Abstract
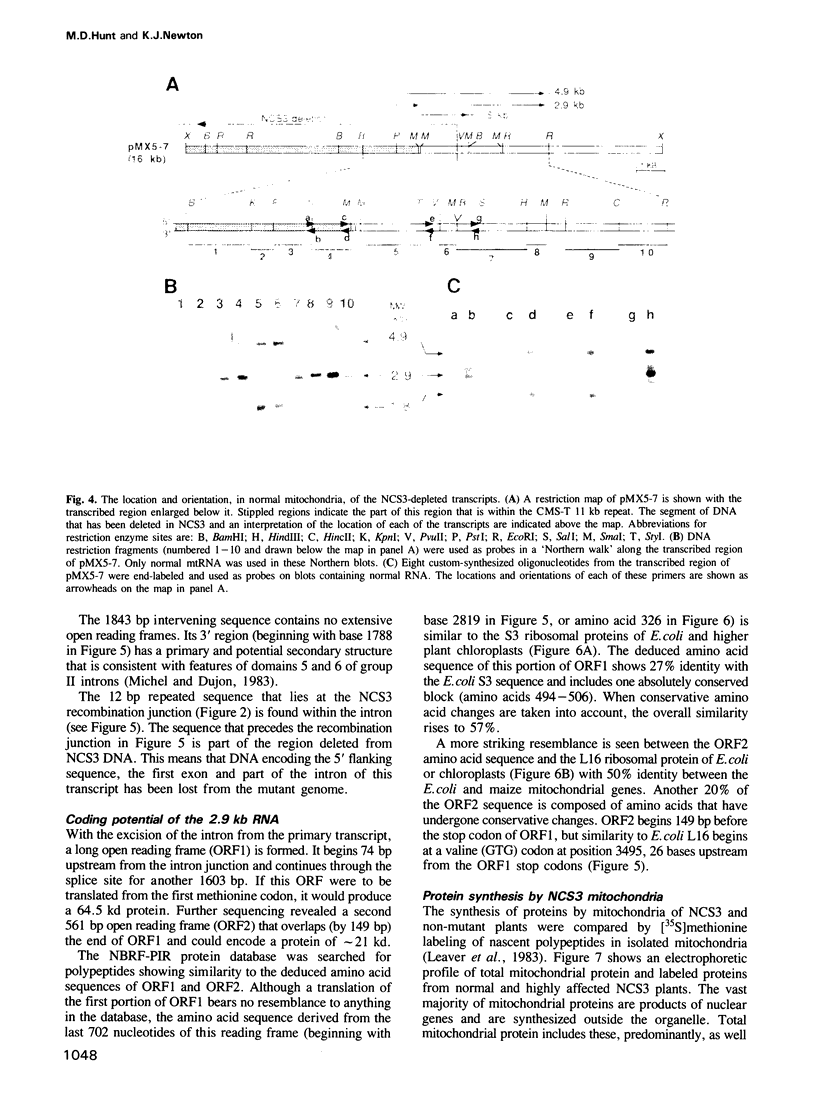
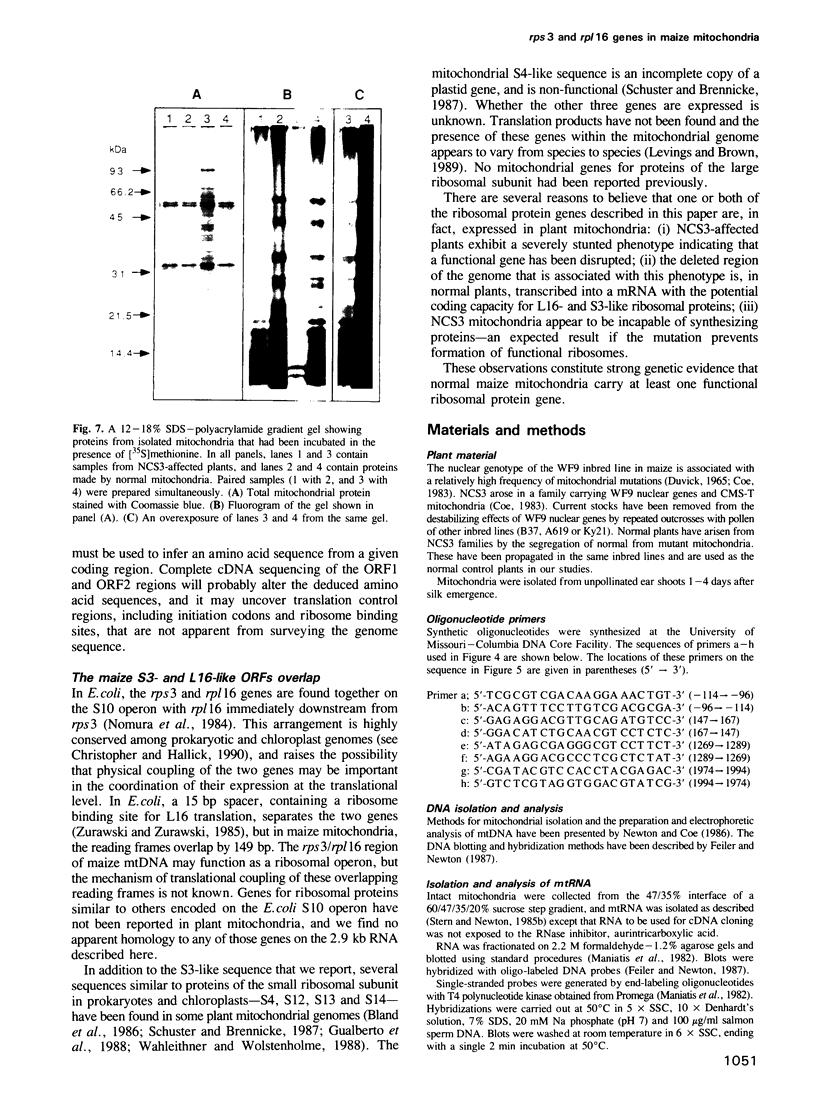
A deletion eliminating part of a transcribed region of mitochondrial DNA (mtDNA) has been found in the maize nonchromosomal stripe 3 (NCS3) mutant. This results in the specific loss of a set of three mitochondrial RNAs consisting, in normal plants, of a 4.9 kb transcript, its 1.8 kb intron and the resulting processed mRNA of approximately 2.9 kb. In the NCS3 mitochondrial genome the DNA encoding the putative promoter and 5' end of the affected RNAs is missing. This transcribed region of normal maize mtDNA has been sequenced and the intron splice junction has been determined. The 2.9 kb processed mRNA carries two overlapping open reading frames (ORFs) with predicted amino acid sequences that show similarity to two Escherichia coli ribosomal proteins, S3 (rps3) and L16 (rpl16). The presence of severe stunting and striping in NCS3 plants correlates absolutely with the molecular changes described here. This fact and the impaired ability for mitochondrial protein synthesis by NCS3 plants indicate that one or both of these reading frames are translated to functional ribosomal proteins in normal maize mitochondria.
Full text
PDF
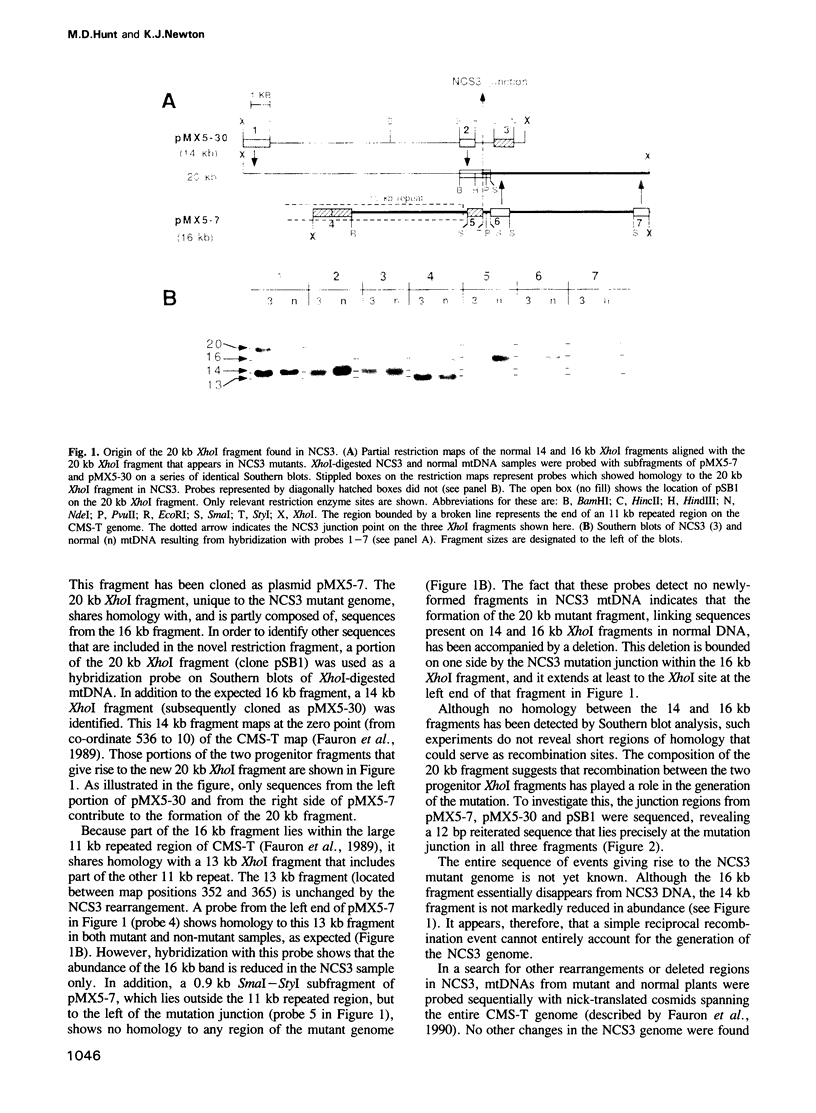
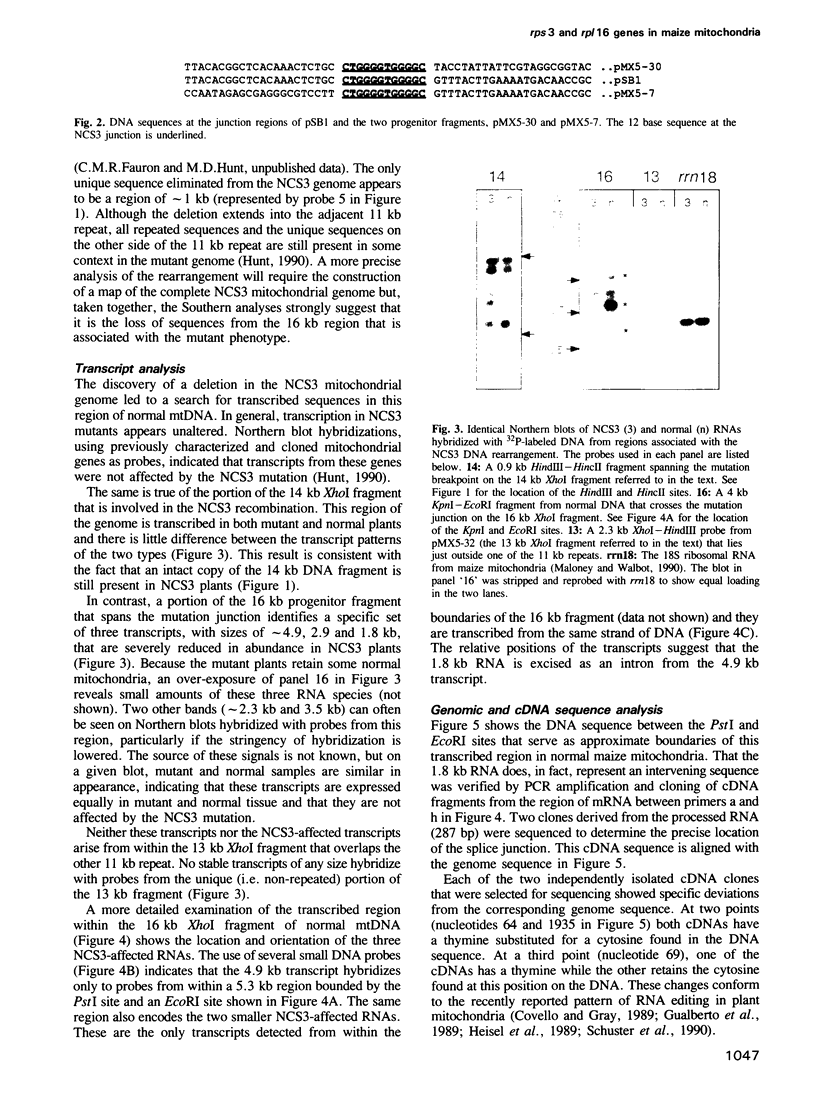



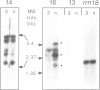
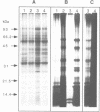
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Bland M. M., Levings C. S., 3rd, Matzinger D. F. The tobacco mitochondrial ATPase subunit 9 gene is closely linked to an open reading frame for a ribosomal protein. Mol Gen Genet. 1986 Jul;204(1):8–16. doi: 10.1007/BF00330180. [DOI] [PubMed] [Google Scholar]
- Christopher D. A., Hallick R. B. Complex RNA maturation pathway for a chloroplast ribosomal protein operon with an internal tRNA cistron. Plant Cell. 1990 Jul;2(7):659–671. doi: 10.1105/tpc.2.7.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P., Newton K. J. Maize nuclear background regulates the synthesis of a 22-kDa polypeptide in Zea luxurians mitochondria. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7423–7426. doi: 10.1073/pnas.86.19.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covello P. S., Gray M. W. RNA editing in plant mitochondria. Nature. 1989 Oct 19;341(6243):662–666. doi: 10.1038/341662a0. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Fauron C. M., Havlik M., Brettell R. I. The mitochondrial genome organization of a maize fertile cmsT revertant line is generated through recombination between two sets of repeats. Genetics. 1990 Feb;124(2):423–428. doi: 10.1093/genetics/124.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiler H. S., Newton K. J. Altered mitochondrial gene expression in the nonchromosomal stripe 2 mutant of maize. EMBO J. 1987 Jun;6(6):1535–1539. doi: 10.1002/j.1460-2075.1987.tb02397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualberto J. M., Lamattina L., Bonnard G., Weil J. H., Grienenberger J. M. RNA editing in wheat mitochondria results in the conservation of protein sequences. Nature. 1989 Oct 19;341(6243):660–662. doi: 10.1038/341660a0. [DOI] [PubMed] [Google Scholar]
- Gualberto J. M., Wintz H., Weil J. H., Grienenberger J. M. The genes coding for subunit 3 of NADH dehydrogenase and for ribosomal protein S12 are present in the wheat and maize mitochondrial genomes and are co-transcribed. Mol Gen Genet. 1988 Dec;215(1):118–127. doi: 10.1007/BF00331312. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hiesel R., Wissinger B., Schuster W., Brennicke A. RNA editing in plant mitochondria. Science. 1989 Dec 22;246(4937):1632–1634. doi: 10.1126/science.2480644. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Morgan-Hughes J. A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988 Feb 25;331(6158):717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- Johns D. R., Rutledge S. L., Stine O. C., Hurko O. Directly repeated sequences associated with pathogenic mitochondrial DNA deletions. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8059–8062. doi: 10.1073/pnas.86.20.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher T. D., Thomas W. K., Meyer A., Edwards S. V., Päbo S., Villablanca F. X., Wilson A. C. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6196–6200. doi: 10.1073/pnas.86.16.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer M., Knudsen C., Newton K. J., Gabay-Laughnan S., Laughnan J. R. A partially deleted mitochondrial cytochrome oxidase gene in the NCS6 abnormal growth mutant of maize. New Biol. 1990 Feb;2(2):179–186. [PubMed] [Google Scholar]
- Leaver C. J., Hack E., Forde B. G. Protein synthesis by isolated plant mitochondria. Methods Enzymol. 1983;97:476–484. doi: 10.1016/0076-6879(83)97156-2. [DOI] [PubMed] [Google Scholar]
- Levings C. S., 3rd, Brown G. G. Molecular biology of plant mitochondria. Cell. 1989 Jan 27;56(2):171–179. doi: 10.1016/0092-8674(89)90890-8. [DOI] [PubMed] [Google Scholar]
- Makaroff C. A., Palmer J. D. Extensive mitochondrial specific transcription of the Brassica campestris mitochondrial genome. Nucleic Acids Res. 1987 Jul 10;15(13):5141–5156. doi: 10.1093/nar/15.13.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney A. P., Walbot V. Structural analysis of mature and dicistronic transcripts from the 18 S and 5 S ribosomal RNA genes of maize mitochondria. J Mol Biol. 1990 Jun 20;213(4):633–649. doi: 10.1016/S0022-2836(05)80252-9. [DOI] [PubMed] [Google Scholar]
- Michel F., Dujon B. Conservation of RNA secondary structures in two intron families including mitochondrial-, chloroplast- and nuclear-encoded members. EMBO J. 1983;2(1):33–38. doi: 10.1002/j.1460-2075.1983.tb01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K. J., Coe E. H. Mitochondrial DNA changes in abnormal growth (nonchromosomal stripe) mutants of maize. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7363–7366. doi: 10.1073/pnas.83.19.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K. J., Knudsen C., Gabay-Laughnan S., Laughnan J. R. An abnormal growth mutant in maize has a defective mitochondrial cytochrome oxidase gene. Plant Cell. 1990 Feb;2(2):107–113. doi: 10.1105/tpc.2.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K. J., Walbot V. Maize mitochondria synthesize organ-specific polypeptides. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6879–6883. doi: 10.1073/pnas.82.20.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster W., Brennicke A. Plastid, nuclear and reverse transcriptase sequences in the mitochondrial genome of Oenothera: is genetic information transferred between organelles via RNA? EMBO J. 1987 Oct;6(10):2857–2863. doi: 10.1002/j.1460-2075.1987.tb02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster W., Wissinger B., Unseld M., Brennicke A. Transcripts of the NADH-dehydrogenase subunit 3 gene are differentially edited in Oenothera mitochondria. EMBO J. 1990 Jan;9(1):263–269. doi: 10.1002/j.1460-2075.1990.tb08104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Voljavec A. S., Soueidan S. A., Costigan D. A., Wallace D. C. Spontaneous Kearns-Sayre/chronic external ophthalmoplegia plus syndrome associated with a mitochondrial DNA deletion: a slip-replication model and metabolic therapy. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7952–7956. doi: 10.1073/pnas.86.20.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small I., Suffolk R., Leaver C. J. Evolution of plant mitochondrial genomes via substoichiometric intermediates. Cell. 1989 Jul 14;58(1):69–76. doi: 10.1016/0092-8674(89)90403-0. [DOI] [PubMed] [Google Scholar]
- Stern D. B., Newton K. J. Isolation of plant mitochondrial RNA. Methods Enzymol. 1986;118:488–496. doi: 10.1016/0076-6879(86)18095-5. [DOI] [PubMed] [Google Scholar]
- Stern D. B., Newton K. J. Mitochondrial gene expression in Cucurbitaceae: conserved and variable features. Curr Genet. 1985;9(5):395–404. doi: 10.1007/BF00421611. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wahleithner J. A., Wolstenholme D. R. Ribosomal protein S14 genes in broad bean mitochondrial DNA. Nucleic Acids Res. 1988 Jul 25;16(14B):6897–6913. doi: 10.1093/nar/16.14.6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeviani M., Servidei S., Gellera C., Bertini E., DiMauro S., DiDonato S. An autosomal dominant disorder with multiple deletions of mitochondrial DNA starting at the D-loop region. Nature. 1989 May 25;339(6222):309–311. doi: 10.1038/339309a0. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Zurawski S. M. Structure of the Escherichia coli S10 ribosomal protein operon. Nucleic Acids Res. 1985 Jun 25;13(12):4521–4526. doi: 10.1093/nar/13.12.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zamaroczy M., Faugeron-Fonty G., Bernardi G. Excision sequences in the mitochondrial genome of yeast. Gene. 1983 Mar;21(3):193–202. doi: 10.1016/0378-1119(83)90002-1. [DOI] [PubMed] [Google Scholar]