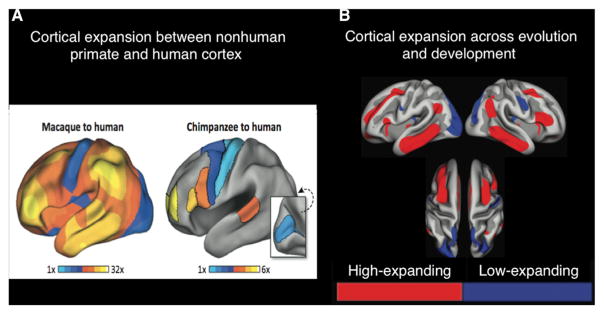
(A) Areas showing greatest (in yellow) amount of cortical expansion from macaques to humans and chimpanzees to humans. Again, the area of greatest cortical expansion between species is anterior prefrontal cortex (BA 10). Numbers in the horizontal bars represent the amount of expansion in humans compared to macaques (left) and chimpanzees (right). Figure adapted with permission from Buckner and Krienen (2013).
(B) Cortical regions showing a shared amount of cortical expansion across evolution (relative to macaques) and across human development. Note the lateral prefrontal and posterior parietal regions are found to be associated with higher cognition in humans. Figure adapted with permission from Fjell et al. (2013).
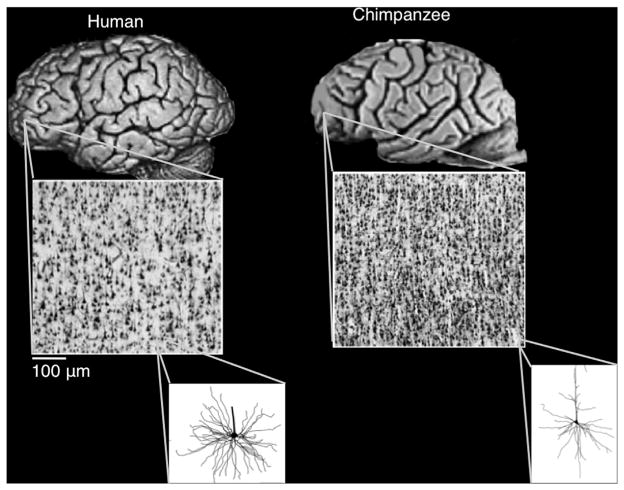
Evidence for cortical expansion in regions supporting higher cognition is corroborated by studies investigating the horizontal spacing distance (see Rakic, 2009). Investigating prefrontal and parietal layer III—a locus for pyramidal cell bodies as well as inter- and intrahemispheric connections—has identified fewer pyramidal neurons, spaced further apart in humans relative to chimpanzees and macaques (Semendeferi et al., 2011; Spocter et al., 2012). A greater horizontal spacing difference suggests that the expanded surface area contains more dendrites and axons, suggesting more of an integrative function of these neurons in humans. Indeed, human pyramidal neurons have greater dendritic length, density, and dendritic spines in prefrontal and parietal cortex than other regions (e.g., primary motor cortex or superior temporal gyrus), thus suggesting a highly integrative role of neurons in this region (see Figure 8; Bianchi et al., 2013).
Figure 8. Cytoarchitectonic Differences in Anterior PFC between Primate Species.
Increased horizontal spacing distance (HSD) between humans and chimpanzee in the anterior PFC (BA 10). Greater HSD is a proxy for increased cortical expansion, and the relatively smaller number of pyramidal cells in these regions for humans likely reflects the extensive density of dendrites and dendritic spines, useful for integrating information. Slices taken from anterior prefrontal cortex (i.e., BA 10 in humans). Figures adapted with permission from Semendeferi et al. (2011). Note: locations for figure insets are approximate.
Differences in Structural Organization of the LFPN between Human and Nonhuman Primates
Until the advent of functional and structural connectivity measures using MRI, most of our understanding of the cytoarchitecture of prefrontal and parietal cortices has relied on the macaque model (Petrides and Pandya, 2007; Petrides et al., 2012). The comparative validity of the macaque model has been called into question (Passingham, 2009). More recently, several studies have used MRI to investigate how functional and structural connections between prefrontal and parietal regions compare between species (Mars et al., 2011; Petrides et al., 2012; Sallet et al., 2013; Goulas et al., 2014; Neubert et al., 2014). As described below, these studies have found a high degree of overlap between humans, macaques, and chimpanzees for certain brain structures, although structural differences have been reported both for lateral PFC and IPL. Indeed, as reviewed below, it has been suggested that RLPFC has no homolog in the macaque brain, although its function may be related more closely to macaque DLPFC than macaque frontopolar cortex (Neubert et al., 2014).
In humans, RLPFC has been hypothesized to sit at the apex of a hierarchically organized PFC (Badre and D’Esposito, 2009; Donoso et al., 2014). Goulas et al. (2014) show that this is not the case in macaques. The authors characterized structural connectivity among PFC regions in the macaque and tested whether the macaque PFC reflects a similar hierarchical caudoraustral gradient as found in humans. To test whether the structural connectivity patterns represented a hierarchy, Goulas et al. employed a simulated annealing technique (Kirkpatrick et al., 1983) as a way to approximate the acyclic graph with the fewest removals of antihierarchical connections.
Although the macaque prefrontal structural organization can be represented as a hierarchy, there were substantial differences in the hierarchical structure as compared with humans. In humans, fMRI studies have placed RLPFC at the top of the hierarchy given its efferent connections with DLPFC as compared to more caudal prefrontal regions (Badre and D’Esposito, 2009). However, in the macaque, frontopolar cortex ranked below both DLPFC and VLPFC in the structural hierarchy (Goulas et al., 2014). These results lend credence to the idea that RLPFC in macaques does not perform the same functional role as RLPFC in humans. Thus, differences between species in hierarchical structural connections within the PFC may explain differences in relational reasoning ability.
Differences in Functional Organization of the LFPN between Human and Nonhuman Primates
The discrepancy in the structural hierarchical organization of the prefrontal cortex between humans and macaques may explain cognitive differences; however, another hypothesis is that there are LFPN connections unique to humans. Differences between humans and macaques were found when investigating functional and structural connections of RLPFC and inferior parietal regions using diffusion-weighted MRI (Li et al., 2013; Miranda-Dominguez et al., 2014). As can be seen in Figure 9A, frontoparietal regions were found to have a larger number of functional connections to other regions, thus demonstrating greater possibility for integration of information within these regions (Miranda-Dominguez et al., 2014). Additional support for this claim comes from several studies comparing functional (Mars et al., 2011; Neubert et al., 2014) and structural (Li et al., 2013) networks between humans and macaques. In one study, researchers used DTI to estimate structural frontoparietal connections, and then used structural connections to constrain possible functional connections among regions using resting-state fMRI (Mars et al., 2011).
Figure 9. Functional Connectivity Differences between Primate Species.
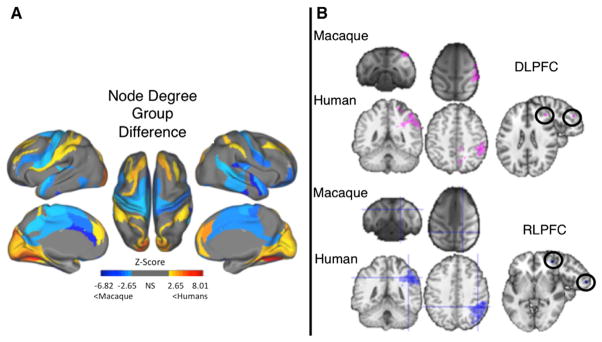
(A) Interspecies comparison of node degree, or the number of functional connections a particular region has to all other regions. Blue colors represent regions where macaques have a higher node degree, and red colors represent regions where humans have higher node degree than macaques. Notice that humans have more highly connected hubs distributed within a lateral frontoparietal network. Figure adapted with permission from Miranda-Dominguez et al. (2014).
(B) Intrinsic functional connectivity maps for DLPFC and RLPFC seeds in humans and macaques. Using DTI, the authors found ten different parcellations of parietal cortex, and used these regions as “seed” regions for resting state fMRI (rsfMRI). Seed regions are regions used in a regression analysis to identify other cortical and subcortical regions sharing a similar time course during a task-free fMRI scan. Although there was much similarity for frontoparietal functional connectivity when using the DLPFC as a seed region, there was no macaque analog for the frontoparietal connections observed between RLPFC and posterior parietal cortex, even when using a much less conservative statistical threshold. Figure adapted with permission from Mars et al. (2011).
Although there was much overlap among patterns of intrinsic frontoparietal functional connectivity between species, this study revealed that humans, but not macaques, exhibit tight resting-state functional connectivity between mid-IPL and RLPFC (see Figure 9B). These results are consistent with the possibility that humans have a direct anatomical projection between IPL and RLPFC that could support higher-order relational thinking (see Figure 5B for results of probabilistic tractography tracing white matter between these regions). These findings suggest that changes in connections between RLPFC and IPL throughout the course of evolution may have resulted in observed differences between human and nonhuman primates (see Rilling, 2014 for a review). Additional research investigating appropriate parcellation of human and chimpanzee neocortex is needed to fully understand how the LFPN may or may not map across species; however, if such findings hold, this would suggest that networks between anterior prefrontal and parietal cortex are unique to humans, and may be a neuroanatomical substrate supporting our ability for higher-order thought.
Conclusion
The ability to represent higher-order relations has been proposed as a cornerstone of human cognition (Penn et al., 2008; Bunge and Preuss, 2010). Here we propose that cortical expansion of RLPFC and IPL and strengthened LFPN connectivity in humans relative to other primates contribute to species differences in relational thinking. Likewise, we propose that age-related strengthening of the LFPN, including RLPFC-IPL coupling, contributes to the development of relational thinking over childhood and adolescence. To conclude, we argue that a little goes a long way: that is, fairly small changes to a core brain network, both over the course of primate evolution and over the course of human development, enabled higher-order relational thinking, which is central to human cognition.
Acknowledgments
This work was funded by a James S. McDonnell Foundation Scholar Award to S.A.B. The authors thank Belén Guerra-Carrillo for input on this manuscript and Carter Wendelken for his input and contributions to this line of research.
References
- Badre D, D’Esposito M. Is the rostrocaudal axis of the frontal lobe hierarchical? Nat Rev Neurosci. 2009;10:659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazargani N, Hillebrandt H, Christoff K, Dumontheil I. Developmental changes in effective connectivity associated with relational reasoning. Hum Brain Mapp. 2014;35:3262–3276. doi: 10.1002/hbm.22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Frith CD, Burgess PW. Rostral prefrontal cortex and the focus of attention in prospective memory. Cereb Cortex. 2012;22:1876–1886. doi: 10.1093/cercor/bhr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi S, Stimpson CD, Bauernfeind AL, Schapiro SJ, Baze WB, McArthur MJ, Bronson E, Hopkins WD, Semendeferi K, Jacobs B, et al. Dendritic morphology of pyramidal neurons in the chimpanzee neocortex: regional specializations and comparison to humans. Cereb Cortex. 2013;23:2429–2436. doi: 10.1093/cercor/bhs239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy AM, McGowen MR, Sherwood CC, Grossman LI, Goodman M, Wildman DE. Comparative analysis of encephalization in mammals reveals relaxed constraints on anthropoid primate and cetacean brain scaling. J Evol Biol. 2012;25:981–994. doi: 10.1111/j.1420-9101.2012.02491.x. [DOI] [PubMed] [Google Scholar]
- Boorman ED, Behrens TE, Rushword MF. Counterfactual choice and learning in a neural network centered on human lateral frontopolar cortex. PLoS Biol. 2011;9:e1001093. doi: 10.1371/journal.pbio.1001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM. The evolution of distributed association networks in the human brain. Trends Cogn Sci. 2013;17:648–665. doi: 10.1016/j.tics.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wendelken C. Comparing the bird in the hand with the ones in the bush. Neuron. 2009;62:609–611. doi: 10.1016/j.neuron.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Preuss TM. Encyclopedia of Behavioral Neuroscience. Oxford: Elsevier; 2010. [Google Scholar]
- Bunge SA, Helskog EH, Wendelken C. Left, but not right, rostrolateral prefrontal cortex meets a stringent test of the relational integration hypothesis. Neuroimage. 2009;46:338–342. doi: 10.1016/j.neuroimage.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Barysheva M, Shattuck DW, Lee AD, Madsen SK, Avedissian C, Klunder AD, Toga AW, McMahon KL, de Zubicaray GI, et al. Genetics of brain fiber architecture and intellectual performance. J Neurosci. 2009;29:2212–2224. doi: 10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Moody TD, Fernandino L, Mumford JA, Poldrack RA, Cannon TD, Knowlton BJ, Holyoak KJ. Common and dissociable pre-frontal loci associated with component mechanisms of analogical reasoning. Cereb Cortex. 2010;20:524–533. doi: 10.1093/cercor/bhp121. [DOI] [PubMed] [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JDE. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14:1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Christoff K, Keramatian K, Gordon AM, Smith R, Mädler B. Prefrontal organization of cognitive control according to levels of abstraction. Brain Res. 2009;1286:94–105. doi: 10.1016/j.brainres.2009.05.096. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention to memory: a hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46:1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9:83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cole MW, Etzel JA, Zacks JM, Schneider W, Braver TS. Rapid transfer of abstract rules to novel contexts in human lateral prefrontal cortex. Front Hum Neurosci. 2011;5:142. doi: 10.3389/fnhum.2011.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, van Leijenhorst L, Honomichl RD, Christoff K, Bunge SA. Neurocognitive development of relational reasoning. Dev Sci. 2009;12:55–66. doi: 10.1111/j.1467-7687.2008.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Thompson PM. Typical and atypical brain development: a review of neuroimaging studies. Dialogues Clin Neurosci. 2013;15:359–384. doi: 10.31887/DCNS.2013.15.3/edennis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso M, Collins AGE, Koechlin E. Human cognition. Foundations of human reasoning in the prefrontal cortex. Science. 2014;344:1481–1486. doi: 10.1126/science.1252254. [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Houlton R, Christoff K, Blakemore SJ. Development of relational reasoning during adolescence. Dev Sci. 2010;13:F15–F24. doi: 10.1111/j.1467-7687.2010.01014.x. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Blair C, Wang J, Lipovsky B, Realmuto J, Baker D, Thorne S, Gamson D, Zimmerman E, Rohrer L, Yang QX. Developmental shifts in fMRI activations during visuospatial relational reasoning. Brain Cogn. 2009;69:1–10. doi: 10.1016/j.bandc.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagot J, Thompson RKR. Generalized relational matching by guinea baboons (Papio papio) in two-by-two-item analogy problems. Psychol Sci. 2011;22:1304–1309. doi: 10.1177/0956797611422916. [DOI] [PubMed] [Google Scholar]
- Fagot J, Wasserman EA, Young ME. Discriminating the relation between relations: the role of entropy in abstract conceptualization by baboons (Papio papio) and humans (Homo sapiens) J Exp Psychol Anim Behav Process. 2001;27:316–328. [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain’s default network. Proc Natl Acad Sci USA. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Peng L, Chang-Quan L, Yi L, Hong L. Relational complexity modulates activity in the prefrontal cortex during numerical inductive reasoning: an fMRI study. Biol Psychol. 2014;101:61–68. doi: 10.1016/j.biopsycho.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Ferrer E, Whitaker KJ, Steele JS, Green CT, Wendelken C, Bunge SA. White matter maturation supports the development of reasoning ability through its influence on processing speed. Dev Sci. 2013;16:941–951. doi: 10.1111/desc.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Tamnes CK, Grydeland H, Engvig A, Espeseth T, Reinvang I, Lundervold AJ, Lundervold A, Walhovd KB. High-expanding cortical regions in human development and evolution are related to higher intellectual abilities. Cereb Cortex. 2013 doi: 10.1093/cercor/bht201. Published online August 19, 2013 http://dx.doi.org/10.1093/cercor/bht201. [DOI] [PubMed]
- Flemming TM, Beran MJ, Thompson RKR, Kleider HM, Washburn DA. What meaning means for same and different: Analogical reasoning in humans (Homo sapiens), chimpanzees (Pan troglodytes), and rhesus monkeys (Macaca mulatta) J Comp Psychol. 2008;122:176–185. doi: 10.1037/0735-7036.122.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming TM, Thompson RKR, Fagot J. Baboons, like humans, solve analogy by categorical abstraction of relations. Anim Cogn. 2013;16:519–524. doi: 10.1007/s10071-013-0596-0. [DOI] [PubMed] [Google Scholar]
- Fry AF, Hale S. Relationships among processing speed, working memory, and fluid intelligence in children. Biol Psychol. 2000;54:1–34. doi: 10.1016/s0301-0511(00)00051-x. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. London: Academic Press; 2008. [Google Scholar]
- Gentner D. Bootstrapping the mind: analogical processes and symbol systems. Cogn Sci. 2010;34:752–775. doi: 10.1111/j.1551-6709.2010.01114.x. [DOI] [PubMed] [Google Scholar]
- Gerlach KD, Spreng RN, Madore KP, Schacter DL. Future planning: default network activity couples with frontoparietal control network and reward-processing regions during process and outcome simulations. Soc Cogn Affect Neurosci. 2014 doi: 10.1093/scan/nsu001. Published online May 12, 2014 http://dx.doi.org/10.1093/scan/nsu001. [DOI] [PMC free article] [PubMed]
- Geschwind DH, Rakic P. Cortical evolution: judge the brain by its cover. Neuron. 2013;80:633–647. doi: 10.1016/j.neuron.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulas A, Uylings HBM, Stiers P. Mapping the hierarchical layout of the structural network of the macaque prefrontal cortex. Cereb Cortex. 2014;24:1178–1194. doi: 10.1093/cercor/bhs399. [DOI] [PubMed] [Google Scholar]
- Gross CG. Hippocampus minor and man’s place in nature: a case study in the social construction of neuroanatomy. Hippocampus. 1993;3:403–415. doi: 10.1002/hipo.450030403. [DOI] [PubMed] [Google Scholar]
- Haász J, Westlye ET, Fjær S, Espeseth T, Lundervold A, Lundervold AJ. General fluid-type intelligence is related to indices of white matter structure in middle-aged and old adults. Neuroimage. 2013;83:372–383. doi: 10.1016/j.neuroimage.2013.06.040. [DOI] [PubMed] [Google Scholar]
- Halford GS, Wilson WH, Phillips S. Processing capacity defined by relational complexity: implications for comparative, developmental, and cognitive psychology. Behav Brain Sci. 1998;21:803–831. doi: 10.1017/s0140525x98001769. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Thompson R, Duncan J, Owen AM. Lateral prefrontal cortex subregions make dissociable contributions during fluid reasoning. Cereb Cortex. 2011;21:1–10. doi: 10.1093/cercor/bhq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haun DBM, Call J. Great apes’ capacities to recognize relational similarity. Cognition. 2009;110:147–159. doi: 10.1016/j.cognition.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Hwang K, Luna B. Principles of Frontal Lobe Function. New York: Oxford University Press; 2013. [Google Scholar]
- Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci. 2007;30:135–154. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Khundrakpam BS, Reid A, Brauer J, Carbonell F, Lewis J, Ameis S, Karama S, Lee J, Chen Z, Das S, Evans AC Brain Development Cooperative Group. Developmental changes in organization of structural brain networks. Cereb Cortex. 2013;23:2072–2085. doi: 10.1093/cercor/bhs187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick S, Gelatt CD, Jr, Vecchi MP. Optimization by simulated annealing. Science. 1983;220:671–680. doi: 10.1126/science.220.4598.671. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318:594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC. The cognition and neuroscience of relational reasoning. Brain Res. 2012;1428:13–23. doi: 10.1016/j.brainres.2010.11.080. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, McClelland MM, Donovan CM, Tillman GD, Maguire MJ. An fMRI investigation of cognitive stages in reasoning by analogy. Brain Res. 2010a;1342:63–73. doi: 10.1016/j.brainres.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, Hanten G, Wilde EA, Li X, Schnelle KP, Merkley TL, Vasquez AC, Cook LG, McClelland M, Chapman SB, Levin HS. Deficits in analogical reasoning in adolescents with traumatic brain injury. Front Hum Neurosci. 2010b doi: 10.3389/fnhum.2010.00062. Published online August 19, 2010 http://dx.doi.org/10.3389/fnhum.2010.00062. [DOI] [PMC free article] [PubMed]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: a parametric study of relational complexity. Cereb Cortex. 2002;12:477–485. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- Langeslag SJE, Schmidt M, Ghassabian A, Jaddoe VW, Hofman A, van der Lugt A, Verhulst FC, Tiemeier H, White TJH. Functional connectivity between parietal and frontal brain regions and intelligence in young children: the Generation R study. Hum Brain Mapp. 2013;34:3299–3307. doi: 10.1002/hbm.22143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Li C, Tian L. Association between resting-state coactivation in the parieto-frontal network and intelligence during late childhood and adolescence. AJNR Am J Neuroradiol. 2014;35:1150–1156. doi: 10.3174/ajnr.A3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Hu X, Preuss TM, Glasser MF, Damen FW, Qiu Y, Rilling J. Mapping putative hubs in human, chimpanzee and rhesus macaque connectomes via diffusion tractography. Neuroimage. 2013;80:462–474. doi: 10.1016/j.neuroimage.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria AR. Higher Cognitive Functions in Man. Oxford: Basic Books; 1966. [Google Scholar]
- Mars RB, Jbabdi S, Sallet J, O’Reilly JX, Croxson PL, Olivier E, Noonan MP, Bergmann C, Mitchell AS, Baxter MG, et al. Diffusion-weighted imaging tractography-based parcellation of the human parietal cortex and comparison with human and macaque resting-state functional connectivity. J Neurosci. 2011;31:4087–4100. doi: 10.1523/JNEUROSCI.5102-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ, Ferrer-Caja E, Hamagami F, Woodcock RW. Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Dev Psychol. 2002;38:115–142. [PubMed] [Google Scholar]
- Miranda-Dominguez O, Mills BD, Grayson D, Woodall A, Grant KA, Kroenke CD, Fair DA. Bridging the gap between the human and macaque connectome: a quantitative comparison of global interspecies structure-function relationships and network topology. J Neurosci. 2014;34:5552–5563. doi: 10.1523/JNEUROSCI.4229-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert FX, Mars RB, Thomas AG, Sallet J, Rushworth MFS. Comparison of human ventral frontal cortex areas for cognitive control and language with areas in monkey frontal cortex. Neuron. 2014;81:700–713. doi: 10.1016/j.neuron.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Passingham R. How good is the macaque monkey model of the human brain? Curr Opin Neurobiol. 2009;19:6–11. doi: 10.1016/j.conb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham RE, Wise SP. The Neurobiology of the Prefrontal Cortex. Oxford: Oxford University Press; 2012. [Google Scholar]
- Penn DC, Holyoak KJ, Povinelli DJ. Darwin’s mistake: explaining the discontinuity between human and nonhuman minds. Behav Brain Sci. 2008;31:109–130. doi: 10.1017/S0140525X08003543. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN. The prefrontal cortex: comparative architectonic organization in the human and the macaque monkey brains. Cortex. 2012;48:46–57. doi: 10.1016/j.cortex.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Smith JAL, Desmond JE, Glover GH, Gabrieli JDE. Neural substrates of fluid reasoning: an fMRI study of neocortical activation during performance of the Raven’s Progressive Matrices Test. Cognit Psychol. 1997;33:43–63. doi: 10.1006/cogp.1997.0659. [DOI] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nature. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Rilling JK. Comparative primate neuroimaging: insights into human brain evolution. Trends Cogn Sci. 2014;18:46–55. doi: 10.1016/j.tics.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Roca M, Parr A, Thompson R, Woolgar A, Torralva T, Antoun N, Manes F, Duncan J. Executive function and fluid intelligence after frontal lobe lesions. Brain. 2010;133:234–247. doi: 10.1093/brain/awp269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Hirata S, Fuwa K, Sugama K, Kusunoki K, Makishima H, Eguchi T, Yamada S, Ogihara N, Takeshita H. Fetal brain development in chimpanzees versus humans. Curr Biol. 2012;22:791–792. doi: 10.1016/j.cub.2012.06.062. [DOI] [PubMed] [Google Scholar]
- Sallet J, Mars RB, Noonan MP, Neubert FX, Jbabdi S, O’Reilly JX, Filippini N, Thomas AG, Rushworth MF. The organization of dorsal frontal cortex in humans and macaques. J Neurosci. 2013;33:12255–12274. doi: 10.1523/JNEUROSCI.5108-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semendeferi K, Teffer K, Buxhoeveden DP, Park MS, Bludau S, Amunts K, Travis K, Buckwalter J. Spatial organization of neurons in the frontal pole sets humans apart from great apes. Cereb Cortex. 2011;21:1485–1497. doi: 10.1093/cercor/bhq191. [DOI] [PubMed] [Google Scholar]
- Song M, Zhou Y, Li J, Liu Y, Tian L, Yu C, Jiang T. Brain spontaneous functional connectivity and intelligence. Neuroimage. 2008;41:1168–1176. doi: 10.1016/j.neuroimage.2008.02.036. [DOI] [PubMed] [Google Scholar]
- Spocter MA, Hopkins WD, Barks SK, Bianchi S, Hehmeyer AE, Anderson SM, Stimpson CD, Fobbs AJ, Hof PR, Sherwood CC. Neuropil distribution in the cerebral cortex differs between humans and chimpanzees. J Comp Neurol. 2012;520:2917–2929. doi: 10.1002/cne.23074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Knight RT. Principles of Frontal Lobe Function. New York: Oxford University Press; 2013. [Google Scholar]
- Tamnes CK, Østby Y, Walhovd KB, Westlye LT, Due-Tønnessen P, Fjell AM. Intellectual abilities and white matter microstructure in development: a diffusion tensor imaging study. Hum Brain Mapp. 2010;31:1609–1625. doi: 10.1002/hbm.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Opstal F, Verguts T. Is there a generalized magnitude system in the brain? Behavioral, neuroimaging, and computational evidence. Front Psychol. 2013;4:435. doi: 10.3389/fpsyg.2013.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CE, Chatterjee A. A bilateral frontoparietal network underlies visuospatial analogical reasoning. Neuroimage. 2012;59:2831–2838. doi: 10.1016/j.neuroimage.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York: The Psychological Corporation: Harcourt Brace & Company; 1999. [Google Scholar]
- Wendelken C, Bunge SA. Transitive inference: distinct contributions of rostrolateral prefrontal cortex and the hippocampus. J Cogn Neurosci. 2010;22:837–847. doi: 10.1162/jocn.2009.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C, Bunge SA, Carter CS. Maintaining structured information: an investigation into functions of parietal and lateral prefrontal cortices. Neuropsychologia. 2008a;46:665–678. doi: 10.1016/j.neuropsychologia.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Wendelken C, Nakhabenko D, Donohue SE, Carter CS, Bunge SA. “Brain is to thought as stomach is to??”: investigating the role of rostrolateral prefrontal cortex in relational reasoning. J Cogn Neurosci. 2008b;20:682–693. doi: 10.1162/jocn.2008.20055. [DOI] [PubMed] [Google Scholar]
- Wendelken C, O’Hare ED, Whitaker KJ, Ferrer E, Bunge SA. Increased functional selectivity over development in rostrolateral prefrontal cortex. J Neurosci. 2011;31:17260–17268. doi: 10.1523/JNEUROSCI.1193-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C, Chung D, Bunge SA. Rostrolateral prefrontal cortex: domain-general or domain-sensitive? Hum Brain Mapp. 2012;33:1952–1963. doi: 10.1002/hbm.21336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker K. Doctoral dissertation. 2012 retrieved from http://escholarship.org/uc/item/3vs463b7?query=Whitaker#.
- Woolgar A, Parr A, Cusack R, Thompson R, Nimmo-Smith I, Torralva T, Roca M, Antoun N, Manes F, Duncan J. Fluid intelligence loss linked to restricted regions of damage within frontal and parietal cortex. Proc Natl Acad Sci USA. 2010;107:14899–14902. doi: 10.1073/pnas.1007928107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolgar A, Bor D, Duncan J. Global increase in task-related fronto-parietal activity after focal frontal lobe lesion. J Cogn Neurosci. 2013;25:1542–1552. doi: 10.1162/jocn_a_00432. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski BA, Prigge MBD, Nielsen JA, Froehlich AL, Abildskov TJ, Anderson JS, Fletcher PT, Zygmunt KM, Travers BG, Lange N, et al. Longitudinal changes in cortical thickness in autism and typical development. Brain. 2014;137:1799–1812. doi: 10.1093/brain/awu083. [DOI] [PMC free article] [PubMed] [Google Scholar]