Abstract
Background
Older adults with dementia are vulnerable to the central deteriorating effects of drugs with anticholinergic properties (DAP). These effects include falls and confusion and may exacerbate dementia-related symptoms. Many individuals with dementia also receive acetylcholinesterase inhibitors (AChEI), indicated for mild to moderate Alzheimer's disease. AChEI have opposing effects to DAP and consequently, concomitant use of DAP and AChEI may further impair cognition among patients with dementia.
Objectives
Our objectives were to 1) evaluate the anticholinergic burden among nursing home (NH) residents with dementia; 2) characterize trends in use of DAP and concomitant use of DAP and AChEI among NH residents with dementia; and 3) identify factors associated with the use of DAP and concomitant use of DAP and AChEI.
Methods
We conducted a retrospective analysis of Medicare data from 2007-2008 linked to the Minimum Data Set.
Results
During the study period, 53,805 (77%) NH residents with dementia used at least one DAP each month. Sixty-seven percent of residents with dementia used Anticholinergic Burden Scale (ACBS) level 1 DAPs, 3% used level 2 and 31% used level 3 DAP. Thirteen percent of NH residents with dementia concomitantly used ACBS levels 2 or3 DAPs and AChEI.
Conclusions
This study sheds new light on the prevalence of DAP use and concomitant use of DAP and AChEI among NH residents with dementia. Clinicians should consider alternatives with lower anticholinergic effects, particularly in patients already taking DAP.
Introduction
Fifty percent of nursing home (NH) residents have Alzheimer's disease or related dementias (ADRD) and 64% of all Medicare beneficiaries living in a nursing home have ADRD.[1-2] Compared to Medicare beneficiaries without ADRD, those with ADRD are more likely to have other chronic conditions such as urinary incontinence, angina, glaucoma, sleep disturbances, heart failure, and psychiatric disorders that are often treated with anticholinergic drugs or drugs with anticholinergic properties.[2-5]
Use of drugs with anticholinergic properties (DAPs) in older adults with ADRD is a concern because the combined effects of polypharmacy, co-morbidities, and age-related changes in pharmacokinetic and pharmacodynamic parameters make patients more vulnerable to the central deteriorating effects of drugs with anticholinergic properties (DAP).[4-7] These effects include falls, confusion, cognitive impairment, sedation, delirium, and increased risk of frailty.[6,8,9] Because age and ADRD are both associated with central cholinergic deficiency, DAP use may exacerbate dementia-related symptoms.[6]
Individuals with ADRD may also receive acetylcholinesterase inhibitors (AChEI), one of the two major drug classes approved by the FDA for the treatment of ADRD.[10] AChEI, including donepezil, galantamine, rivastigmine and tacrine, are the only drugs with indications for mild to moderate Alzheimer's disease and have become the standard of care for managing dementia.[10,11] AChEI improve cholinergic neurotransmission, primarily through inhibition of catabolic enzyme acetylcholinesterase, which degrades acetylcholine in the synaptic cleft.[10] Due to their opposing effects, concomitant use of DAP and AChEI may further impair cognition among patients with dementia, yet several studies have reported that concomitant use of DAP and AChEI is common among older residents of nursing homes.[3,12-17]
Previous studies that examined anticholinergic use among NH residents with ADRD evaluated prevalence and predictors of DAP use during a single year or concomitant use of DAP and AChEI, but none have evaluated time trends in DAP use or predictors of concomitant DAP and AChEI use.[6,12-17] The goals of this study were to: 1) evaluate the anticholinergic burden among NH residents with dementia; 2) characterize trends in use of DAPs and concomitant use of DAP and AChEI among NH residents with dementia; and 3) identify factors associated with the use of DAP and concomitant use of DAP and AChEI.
Methods
Study Design and Data Source
This retrospective study used data from a 5% random sample of Medicare beneficiaries from 2006-2008. Medicare administrative data were obtained from the Centers for Medicare & Medicaid Services (CMS) Chronic Condition Data Warehouse (CCW). Medicare enrollment and claims data were linked to the Minimum Data Set 2.0 (MDS). The MDS is part of the U.S. federally mandated process for clinical assessment of all residents in Medicare or Medicaid certified nursing homes and provides comprehensive assessments of each resident's physical, psychological and psychosocial functioning. These assessments are required for residents on admission to the nursing facility and then periodically, with discharges, quarterly audits, or transfers.
Cohort selection
The CCW identifies 27 chronic conditions including ADRD and contains an annual flag for each condition. An annual flag for ADRD requires at least one inpatient, skilled nursing facility (SNF), home health agency, hospital outpatient, or carrier (physician) claim with a dementia diagnosis (ICD-9-CM codes: 331.0, 331.1×, 331.2, 331.7, 290.0, 290.1×, 290.2×, 290.3, 290.4×, 294.0, 294.1×, 294.8, 797) during the preceding three years.[18] The study population included long-stay NH residents aged 65 and older with ADRD, defined as a diagnosis of ADRD at any time during their Medicare enrollment (back to 1999) through the end of 2008. ADRD was operationalized using the CCW ADRD algorithm applied to Medicare claims, the presence of an ADRD diagnosis on an MDS assessment, or a prescription for an AChEI or memantine, a n-methyl-d-aspartate receptor antagonist indicated for moderate to severe dementia, in the Medicare Part D Prescription Drug Event (PDE) files in 2006, 2007 or 2008. We restricted our analyses to beneficiaries with ADRD who had evidence of long-stay NH residence based on MDS assessments during 2007-2008. We used 2006 as a baseline period to collect information on covariates. NH residents with a qualifying hospital stay may be eligible for skilled nursing care for a period of up to 100 days, during which drug use can't be consistently observed in claims data. In this study, monthly prevalence of DAP and AChEI was calculated across person months of all beneficiaries. Those person-months corresponding to hospital or SNF stays would be excluded from the numerator and the denominator for that month and hence would not impact our overall estimates. Beneficiaries who did not have continuous Part D Prescription Drug coverage during the study period were excluded from these analyses. This study was approved by the Institutional Review Board of the University of Maryland, Baltimore.
Drug Measures / Drug Use
The primary exposures for this study were DAP and AChEI use. In this study of NH residents with dementia, we were interested in the cognitive burden of DAP. Therefore, we identified and classified DAP based on the Indiana Drug Discovery Network Anticholinergic Cognitive Burden Scale (ACBS), which, unlike other anticholinergic drug scales, specifically targets the cognitive effects of DAP.[19,20,21] The ACBS is derived from a list of 82 DAP that have an acute negative impact on cognition. DAP are scored from 1 – 3.[19,20] Medications with an ACBS score of 1 have serum anticholinergic activity, but their clinical impact on cognition has not been confirmed in research studies. Medications with established and clinically relevant cognitive anticholinergic effects have scores of 2 or 3 based on increasing anticholinergic activity.[19,20] The sum of ACBS for each DAP equals the monthly ACBS for each resident. In this study, AChEI included donepezil, galantamine and rivastigmine.
We searched for all DAP and AChEI in the Medicare Part D prescription drug events file and calculated the proportion of days covered (number of daily doses/30-day month) for all DAP and AChEI in each month. DAP and AChEI use were dichotomous variables defined as a proportion of days covered greater than zero during the month. We also created variables indicating any definite DAP (ACBS score 2-3) use and concomitant definite DAP and AChEI use in each month. We used three methods to calculate average monthly anticholinergic burden: 1) ACBS; 2) Count of DAP: the number of unique DAP used each month; 3) Daily anticholinergic burden (DACB): average daily burden in each month, calculated by dividing the sum of each day's ACBS by the total number of days in the month.
Covariates
Demographic characteristics and comorbidities were obtained from the CCW file. Baseline comorbid conditions were based on CCW flags from the prior year. Behavioral, cognitive, and functional factors derived from MDS assessments included the Aggressive Behavior Scale (ABS), Cognitive Performance Scale (CPS), and Activities of Daily Living (ADL).[22-24] Baseline values were from the first MDS assessment during the NH stay, and were carried forward until the next MDS assessment. The ABS is a measure of aggressive behavior based on the occurrence of verbal abuse, physical abuse, socially disruptive behavior and resistance of care. Scale scores range from 0-12 with higher scores indicative of greater frequency and diversity of aggressive behavior.[22] The CPS combines information on memory impairment, level of consciousness, and executive function, with scores ranging from 0 (intact) to 6 (very severe impairment).[23] Activities of Daily Living (ADL) refers to daily self-care activities an individual performs and has scores ranging from 0-28, with lower scores indicating greater independence in physical functioning.[24]
Statistical analysis
We calculated the average monthly prevalence of DAP and AChEI use and tested for time trends over the two-year study period using Cochran-Armitrage tests. We also calculated the average monthly anticholinergic burden over the study period and the average monthly prevalence of DAP use by drug class among nursing home residents for Level 1, 2 and 3 DAPs. To identify factors associated with the use of ACBS Level 2 or 3 DAPs, we used generalized estimating equations (GEE) with a Poisson distribution to account for clustering within residents and generated risk ratios and 95% confidence intervals. Similarly, we identified factors associated with ACBS Level 2 or 3 DAP and concomitant use of AChEI. Because resident characteristics may change over the study period, we used time-varying ADL, ACBS, and CPS scores based on the most recent prior MDS assessment in our regression models. Due to large sample size, statistical significance was defined a priori as p<0.001. All analyses were performed with SAS® version 9.2 (SAS Institute, Cary, NC).
Results
We identified 395,131 beneficiaries with ADRD during 2006-2008 based on Medicare claims, MDS assessments, or Part D claims for ADRD medication. Among these, 179,773 had at least one MDS assessment following ADRD diagnosis. After applying our continuous Medicare Part D coverage criteria, our final sample comprised 69,877 beneficiaries with ADRD. Average age was 83.7 (standard deviation (s.d.) 7.5) years and the majority were white (84%) and female (79%)(Table 1). Average NH stay was 12.8 (s.d.8.5) months. At the baseline MDS assessment, beneficiaries had low levels of aggressive behavior symptoms (ABS mean 0.7 (s.d. 1.5)), moderate physical functioning (ADL mean 16.7 (s.d. 8.1)) and moderate cognitive impairment (CPS mean 3.2 (s.d. 1.6)).
Table 1. Characteristics of Long-Stay Nursing Home Residents with Alzheimer's disease and Related Dementias 2007-2008 (N = 69,877).
| Characteristic | |
|---|---|
| Age in years, mean (sd) | 83.7 (7.5) |
| Sex, n (%) | |
| Male | 14,837 (21) |
| Female | 55,040 (79) |
| Race, n (%) | |
| White | 58,480 (84) |
| African American | 8,178 (12) |
| Other | 3,219 (4) |
| Cognitive Performance Score, mean (sd) | 3.2 (1.6) |
| Activities of Daily Living Score, mean (sd) | 16.7 (8.1) |
| Aggressive Behavior Score, mean (sd) | 0.7 (1.5) |
| Comorbid Conditions, n(%) | |
| Atrial Fibrillation | 14,895 (21) |
| Chronic Kidney Disease | 17,955 (26) |
| Chronic Obstructive Pulmonary Disease | 21,512 (31) |
| Depression | 22,930 (33) |
| Diabetes | 27,702 (40) |
| Heart Failure | 36,919 (53) |
| Ischemic Heart Disease | 40,669 (58) |
| Stroke/TIAa | 24,268 (35) |
| Nursing Home Stay (months), mean (SD) | 12.8 (8.5) |
Transient ischemic attack
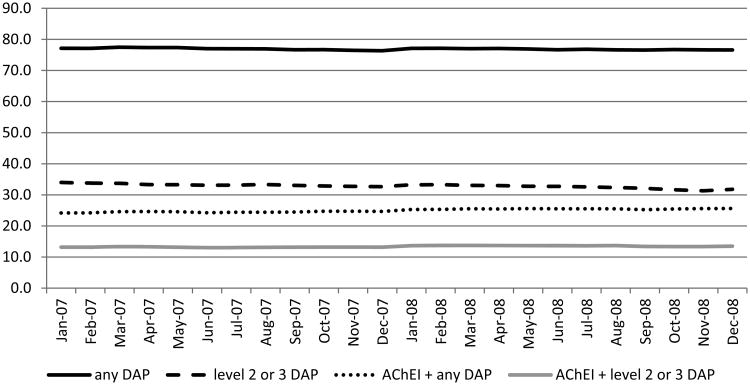
During the study period, seventy-seven percent of NH residents with ADRD used at least one DAP each month on average (Figure 1). Sixty-seven percent of NH residents with ADRD used ACBS level 1 DAP, 3% used level 2and 31% used level 3 DAP. Thirteen percent of NH residents with ADRD concomitantly used ACBS levels 2 or 3 DAP and AChEI within the same month. Among AChEI users, the average monthly prevalence of DAP use was 81% and 37% used level 2 or 3 DAP. There were small but significant negative trends over the two-year study period in any DAP use (77.1% in 2007 to 76.6% in 2008, p=0.001) and in level 3DAP use (32.3% in 2007 to 30.0% in 2008, p<0.001). On the other hand, there was a significant increase in concomitant DAP and AChEI use over time (24.2% in 2007 to 25.0% in 2008, p<0.0001) (Figure 1). Among DAP users, the average monthly ACBS was moderate at 3.1 (s.d.2.1) and the average daily anticholinergic burden each month was 2.6 (s.d.1.8)(Table 2).The average number of DAP used per month was 2.1 (s.d. 1.2).
Figure 1. Use of Drugs with Anticholinergic Properties among Nursing Home Residents with Alzheimer's Disease and Related Dementias, N = 69, 877.
AChEI – acetylcholinesterase Inhibitor; DAP – drug with anticholinergic properties
Table 2. Average Monthly Anticholinergic Burden Among Long-Stay Nursing Home Residents with Alzheimer's Disease and Related Dementias Who used Drugs with Anticholinergic Properties in 2007-2008, N = 69,877.
| Burden Measurement | Mean (standard deviation) | Range |
|---|---|---|
| ACBSa Score | 3.1 (2.1) | 1-22 |
| Count of DAPb | 2.1 (1.2) | 1-10 |
| DACBc | 2.6 (1.8) | <1-22 |
ACBS – Anticholinergic cognitive burden scale
DAP – Drugs with anticholinergic properties
DACB – Daily anticholinergic burden
Among DAP level 1 users, the most commonly used drug classes were antipsychotics (16%) and bladder antispasmodics (8%). Among DAP level 2 users, the most commonly used drug classes were antiepileptics (48%), antipsychotics (22%), and central anticholinergics (16%). The most commonly used drug classes among Level 3 DAP users were antipsychotics (55%), bladder antispasmodics (23%), and antidepressants (17%).
The final regression model for ACBS level 2 or 3 DAP use contained age, ABS, CPS, ADL, time, and indicator variables for sex, race, atrial fibrillation, chronic kidney disease, chronic obstructive pulmonary disease, depression, diabetes, heart failure, ischemic heart disease, stroke, Medicare coverage, and low income subsidy. Women were less likely to use DAPs (risk ratio (RR) 0.91, 95% confidence interval (CI) 0.88-0.95) compared to men (Table 3). Residents aged ≥95 years (RR: 0.56; 95% CI 0.52 – 0.59), 85-94 years (RR: 0.70, 95% CI 0.67-0.72), and 75-84 years (RR: 0.83; CI 0.80-0.86) were less likely to use DAPs than those aged 66-74 years. African Americans (RR 0.77; 95% CI 0.74-0.80) had less DAP use compared to Whites. Those with depression were less likely to use DAPs (RR 0.83; 95% CI 0.81-0.86). Those with high CPS scores (4-6), indicative of more severe cognitive impairment, were less likely to use DAPs than those with low (0-1) scores (RR: 0.89, 95% CI 0.85-0.94). Higher scores on the ABS, indicating more aggressive behavior, were associated with DAP use (severe RR: 1.44, CI 1.38-1.49) and (moderate RR: 1.22; 95% CI 1.19-1.26) compared to no aggressive behavior.
Table 3. Adjusted Risk Ratios and 95% Confidence Intervals of Factors Associated with Use of Level 2 or 3 Drugs with Anticholinergic Properties (DAP) alone and combined with Acetylcholinesterase Inhibitors (AChEIs) use among Medicare Beneficiaries with Alzheimer's Disease and Related Dementias residing in a Nursing Home 2007-2008 (N = 69, 877).
| Predictor | Level 2 or 3 DAP | Level 2 or 3 DAP and AChEI |
|---|---|---|
| Sex | ||
| Male | Reference | Reference |
| Female | 0.91 (0.88 – 0.95) | 0.91 (0.85 – 0.97) |
| Age in years | ||
| 66-74 | Reference | Reference |
| 75-84 | 0.83 (0.80 – 0.86) | 1.12 (1.03 – 1.22) |
| 85-94 | 0.70 (0.67 – 0.72) | 0.84 (0.77 – 0.91) |
| ≥95 | 0.56 (0.52 – 0.59) | 0.44 (0.38 – 0.53) |
| Race | ||
| White | Reference | Reference |
| African American | 0.77 (0.74 – 0.80) | 0.81 (0.74 – 0.89) |
| Other | 0.89 (0.83 – 0.94) | 0.93 (0.81 – 1.05) |
| Cognitive Performance Score | ||
| Low impairment (0-1) | Reference | Reference |
| Moderate impairment (2-3) | 1.01 (0.97 – 1.05) | 1.09 (1.05 – 1.13) |
| High impairment (4-6) | 0.89 (0.85 – 0.94) | 1.03 (0.98 – 1.09) |
| Activities of Daily Living Scorea | ||
| Low (0-7) | Reference | Reference |
| Mild (8-14) | 0.99 (0.96 – 1.03) | 0.97 (0.937 – 1.01) |
| Moderate (15-21) | 0.94 (0.91 – 0.98) | 0.92 (0.88 – 0.96) |
| High (22-28) | 0.78 (0.74 – 0.81) | 0.82 (0.78 – 0.86) |
| Aggressive behavior | ||
| None (0) | Reference | Reference |
| Moderate (1-2) | 1.22 (1.19 – 1.26) | 1.04 (1.02 – 1.06) |
| Severe (≥ 3) | 1.44 (1.38 – 1.49) | 1.08 (1.05 – 1.12) |
| Coverage | ||
| Fee for service | Reference | Reference |
| Medicare Advantage | 0.85 (0.81 – 0.88) | 0.73 (0.73 – 0.81) |
| Both | 0.92 (0.87 – 0.97) | 0.90 (0.81 – 1.00) |
| Comorbidities | ||
| Atrial fibrillation | 1.06 (1.02 – 1.10) | 1.06 (0.99 - 1.13) |
| Congestive heart failure | 0.98 (0.95 – 1.01) | 1.08 (1.04 – 1.11) |
| COPDb | 0.94 (0.91 – 0.97) | 1.00 (0.95 – 1.06) |
| Depression | 0.83 (0.81 – 0.86) | 0.97 (0.94 – 0.99) |
| Diabetes | 1.00 (0.97 – 1.03) | 1.05 (0.99 – 1.10) |
| Ischemic heart disease | 0.98 (0.94 – 1.01) | 0.96 (0.91 – 1.02) |
| Kidney disease | 1.04 (1.00 – 1.07) | 1.07 (1.01 – 1.14) |
| Stroke/TIAc | 1.01 (0.98 – 1.04) | 1.06 (1.00 – 1.12) |
| Low income subsidy | 0.91 (0.87 – 0.95) | 0.99 (0.94 – 1.04) |
low scores indicate more independence;
Chronic obstructive pulmonary disorder;
Transient ischemic attack
The final regression model for ACBS level 2 or 3 DAP and concomitant AChEI use contained age, ABS, CPS, ADL, time, and indicator variables for sex, race, atrial fibrillation, chronic kidney disease, chronic obstructive pulmonary disease, depression, diabetes, heart failure, ischemic heart disease, stroke, Medicare coverage, and low income subsidy. Women were less likely to have concomitant use (RR: 0.91; CI 0.85-0.97) compared to men. Residents aged ≥95 years (RR: 0.44; 95% CI 0.38 – 0.53) and 85-94 years (RR: 0.84; 95% CI 0.77 – 0.91) were less likely to have concomitant use compared to those aged 66-74 years. However, those 75-84 years were more likely to have concomitant use compared to those 66-74 years (RR: 1.12; 95% CI 1.03 – 1.22). African Americans were less likely to have concomitant use compared to whites (RR: 0.81; 95% 0.74-0.89). Higher scores on the ABS were associated with concomitant use (severe RR: 1.08; CI 1.05-1.12) and (moderate RR: 1.04; 95% CI 1.02-1.06) compared to no aggressive behavior.
Discussion
DAP use was common among NH residents with ADRD. One-third used DAPs with known anticholinergic effects (ACBS Level 2 and 3) but concomitant use of ACBS level 2 or 3 DAP and AChEI use was less common. We identified several patient factors associated with use of ACBS level 2 or 3DAP and concomitant use of ACBS level 2 or 3DAP and AChEI.
The average monthly prevalence of DAP use among NH residents with dementia (77%) reported here is consistent with prior studies (range 74%-82%) and updates estimates for 2007-2008.[6,16] A prior study reporting anticholinergic burden used the Anticholinergic Drug Scale rather than the ACBS to categorize anticholinergic activity, making comparisons with our study on the levels of DAPs used difficult.[6,25]
Few studies have examined the prevalence of AChEI use among DAP users. A study conducted among individuals in residential care in Finland reported 27% concomitant use of AChEI or memantine among DAP users.[12] This is consistent with our reported estimate (25%), although our study did not include memantine except to identify NH residents with ADRD. Two prior studies examined the prevalence of DAP use among users of AChEIs.[15,26] In one conducted in nursing homes in Indiana, 47% of AChEI users with dementia used DAPs with known cognitive effects during the study period.[15] In the other, 37% of community dwelling AChEI users aged 50 and older also used DAPs during the study period. [26] Our lower estimate of concomitant ACBS Level 2 or 3 DAP use among NH residents with dementia who used AChEIs (37%) is likely because of differences in measurement (any use vs. average monthly prevalence of use in our study).
In this cross-sectional study we identified factors associated with DAP and AChEI use, but determining predictors of use is more difficult. Nonetheless, antipsychotics constitute a large percentage of level 2 and 3 DAPs; hence, factors associated with level 2 or 3 DAP use may actually represent factors associated with antipsychotic use. In this context, the association between aggressive behavior and level 2 or 3 DAP use is likely due to the use of antipsychotics to control dementia-related behavioral symptoms.[27,28] This study took place after the FDA issued the first black box warning on antipsychotics for the control of dementia-related behavioral symptoms in 2005.[29] Therefore, beneficiaries with greater cognitive and physical impairment may have been less likely to receive antipsychotics, consistent with our results. Other observed associations between age and sex cognition, and aggressive behavior and DAP or concomitant DAP/AChEI use are in accordance with previous research.[6,15,26]
Strengths of this study include use of nationally representative data to assess DAP use among Medicare beneficiaries with ADRD residing in a NH. In addition, this study is the first to report factors associated with concomitant use of DAP and AChEI among NH residents with ADRD. Finally, we used the ACBS, a well-validated scale used to measure ant cholinergic activity that was developed based on a systematic review of literature and expert professional input.[19-21] Nonetheless, administrative claims data do not capture use of over the counter DAPs such as first generation antihistamines which may have resulted in an underestimation of the prevalence of DAP use. Furthermore, claims data are created for billing purposes and may be missing information, such as comorbidities like ADRD. To address this limitation, we used three independent criteria to define our study population, increasing the specificity of our dementia definition. Finally, this study did not examine outcomes of DAP or concomitant DAP and AChEI use. Future longitudinal studies should determine if DAP use is associated with adverse events such as falls or fractures.
Conclusions
This study sheds new light on the prevalence of DAP use and concomitant use of DAP and AchEI among NH residents with ADRD. Older dementia patients have a higher sensitivity to anti cholinergic side effects which may be particularly detrimental.[6,8] Nonetheless, DAP are indicated for many of the comorbidities common in this population. Many ACBS level 2 and 3 DAP come from drug classes that contain options that do not have anticholinergic effects. For example, second generation antihistamines have fewer anticholinergic affects than first generation antihistamines. Antidepressants such as sertraline or citalopram are not DAP, while amitriptyline and paroxetine are level 3 DAP. For neuropathic pain, gabapentin or levetiracetam are possible alternatives to definite DAP such as carbamazepine or oxacarbazine. When adding or changing medication(s) to a patients' regimen, it is important for clinicians to consider the potential cognitive burden and look for alternatives with lower anticholinergic effects, particularly in patients already taking DAP.
Key Points.
Seventy-seven percent of nursing home residents with dementia used at least one drug with anticholinergic properties (DAP) each month
Thirteen percent of nursing home residents with dementia concomitantly used Anticholinergic Burden Scale levels 2 or 3 DAP and acetylcholinesterase inhibitors each month
When adding or changing medication to a patients' regimen, it is important for clinicians to consider the potential cognitive burden and look for alternatives with lower anticholinergic effects, particularly in patients already taking DAP
Acknowledgments
Drs. Palmer, Albrecht, Park, Dutcher, Rattinger, Simoni-Wastila, and Zuckerman, and Ms. Walker would like to thank Dr. Xingang Liu and Ms. Xian Shen for SAS coding assistance and acknowledge Ms. Zippora Kiptanui for assistance with the IRB protocol. This work was supported in part by the Food and Drug Administration (Collaborative agreement U01FD004320). Dr. Albrecht is supported by National Institutes of Health grant T32AG000262-14 (Magaziner, PI). Drs. Palmer and Park are supported by a fellowship grant from Novartis.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Harris-Kojetin L, Sengupta M, Park-Lee E, Valverde R. Long-term care services in the United States: 2013 overview. Hyattsville, MD: National Center for Health Statistics; 2013. [PubMed] [Google Scholar]
- 2.Alzheimer's Association. 2014 Alzheimer's disease facts and figures. Alzheimers Dement. 2014 Mar;10(2):e47–92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Carnahan RM, Lund BD, Perry PJ, Chrischilles EA. The concurrent use of anticholinergics and cholinesterase inhibitors: rare event or common practice? J Am Geriatr Soc. 2004 Dec;52(12):2082–7. doi: 10.1111/j.1532-5415.2004.52563.x. [DOI] [PubMed] [Google Scholar]
- 4.Mintzer J, Burns A. Anticholinergic side-effects of drugs in elderly people. J R Soc Med. 2000 Sep;93(9):457–62. doi: 10.1177/014107680009300903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doraiswamy PM, Husain MM. Anticholinergic drugs and elderly people: a no brainer? Lancet Neurol. 2006 May;5(5):379–80. doi: 10.1016/S1474-4422(06)70421-5. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee S, Mehta S, Sherer JT, Aparasu RR. Prevalence and predictors of anticholinergic medication use in elderly nursing home residents with dementia: analysis of data from 2004 National Nursing Home Survey. Drugs Aging. 2010 Dec 1;27(12):987–97. doi: 10.2165/11584430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Uusvaara J, Pitkala KH, Kautinainen H, Tilvis RS, Strandberg TE. Detailed cognitive function and use of drugs with anticholinergic properties in older people: a community-based cross-sectional study. Drugs Aging. 2013 Mar;30(3):177–82. doi: 10.1007/s40266-013-0055-2. [DOI] [PubMed] [Google Scholar]
- 8.Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Cumming RG, Handelsman DJ, McLachlan AJ, Abernethy DR, Banks E, Le Couteur DG. High-Risk Prescribing and Incidence of Frailty Among Older Community-Dwelling Men. Clin Pharmacol Ther. 2012;91(3):521–8. doi: 10.1038/clpt.2011.258. [DOI] [PubMed] [Google Scholar]
- 9.Fox C, Smith T, Maidment I, Chan WY, Bua N, Myint PK, Boustani M, Kwok CS, Glover M, Koopmans I, Campbell N. Effect of medications with anti-cholinergic properties on cognitive function, delirium, physical function and mortality: a systematic review. Age and Ageing. 2014;43:604–615. doi: 10.1093/ageing/afu096. [DOI] [PubMed] [Google Scholar]
- 10.Ellis JM. Cholinesterase inhibitors in the treatment of dementia. J Am Osteopath Assoc. 2005;105:145–158. [PubMed] [Google Scholar]
- 11.Forchetti CM. Treating patients with moderate to severe Alzheimer's disease: Implications of recent pharmacologic studies. Prim Care Companion J Clin Psychiatry. 2005;7:155–161. doi: 10.4088/pcc.v07n0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teramura-Gronblad M, Muurinen S, Soini H, Suominen M, Pitkala KH. Use of anticholinergic drugs and cholinesterase inhibitors and their association with psychological well-being among frail older adults in residential care facilities. Ann Pharmacother. 2011 May;45(5):596–602. doi: 10.1345/aph.1P650. [DOI] [PubMed] [Google Scholar]
- 13.Johnell K, Fastborn J. Concurrent use of anticholinergic drugs and cholinesterase inhibitors: register-based study of over 70,000 elderly people. Drugs Aging. 2008;25(10):871–7. doi: 10.2165/00002512-200825100-00006. [DOI] [PubMed] [Google Scholar]
- 14.Gill SS, Mamdani M, Naglie G, Streiner DL, Bronskill SE, Kopp A, Shulman KI, Lee PE, Rochon PA. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med. 2005 Apr 11;165(7):808–13. doi: 10.1001/archinte.165.7.808. [DOI] [PubMed] [Google Scholar]
- 15.Sink KM, Thomas J, 3rd, Xu H, Craig B, Kritchevsky S, Sands LP. Dual use of bladder anticholinergics and cholinesterase inhibitors: long-term functional and cognitive outcomes. J Am Geriatr Soc. 2008 May;56(5):847–53. doi: 10.1111/j.1532-5415.2008.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Modi A, Weiner M, Craig BA, Sands LP, Rosenman MB, Thomas J., 3rd Concomitant use of anticholinergics with acetylcholinesterase inhibitors in Medicaid recipients with dementia and residing in nursing homes. J Am Geriatr Soc. 2009 Jul;57(7):1238–44. doi: 10.1111/j.1532-5415.2009.02258.x. [DOI] [PubMed] [Google Scholar]
- 17.Kolanowski A, Fick DM, Campbell J, Litaker M, Boustani MA. Preliminary Study of Anticholinergic Burden and Relationship to a Quality of Life Indicator, Engagement in Activities, in Nursing Home Residents with Dementia. J Am Med Dir Assoc. 2009;10(4):252–257. doi: 10.1016/j.jamda.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Medicare and Medicaid Services. Chronic Condition Data Warehouse. [Accessed 7/31/14]; Available at http://www.ccwdata.org/web/guest/condition-categories.
- 19.Boustani MA, Campbell NL, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008 Jun;4(3):311–320. [Google Scholar]
- 20.Aging Brain Program of the Indiana University Center for Aging Research. Anticholinergic Cognitive Burden Scale (2012 update) Aging Brain Care. 2012 Web. 7 Jul 2014. Available at: http://www.agingbraincare.org/uploads/products/ACB_scale_-_legal_size.pdf.
- 21.Kersten H, Wyller TB. Anticholinergic Drug Burden in Older People's Brain – How well is it Measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151–9. doi: 10.1111/bcpt.12140. [DOI] [PubMed] [Google Scholar]
- 22.Perlman CM, Hirdes JP. The aggressive behavior scale: a new scale to measure aggression based on the minimum data set. Am Geriatr Soc. 2008 Dec;56(12):2298–303. doi: 10.1111/j.1532-5415.2008.02048.x. [DOI] [PubMed] [Google Scholar]
- 23.Morris JN, Fries BE, Mehr DR, Hawes C, Phillips C, Mor V, Lipsitz LA. MDS Cognitive Performance Scale. J Gerontol. 1994 Jul;49(4):M174–82. doi: 10.1093/geronj/49.4.m174. [DOI] [PubMed] [Google Scholar]
- 24.Morris JN, Fries BE, Morris SA. Scaling ADLs within MDS. J Gerontol A Biol Sci Med Sci. 1999 Nov;54(11):M546–53. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 25.Carnahan RM, Lund BD, Perry PJ, Pollock BG, Culp KR. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006 Dec;46(12):1281–6. doi: 10.1177/0091270006292126. [DOI] [PubMed] [Google Scholar]
- 26.Boudreau DM, Yu O, Gray SL, Raebel MA, Johnson J, Larson EB. Concomitant Use of Cholinesterase Inhibitors and Anticholinergics: Prevalence and Outcomes. J Am Geriatr Soc. 2011;59:2069–2076. doi: 10.1111/j.1532-5415.2011.03654.x. [DOI] [PubMed] [Google Scholar]
- 27.Briesacher BA, Limcangco MR, Simoni-Wastila L, Doshi JA, Levens SR, Shea DS, Stuart B. The Quality of Antipsychotic Drug Prescribing in Nursing Homes. Arch Intern Med. 2005:165, 1280–1285. doi: 10.1001/archinte.165.11.1280. [DOI] [PubMed] [Google Scholar]
- 28.Shekelle P, Maglione M, Bagley S, et al. Efficacy and Comparative Effectiveness of Off-Label Use of Atypical Antipsychotics. Agency for Healthcare Research and Quality (US); 2007. Jan, (Comparative Effectiveness Reviews, No. 6.) AHRQ Publication No. 07-EHC003-EF. [PubMed] [Google Scholar]
- 29.United States Food and Drug Administration. Public Health Advisory: Deaths with Antipsychotics in Elderly Patients with Behavioral Disturbances. [Accessed 10/22/14];2005 Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm053171.htm.