Abstract
Background:
Excision followed by adjuvant irradiation is considered safe and most efficacious for treatment of keloid scars. Recently, different authors published successful treatment protocols and recommended the following: (1) the use of high-dose-rate brachytherapy instead of low-dose-rate brachytherapy or external radiation; (2) a short-time interval between operation and irradiation; (3) single fraction instead of multifraction irradiation; and (4) a minimum of 12- to 24-month follow-up post treatment.
Methods:
This study evaluates the above recommendations with a systematic review of the English-language literature, based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement. Both PubMed and EMBASE were searched. Studies were graded according to the American Society of Plastic Surgeons Rating Levels of Evidence.
Results:
Thirty-three studies were selected. Six studies were graded as level of evidence type II studies and 27 as type III. High-dose-rate brachytherapy showed lower recurrence rates compared with low-dose-rate brachytherapy and external radiation. A short-time (<7 hours) interval between scar excision and irradiation results in a lower recurrence rate compared with long-time intervals (>24 hours). Single-fraction irradiation showed promising results in terms of recurrence rate and patient convenience. Finally, scar recurrences were seen between 2 and 36 months, with a mean of 15 months.
Conclusions:
Based on this systematic review of the literature, the evidence confirms the recommendations stated by authors in the recent years. However, due to the lack of high-quality randomized studies, the quality of this evidence is limited. More randomized studies will generate stronger recommendations.
Keloid scars are a benign fibroproliferative disease impairing the quality of life of patients by causing cosmetic disfigurement and complaints of pain and pruritus.1,2 Treatment is difficult with high recurrence rates and even growth stimulus as the main issue.1 According to the international advisory panel on scar management, surgical excision with postoperative radiation therapy is considered the most efficacious treatment.3
Radiation therapy for treatment of keloid scars was first described by Sequeira4 in 1909. Traditionally, it was applied externally by a variety of devices.5 Although good results were achieved, external radiation therapy requires a relatively high irradiation dose due to the large distance between the radiation source and the scar. Also, the surrounding healthy skin is unnecessarily exposed to radiation.6
To solve these problems, Malaker et al6 introduced a technique called “brachytherapy” (also called interstitial or internal radiation) in 1976. Nowadays, it is available as low-dose-rate (LDR) or high-dose-rate (HDR) brachytherapy. In both methods, a hollow catheter is incorporated in the surgical lesion after excision of the scar, through which a radioactive source is directed. In this way, irradiation is effectively localized from inside the lesion, only targeting the desired area.6 With LDR brachytherapy, a low-dose radioactive source is used and removed after typically 20–72 hours.7 In contrast, with HDR brachytherapy, a high radioactive source is applied for a short period of 5–10 minutes.8 Due to the short treatment time, HDR brachytherapy is an outpatient procedure enhancing patient convenience, whereas LDR brachytherapy requires hospitalization (Fig. 1).
Figure 1.
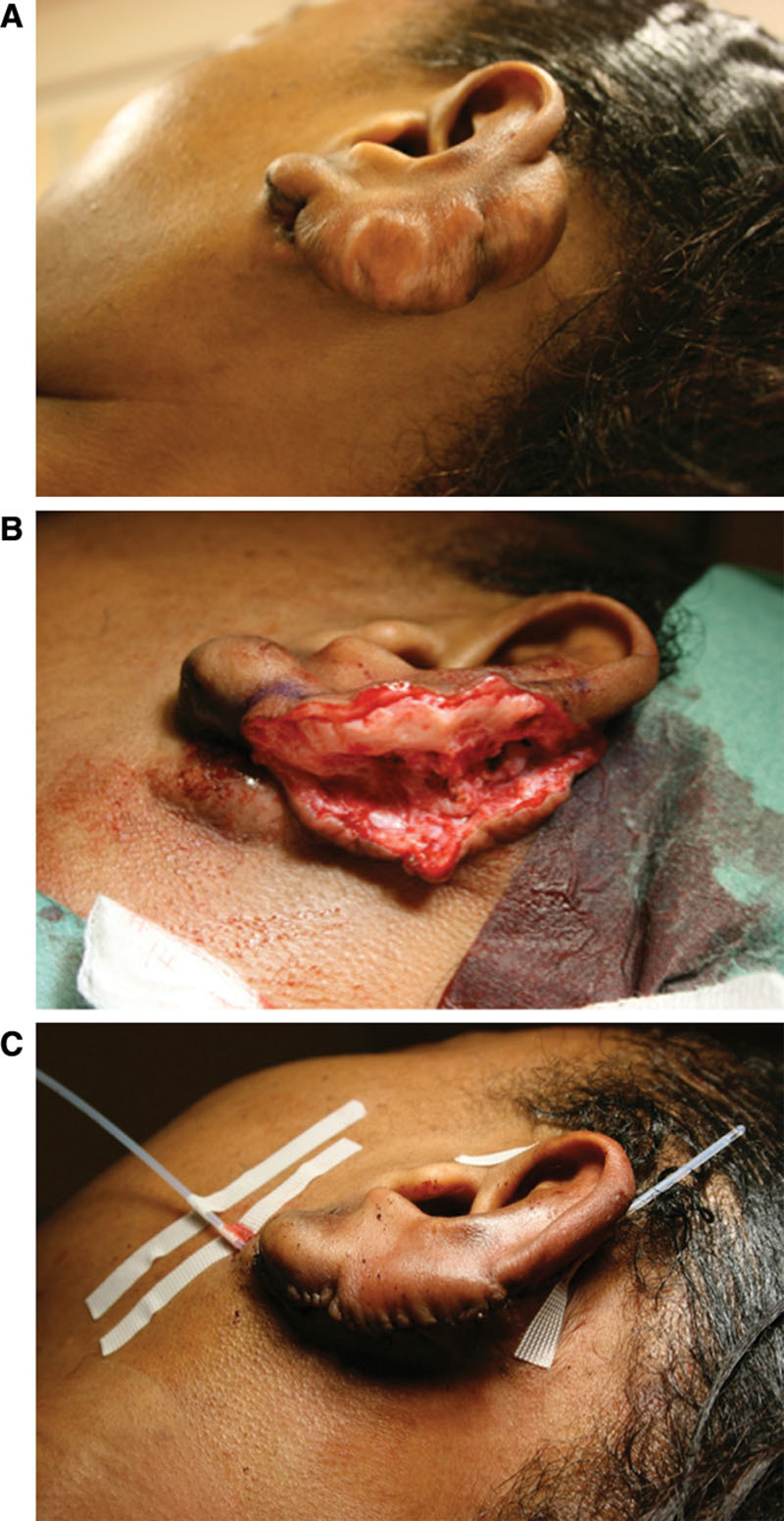
Surgical excision with adjuvant brachytherapy. Example of an auricular keloid scar (A) with surgical excision (B). C, A catheter is positioned between the dermal edges of the wound, below the surface of the skin and extending out of the skin beyond the wound. Postoperatively, the patient will be transferred to the radiation department for the adjuvant high-dose-rate brachytherapy (Source: van Leeuwen MC, Stokmans SC, Bulstra AE, et al. High-dose-rate brachytherapy for the treatment of recalcitrant keloids: a unique, effective treatment protocol. Plast Reconstr Surg. 2014;134:527–534).
Recently, different authors described new protocols aiming to reduce keloid recurrence and improve patient convenience.7–14 They recommended the following: (1) the use of HDR brachytherapy instead of LDR brachytherapy or external radiation8,9; (2) a short-time interval between operation and irradiation7,8; (3) single fraction instead of multifraction irradiation10–13; and (4) a minimum of a 12- to 24-month follow-up post treatment.14,15 This systematic review evaluates these recommendations.
METHODS
Search Strategy
A comprehensive systematic review of the English-language literature was performed, based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement. PubMed and EMBASE were searched from inception to January 14, 2014, and January 23, 2014, respectively. The following terms were used as index terms or free-text words: “cicatrix” or “scars” (including synonyms and closely related words as hypertrophic scar and keloid scar) and “brachytherapy” or “x-ray therapy” or “surface radiotherapy.”
References of retrieved articles were scanned for additional studies. Inclusion criteria consisted of the following: (1) any English-language randomized controlled trials (RCTs), controlled clinical trials, or prospective or retrospective cohort studies reporting surgical excision (primary closure, no use of skin grafts) with adjuvant radiotherapy for treatment of keloid scars; (2) a minimum follow-up duration of 1 year for all lesions; (3) studies including solely keloid scars or studies with a clear definitions distinguishing hypertrophic and keloid scars and separate analysis for both lesions; (4) no adjuvant interventions following surgical excision other than radiation therapy; (5) studies measuring recurrence rate as outcome, based on the regrowth of the keloid scars with or without functional complaints8; and (6) poster abstracts, case reports, or letters to the editor were not included. In case of duplicate articles, only one was included.
The article screening process was performed as follows: 3 investigators (M.C.E.v.L., S.C.S., and J.C.F.K.) carried out the initial searches and 2 investigators (M.C.E.v.L. and S.C.S.) independently reviewed the studies for eligibility. Investigators were blinded to each other, meeting only to compare findings after completing the extraction process. Decisions about eligibility were resolved by discussion. Seventy potentially relevant studies were identified from the initial searches. Subsequently, 2 authors (M.C.E.v.L. and S.C.S.) independently screened the full-text articles for eligibility using a standardized data abstraction form with inclusion and exclusion criteria. Disagreement was resolved by discussion. This eventually resulted in 33 articles (Fig. 2).
Figure 2.
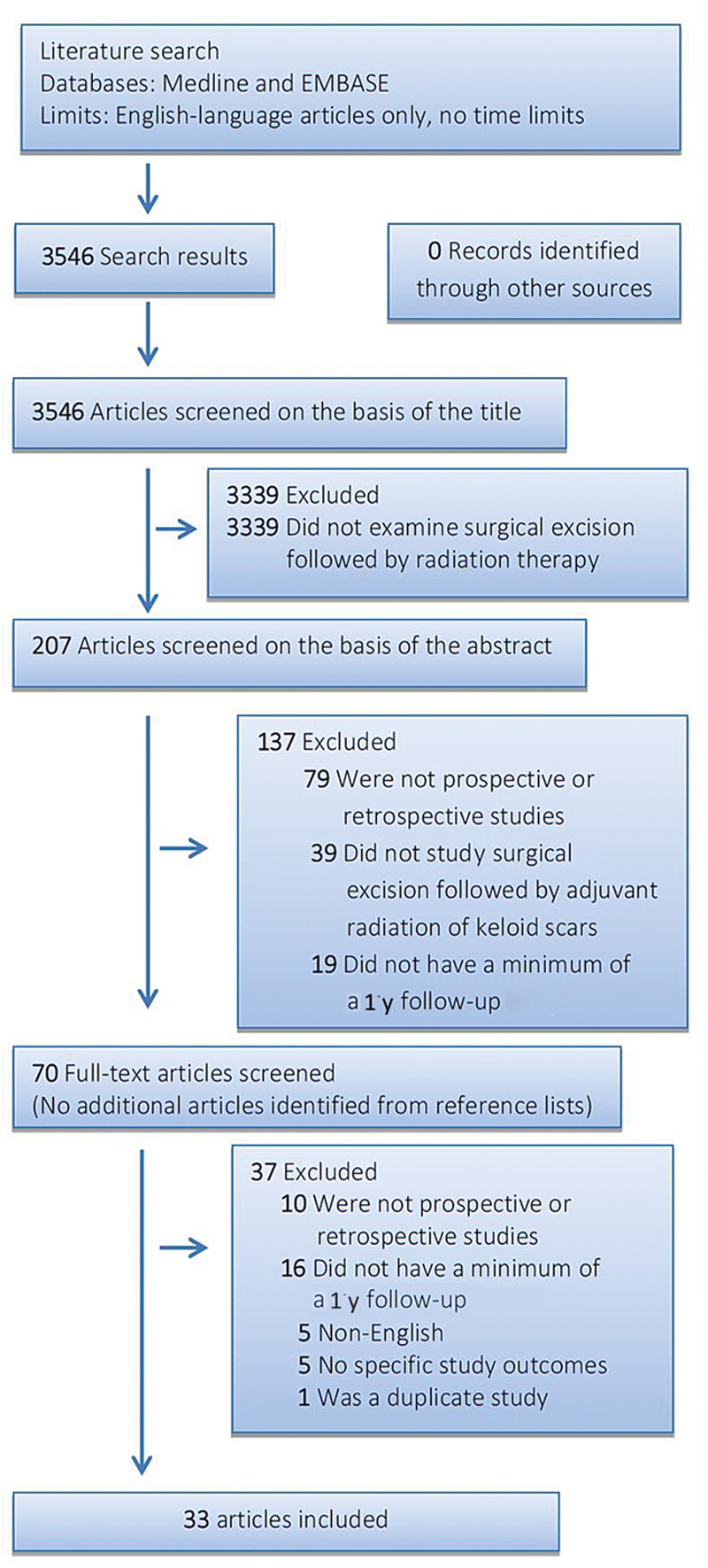
Flow diagram of the search and selection process according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Data Extraction
One reviewer extracted data and a second review author verified the accuracy of the extracted data. Discrepancies in opinion about an article were reviewed, and consensus was achieved through discussion. A standardized data form was used to obtain the following information: (1) study characteristics; (2) study participants (including origin or Fitzpatrick score); (3) study design (prospective/retrospective and follow-up duration); (4) intervention, including type of radiation. Type of radiation was divided into external radiation (all different external devices including the surface applicator), LDR brachytherapy, and HDR brachytherapy. Also, radiation dosage and radiation scheme were extracted; 5) study results, of which the recurrence rate was the main outcome. Thereafter, data were arranged in evidence tables according to type of radiation.
Methodological Quality Assessment
Heterogeneity in study design and outcome measures did not allow for quantitative pooling of data for meta-analysis. The extracted studies were graded according to the American Society of Plastic Surgeons Rating Levels of Evidence.16 This classification assigns each article to a corresponding level of evidence ranging from I (highest) to V (lowest). We classified a level II study to prospective studies, with a clear definition of keloid scars17 and recurrence.18
RESULTS
Study Characteristics
Initial database searches identified 3546 articles. A flow diagram of the search and selection process is shown in Figure 2; 3339 articles were eliminated based on the title of the article because there was no relation between radiation therapy and keloid scars. Next, 207 abstracts were screened, of which 137 were excluded for not meeting with the selection criteria. Thus, 70 full-text articles were analyzed. Sixteen studies were excluded because they did not have a minimum of a 1-year follow-up, 10 studies were not prospective or retrospective, 5 were not in English, 5 had no specific outcome measures, and there was 1 duplicate study. Finally, 33 articles met all inclusion criteria (Fig. 2).
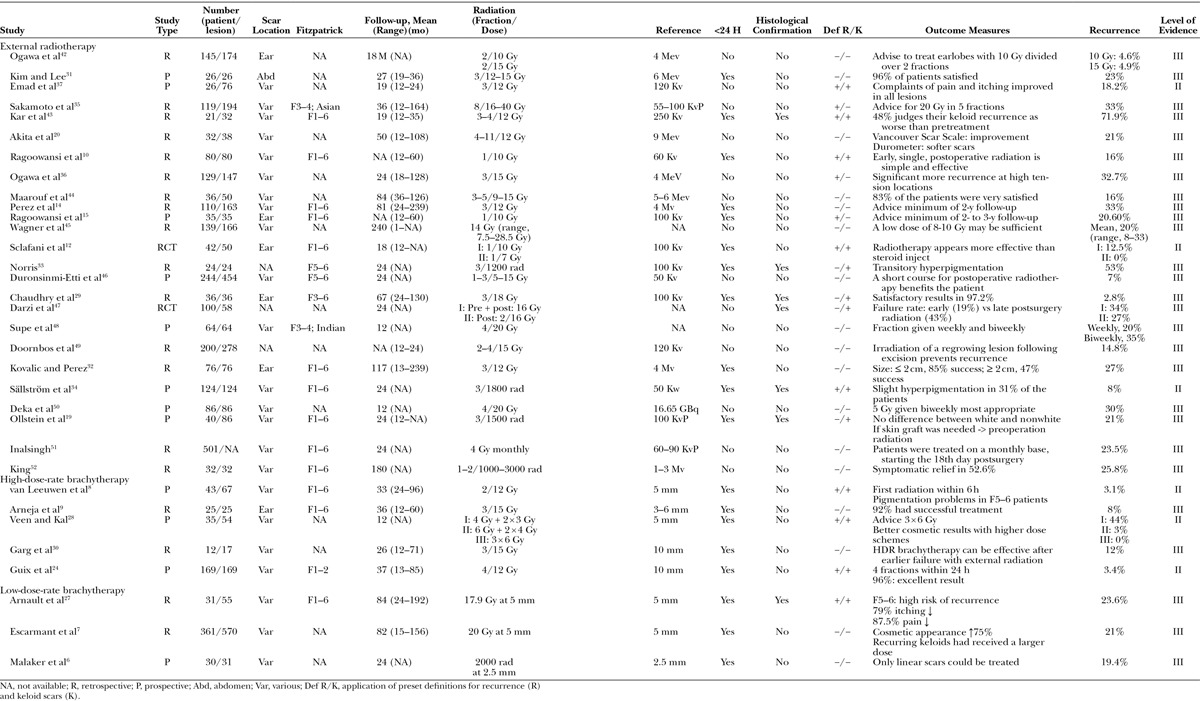
A summary of the characteristics of the included studies is given in Table 1. Of the included articles, 10 were from the United States, 4 from Japan, and 4 from India. The remaining 15 studies were from 9 different countries. Twenty-five studies used external radiation, 5 used HDR brachytherapy, and 3 used LDR brachytherapy. The mean publication date was most recent for studies using HDR brachytherapy (HDR: 2005, range, 2001–2008; external: 1996, range, 1970–2013; LDR: 1992, range, 1976–2009).
Table 1.
Characteristics
Methodological Quality
We classified 6 studies with level of evidence type II and 27 studies with level of evidence type III. There were 2 RCT studies, randomizing different keloid treatment options, of which radiation was one. Twelve studies were prospective and 19 retrospective (Table 1).
Patient Characteristics
The sample size of the included studies ranged from 12 to 501 patients (mean, 97.8 ± 18.8) with a total of 17–570 scars (mean, 111.9 ± 22.4). In total, 3130 patients with 3470 keloid scars were treated. The follow-up ranged from 12 to 239 months (mean, 49 ± 9.5 months). Although all included studies mentioned a minimum of a 1-year follow-up, 42% did not describe the range of the follow-up completely. Patients’ origin was noted in 57.6% of the studies: the majority treated a mixed population (74%), although origin or skin type was not always specified. Others treated solely white (5%), Asian (10.5%), or Afro-American patients (10.5%). The location of the keloid scars was mixed in 67% of the studies. In 18% of the studies, only keloid scars located on the earlobes were treated and 15% of the studies did not specify scar location. The mean age of the patients was 28.7 ± 1.3 years (range, 2–82 years). In 35% of the studies, age was not described.
Excision and Radiation Type
Most studies used an extralesional approach to excise the scar (n = 12), only one study19 excised the scar intralesionally. Other studies did not specify their excision approach.
Studies using external radiation, HDR brachytherapy, or LDR brachytherapy were compared on study characteristics and study outcomes (Table 2). When analyzing the patient populations per radiation type group, no major differences in patient characteristics were seen.
Table 2.
Study Characteristics Analyzed between External Radiation and HDR and LDR Brachytherapy
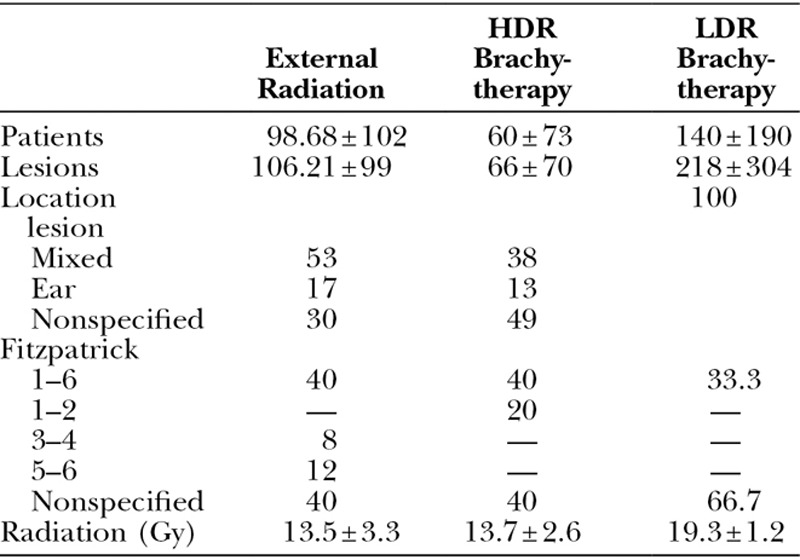
The mean total radiation dose for studies investigating external radiation and HDR brachytherapy was the same. Studies using LDR brachytherapy applied a higher radiation dose (external, 13.5 ± 3.3; HDR, 13.7 ± 2.6; LDR, 19.3 ± 1.2).
HDR brachytherapy was associated with the lowest mean recurrence rate, followed by LDR brachytherapy and external radiation therapy (HDR, 10.5 ± 15; range, 0–44; LDR, 21.3 ± 2.1; range, 19.4–23.6; external, 22.2 ± 16; range, 0–72). When looking only at level of evidence type II studies, HDR brachytherapy showed the lowest recurrence rate as well.
Only one study used a device to measure scar quality: Akita et al20 described the use of a Durometer to measure scar hardness, which improved with 50% posttreatment compared with pretreatment. No other studies used objective devices measuring scar elasticity, scar volume, or scar color.
Three studies5,8,20 used standardized assessment methods as the Patient and Observer Scar Assessment Scale (POSAS)21,22 or the Vancouver Scar Scale.23 Kreulen and Van de Kar et al5 reported high POSAS scores (the higher the score, the less the scar resembles normal skin) after treatment using external radiation. In contrast, van Leeuwen et al8 reported low POSAS scores after treatment using HDR brachytherapy.
Akita et al20 reported a significantly better improvement after external radiotherapy on all categories using the Vancouver Scar Scale compared with pretreatment. Other studies used different, nonvalidated, assessment tools.24
Short Interval
Many authors used a time interval of less than 24 hours between excision and irradiation.8,25,26 Especially with the use of brachytherapy, authors described an immediate transfer to the radiation department after surgery, resulting in an interval of less than 7 hours.7,8
Table 3 shows the differences in recurrence rate for radiation following excision within 7 hours,7,8,12,19,24,27,28 within 24 hours,5,9,10,14,15,29–34 or a longer period between excision and radiation.
Table 3.
Recurrence Rate of the Different Radiation Types Related to the Time Interval between Surgery and Adjuvant Radiotherapy
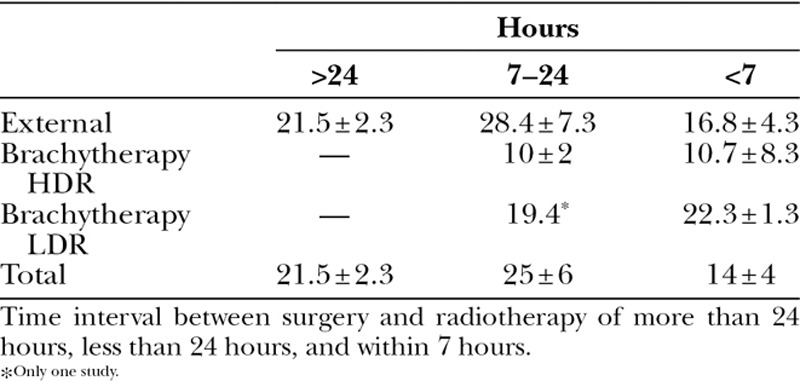
In the external radiation group of studies, the rate of recurrence of keloid scars decreased when radiation was applied within 7 hours, compared with 24 hours or longer (external radiotherapy: <7 hours, 17 ± 4; 7–24 hours, 28 ± 7; >24 hours, 21 ± 2). With HDR brachytherapy, radiation within 7 hours showed no difference in recurrence rate compared with HDR brachytherapy applied within 24 hours. Within the LDR brachytherapy group, comparison was not possible because of the low number of included studies.
Single Fraction
Of the included studies, Ragoowansi et al10,15 and Sclafani et al12 promoted a single-fraction radiation therapy using external radiation. When looking at the mean recurrence rate for these single-fraction protocols, a lower recurrence rate (12 ± 8.8) was seen compared with the mean recurrence rate within the total external radiation group (22.2 ± 16). In addition, no complications were described, and good results were achieved in terms of scar quality and patient’s satisfaction.
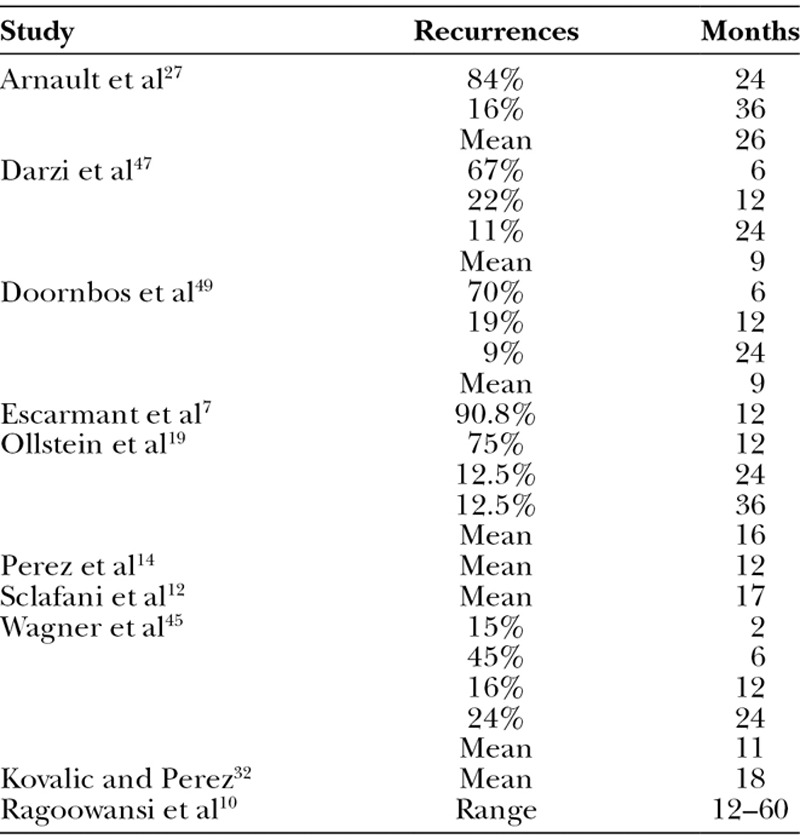
Recurrence
Ten studies (30%) provided information about the incidence of recurrence. The mean time for the incidence of recurrence after treatment was 14.8 ± 6.7 months with a range of 2–36 months (Table 4). Twelve studies described a definition for recurrence. Authors defined recurrence as any regrowth of tissue8,12,24,35,36; mild or failure relapse38; a symptomatic reappearance28; a regrowth extending beyond the original surgical field5; pain, itch from the scar, clinical evidence of a mass; obvious return of the lesion10,15; or just as impairment.34
Table 4.
Recurrence Percentages in Months
Complications
In all selected studies, no relation between scar radiation and malignancies was found. This is in accordance with other literature.38–40
DISCUSSION
The use of excision followed by adjuvant irradiation for the treatment of keloid scars is mostly based on research performed in the 1960s by Van den Brenk and Minty25 and Cosman et al.27 They were the first to compare different radiation protocols for the treatment of keloid scars.25,26
In their studies, the treatment options were divided in 2 categories: (1) primary irradiation without surgery and (2) lesions treated by excision combined with planned early and late prophylactic irradiation. Both authors draw comparative conclusions stating that (1) primary irradiation without surgery may relieve symptoms but fails to cause resolution of the actual lesion. (2) Late postoperative radiation is associated with higher recurrence rates compared with early postoperative radiation.25,26
In 1967, Nicoletis and Chassagne41 were the first to introduce interstitial (or internal) radiation, also called brachytherapy. Hereby, radiation is effectively localized inside the scar lesion, only targeting the area which is desired to irradiate. This in contrast to external radiotherapy in which considerable radiation of adjacent tissue is inevitable. This is undesirable, as exposure to radiation should be minimized in this often young population suffering from a benign disease which only needs radiation in a small area.
The conclusions of Van den Brenk and Minty25 and Cosman et al26 combined with the introduction of brachytherapy led to several recent publications in which protocols were described resulting in low recurrence rates and enhanced patient convenience.9,13,23,28,53 These protocols used HDR brachytherapy in one or more fractions, applied immediately after excision. We evaluated these results and recommendations with a comprehensive review based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Radiation Modality
External radiation resulted in a higher recurrence percentage compared with HDR and LDR brachytherapy. This can be explained by the fact that brachytherapy, in contrast to external radiation: (1) has more focused and efficient radiation of the targeted area; (2) requires a lower dose of radiation to achieve the same therapeutic effect, thereby reducing radionecrosis; and (3) provides less irradiation to surrounding healthy tissue.
When looking at brachytherapy, HDR brachytherapy scored lower recurrence rates compared with LDR brachytherapy. Although both techniques are considered brachytherapy, with HDR brachytherapy the total radiation is given in several minutes and ends shortly (<24 hours) following the operation. With LDR brachytherapy, however, this dose is spread out over 20–72 hours, which is actually a delayed treatment. In addition, HDR brachytherapy is an outpatient procedure enhancing patient convenience, whereas LDR brachytherapy requires hospitalization in lead-coated radioprotection chambers.
Short Interval
Van den Brenk and Minty25 and Cosman et al26 showed that early irradiation, within 24 hours, results in lower recurrence rates, compared with a more delayed irradiation. Other authors, however, hypothesized that early radiation within 7 hours could lower the recurrence rate even further.8 As Table 3 shows, this hypothesis was confirmed with the external radiation group of studies.
Surprisingly, this hypothesis was not confirmed in the HDR brachytherapy group. Notably, this discrepancy was caused by one study, which showed a very high recurrence percentage.28 When analyzing this RCT study by Veen and Kal,28 there were 3 groups receiving different radiation doses within 6 hours. The group receiving the smallest amount of gray (10 Gy) scored a high recurrence percentage of 44%. The other 2 groups in this study receiving 14 and 18 Gy showed a 3% and 0% recurrence rate, respectively. The authors hypothesized that this high recurrence rate was due to the low radiation dose of 10 Gy. This is in accordance with other HDR brachytherapy studies, which all applied a dose of 12 Gy or more. Thus, when excluding this deviant rate, an average recurrence rate of 2.3% was seen in with studies applying brachytherapy within 7 hours. This confirms the trend already seen in the external radiation group toward a low recurrence rate when irradiation is applied within 7 hours. Also, it may show that a minimum of 12-Gy irradiation is required.
The mechanism behind immediate irradiation following scar excision remains unclear. Many studies explain the effect of irradiation by the prevention of keloidal fibroblasts to repopulate.54,55 This seems illogical, as extralesional scar excision already removed all keloid fibroblasts. Another explanation could be that surgical scar excision will attract local fibroblasts. Stimulated by humoral or cellular factors, these local fibroblasts lead to a disturbed proliferation homeostasis, which eventually can lead to recurring of the scar. Irradiation may modulate these humoral or cellular factors, leading to a disruption of this cascade, thereby preventing scar recurrence. As this process starts directly after the operation, it is important to start the irradiation as quickly as possible, that is, transferring the patient immediately after surgery to the radiation department.
Single Fraction
Surgical excision followed by a single-fraction radiation dose would prevent repeated (outpatient) consults, thereby increasing the patient convenience and therapy adherence. Moreover, Van Den Brenk and Minty25 stated that there is no place for the use of fractionated small doses of radiation to attain a larger cumulative dose. They state that the dose-effect relationship is strictly threshold and that doses of less than 10 Gy substantially fail to inhibit the growth of regeneration of the scar.25 Out of the studies in this review, 3 studies administered a single- fraction dose with external radiation. They showed low recurrence percentages with good results in terms of scar quality and patient’s satisfaction. Importantly, no complications were noted.10,12 In our opinion, these results are promising. However, a RCT is required to confirm these results and prove the safety and efficacy of a single-fraction radiation therapy.
Study Protocol
A large part of the initially selected studies were excluded because they did not describe the minimum follow-up of the study. This review reports a mean scar recurrence after a mean of 15 months posttreatment with a maximum range of 36 months. Therefore, we recommend a minimum of 15-month follow-up, but preferably a period of 2 or even 3 years.
In addition, most studies did not clearly define keloid characteristics, keloid recurrence, and study outcomes. Defining keloid characteristics prevents inclusion of nonkeloid scars, such as hypertrophic scars. This is relevant as hypertrophic scars have better prognostic factors than keloid scars. We advise to use the following definition for inclusion of keloid scars as stated by Ogawa18: “A fibroproliferative disorder of the skin that grows beyond the boundaries of the original wound or has an unrecognized origin.” Also, posttreatment histology of the excised lesion may be used to confirm the nature of the scar.
As described in the result sections, only 12 studies (36%) defined keloid scar recurrence. Most studies, evaluating scar recurrences, use the definition as stated by Cosman and Wolff18 in 1974: “A growing, pruritic, nodular scar constituted a recurrence; a flat, nonpruritic scar was considered a good result.” Furthermore, to define the (residual) scar quality, validated measurement devices can be used. Examples are the Cutometer for scar elasticity,56 the Dermaspectrometer for scar color,57 and the POSAS for general scar assessment.21,22 Finally, inclusion of a variety of Fitzpatrick (F1–6)58 score patients is preferable as Afro-American patients (F5+6) are more prone to pigmentation disorders59, 60 and scar recurrence27 compared with white patients (F1–3).
Primary Closure
The recommendations in this article are based on the included studies, which included all keloid subtypes that could be closed primarily after excision. In the case of large or high-tension keloid scars, which cannot be closed primarily, we advise to use skin grafts as described by Li et al.61
Comparison with Other Treatment Modalities
As demonstrated in this systematic review, excision with adjuvant HDR brachytherapy offers total scar eradication with low recurrence rates (mean, 10.5%). Other treatment modalities will not always result in a complete volume reduction and demonstrated higher recurrence rates, corticosteroid injections monotherapy (>50%17), surgery with corticosteroid injections (<50%3), and intralesional cryotherapy (24%62). On the other hand, surgical excision with irradiation is not always possible due to the patient characteristics (pregnant or age, <12 years) or the location of the keloid scar (radiosensitive locations such as the thyroid gland). Finally, it should be mentioned that the costs of excision with irradiation exceed the costs of other treatments significantly. Therefore, excision with adjuvant radiation therapy should be regarded as a “last resort” for (recalcitrant) large keloid scars, when other nonsurgical treatments have failed.
CONCLUSIONS
Based on this systematic review of the literature, the use of HDR brachytherapy following scar excision, preferably applied within 7 hours, results in a low recurrence rate. Also, single-fraction irradiation appears safe and enhances patient convenience. However, the quality of this evidence is limited. There is a paucity of high-quality studies with clearly defined methods and study outcomes. More randomized studies comparing different radiation protocols will generate stronger recommendations.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. A portion of the Article Processing Charge was paid for by PRS Global Open at the discretion of the Editor-in-Chief. The remainder of the Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Niessen FB, Spauwen PH, Schalkwijk J, et al. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999;104:1435–1458. doi: 10.1097/00006534-199910000-00031. [DOI] [PubMed] [Google Scholar]
- 2.Bock O, Schmid-Ott G, Malewski P, et al. Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res. 2006;297:433–438. doi: 10.1007/s00403-006-0651-7. [DOI] [PubMed] [Google Scholar]
- 3.Mustoe TA, Cooter RD, Gold MH, et al. International Advisory Panel on Scar Management. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560–571. doi: 10.1097/00006534-200208000-00031. [DOI] [PubMed] [Google Scholar]
- 4.Sequeira JH. Case illustrating the effects of x-rays on scar-keloid. Proc R Soc Med. 1909;2:96–98. [PMC free article] [PubMed] [Google Scholar]
- 5.Kreulen M, Van De Kar A. Long-term follow-up in the treatment of keloids by combined surgical excision and immediate postoperative adjuvant irradiation: reply. Plast Reconstr Surg. 2008;121:700–701. doi: 10.1097/01.prs.0000294956.63159.0c. [DOI] [PubMed] [Google Scholar]
- 6.Malaker A, Ellis F, Paine CH. Keloid scars: a new method of treatment combining surgery with interstitial radiotherapy. Clin Radiol. 1976;27:179–183. doi: 10.1016/s0009-9260(76)80141-9. [DOI] [PubMed] [Google Scholar]
- 7.Escarmant P, Zimmermann S, Amar A, et al. The treatment of 783 keloid scars by iridium 192 interstitial irradiation after surgical excision. Int J Radiat Oncol Biol Phys. 1993;26:245–251. doi: 10.1016/0360-3016(93)90204-9. [DOI] [PubMed] [Google Scholar]
- 8.van Leeuwen MC, Stokmans SC, Bulstra AE, et al. High-dose-rate brachytherapy for the treatment of recalcitrant keloids: a unique, effective treatment protocol. Plast Reconstr Surg. 2014;134:527–534. doi: 10.1097/PRS.0000000000000415. [DOI] [PubMed] [Google Scholar]
- 9.Arneja JS, Singh GB, Dolynchuk KN, et al. Treatment of recurrent earlobe keloids with surgery and high-dose-rate brachytherapy. Plast Reconstr Surg. 2008;121:95–99. doi: 10.1097/01.prs.0000293755.64918.22. [DOI] [PubMed] [Google Scholar]
- 10.Ragoowansi R, Cornes PG, Moss AL, et al. Treatment of keloids by surgical excision and immediate postoperative single-fraction radiotherapy. Plast Reconstr Surg. 2003;111:1853–1859. doi: 10.1097/01.PRS.0000056869.31142.DE. [DOI] [PubMed] [Google Scholar]
- 11.Lo TC, Seckel BR, Salzman FA, et al. Single-dose electron beam irradiation in treatment and prevention of keloids and hypertrophic scars. Radiother Oncol. 1990;19:267–272. doi: 10.1016/0167-8140(90)90153-n. [DOI] [PubMed] [Google Scholar]
- 12.Sclafani AP, Gordon L, Chadha M, et al. Prevention of earlobe keloid recurrence with postoperative corticosteroid injections versus radiation therapy: a randomized, prospective study and review of the literature. Dermatol Surg. 1996;22:569–574. doi: 10.1111/j.1524-4725.1996.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 13.Song C, Wu HG, Chang H, et al. Adjuvant single-fraction radiotherapy is safe and effective for intractable keloids. J Radiat Res. 2014;55:912–916. doi: 10.1093/jrr/rru025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez CA, Lockett MA, Young G. Radiation therapy for keloids and plantar warts. Front Radiat Ther Oncol. 2001;35:135–146. doi: 10.1159/000061273. [DOI] [PubMed] [Google Scholar]
- 15.Ragoowansi R, Cornes PG, Glees JP, et al. Ear-lobe keloids: treatment by a protocol of surgical excision and immediate postoperative adjuvant radiotherapy. Br J Plast Surg. 2001;54:504–508. doi: 10.1054/bjps.2001.3656. [DOI] [PubMed] [Google Scholar]
- 16.American Society of Plastic Surgeons. ASPS Evidence Rating Scales. Available at: http://www.plasticsurgery.org/Documents/medical-professionals/ health-policy/evidence-practice/ASPS-Rating-Scale-March-2011.pdf. Accessed January 2014. [Google Scholar]
- 17.Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. Plast Reconstr Surg. 2010;125:557–568. doi: 10.1097/PRS.0b013e3181c82dd5. [DOI] [PubMed] [Google Scholar]
- 18.Cosman B, Wolff M. Bilateral earlobe keloids. Plast Reconstr Surg. 1974;53:540–543. doi: 10.1097/00006534-197405000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Ollstein RN, Siegel HW, Gillooley JF, et al. Treatment of keloids by combined surgical excision and immediate postoperative x-ray therapy. Ann Plast Surg. 1981;7:281–285. doi: 10.1097/00000637-198110000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Akita S, Akino K, Yakabe A, et al. Combined surgical excision and radiation therapy for keloid treatment. J Craniofac Surg. 2007;18:1164–1169. doi: 10.1097/scs.0b013e3180de62a1. [DOI] [PubMed] [Google Scholar]
- 21.Draaijers LJ, Tempelman FR, Botman YA, et al. The Patient and Observer Scar Assessment Scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg. 2004;113:1960–1965; discussion 1966. doi: 10.1097/01.prs.0000122207.28773.56. [DOI] [PubMed] [Google Scholar]
- 22.van der Wal MB, Tuinebreijer WE, Bloemen MC, et al. Rasch analysis of the Patient and Observer Scar Assessment Scale (POSAS) in burn scars. Qual Life Res. 2012;21:13–23. doi: 10.1007/s11136-011-9924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baryza MJ, Baryza GA. The Vancouver Scar Scale: an administration tool and its interrater reliability. J Burn Care Rehabil. 1995;16:535–538. doi: 10.1097/00004630-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Guix B, Henríquez I, Andrés A, et al. Treatment of keloids by high-dose-rate brachytherapy: a seven-year study. Int J Radiat Oncol Biol Phys. 2001;50:167–172. doi: 10.1016/s0360-3016(00)01563-7. [DOI] [PubMed] [Google Scholar]
- 25.Van Den Brenk HA, Minty CC. Radiation in the management of keloids and hypertrophic scars. Br J Surg. 1960;47:595–605. doi: 10.1002/bjs.18004720603. [DOI] [PubMed] [Google Scholar]
- 26.Cosman B, Crikelair GF, Ju DMC, et al. The surgical treatment of keloids. Plast Reconstr Surg. 1961;27:335–358 [Google Scholar]
- 27.Arnault JP, Peiffert D, Latarche C, et al. Keloids treated with postoperative iridium 192* brachytherapy: a retrospective study. J Eur Acad Dermatol Venereol. 2009;23:807–813. doi: 10.1111/j.1468-3083.2009.03190.x. [DOI] [PubMed] [Google Scholar]
- 28.Veen RE, Kal HB. Postoperative high-dose-rate brachytherapy in the prevention of keloids. Int J Radiat Oncol Biol Phys. 2007;69:1205–1208. doi: 10.1016/j.ijrobp.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhry MR, Akhtar S, Duvalsaint F, et al. Ear lobe keloids, surgical excision followed by radiation therapy: a 10-year experience. Ear Nose Throat J. 1994;73:779–781. [PubMed] [Google Scholar]
- 30.Garg MK, Weiss P, Sharma AK, et al. Adjuvant high dose rate brachytherapy (Ir-192) in the management of keloids which have recurred after surgical excision and external radiation. Radiother Oncol. 2004;73:233–236. doi: 10.1016/j.radonc.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Lee SH. Therapeutic results and safety of postoperative radiotherapy for keloid after repeated Cesarean section in immediate postpartum period. Radiat Oncol J. 2012;30:49–52. doi: 10.3857/roj.2012.30.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kovalic JJ, Perez CA. Radiation therapy following keloidectomy: a 20-year experience. Int J Radiat Oncol Biol Phys. 1989;17:77–80. doi: 10.1016/0360-3016(89)90373-8. [DOI] [PubMed] [Google Scholar]
- 33.Norris JE. Superficial x-ray therapy in keloid management: a retrospective study of 24 cases and literature review. Plast Reconstr Surg. 1995;95:1051–1055. doi: 10.1097/00006534-199505000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Sällström KO, Larson O, Hedén P, et al. Treatment of keloids with surgical excision and postoperative x-ray radiation. Scand J Plast Reconstr Surg Hand Surg. 1989;23:211–215. doi: 10.3109/02844318909075120. [DOI] [PubMed] [Google Scholar]
- 35.Sakamoto T, Oya N, Shibuya K, et al. Dose-response relationship and dose optimization in radiotherapy of postoperative keloids. Radiother Oncol. 2009;91:271–276. doi: 10.1016/j.radonc.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa R, Mitsuhashi K, Hyakusoku H, et al. Postoperative electron-beam irradiation therapy for keloids and hypertrophic scars: retrospective study of 147 cases followed for more than 18 months. Plast Reconstr Surg. 2003;111:547–553; discussion 554. doi: 10.1097/01.PRS.0000040466.55214.35. [DOI] [PubMed] [Google Scholar]
- 37.Emad M, Omidvari S, Dastgheib L, et al. Surgical excision and immediate postoperative radiotherapy versus cryotherapy and intralesional steroids in the management of keloids: a prospective clinical trial. Med Princ Pract. 2010;19:402–405. doi: 10.1159/000316381. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa R, Yoshitatsu S, Yoshida K, et al. Is radiation therapy for keloids acceptable? The risk of radiation-induced carcinogenesis. Plast Reconstr Surg. 2009;124:1196–1201. doi: 10.1097/PRS.0b013e3181b5a3ae. [DOI] [PubMed] [Google Scholar]
- 39.De Lorenzi F, Tielemans HJ, van der Hulst RR, et al. Is the treatment of keloid scars still a challenge in 2006? Ann Plast Surg. 2007;58:186–192. doi: 10.1097/01.sap.0000237761.52586.f9. [DOI] [PubMed] [Google Scholar]
- 40.Kal HB, Veen RE, Jürgenliemk-Schulz IM. Dose-effect relationships for recurrence of keloid and pterygium after surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 2009;74:245–251. doi: 10.1016/j.ijrobp.2008.12.066. [DOI] [PubMed] [Google Scholar]
- 41.Nicoletis C, Chassagne D. [Interstitial irradiation by iridium 192 in the prevention of recurrence after surgical excision of keloid cicatrices]. Ann Chir Plast. 1967;12:237–242. [PubMed] [Google Scholar]
- 42.Ogawa R, Huang C, Akaishi S, et al. Analysis of surgical treatments for earlobe keloids: analysis of 174 lesions in 145 patients. Plast Reconstr Surg. 2013;132:818e–825e. doi: 10.1097/PRS.0b013e3182a4c35e. [DOI] [PubMed] [Google Scholar]
- 43.Van de Kar AL, Kreulen M, van Zuijlen PPM, Oldenburger F. The results of surgical excision and adjuvant irradiation for therapy-resistant keloids: a prospective clinical outcome study. Plast Reconstr Surg. 2007;119:2248–2254. doi: 10.1097/01.prs.0000260751.20217.28. [DOI] [PubMed] [Google Scholar]
- 44.Maarouf M, Schleicher U, Schmachtenberg A, Ammon J. Radiotherapy in the management of keloids. Clinical experience with electron beam irradiation and comparison with X-ray therapy. Strahlenther Onkol Organ Dtsch Röntgenges Al. 2002;178:330–335. doi: 10.1007/s00066-002-0935-6. [DOI] [PubMed] [Google Scholar]
- 45.Wagner W, Alfrink M, Micke O, Schafer U, Schuller P, Willich N. Results of prophylactic irradiation in patients with resected keloids - A retrospective analysis. Acta Oncol. 2000;39:217–220. doi: 10.1080/028418600430806. [DOI] [PubMed] [Google Scholar]
- 46.Durosinmi-Etti FA, Olasinde TA, Solarin EO. A short course postoperative radiotherapy regime for keloid scars in Nigeria. West Afr J Med. 1994;13:17–19. [PubMed] [Google Scholar]
- 47.Darzi MA, Chowdri NA, Kaul SK, Khan M. Evaluation of various methods of treating keloids and hypertrophic scars: A 10-year follow-up study. Br J Plast Surg. 1992;45:374–379. doi: 10.1016/0007-1226(92)90008-l. [DOI] [PubMed] [Google Scholar]
- 48.Supe SS, Supe SJ, Rao SM, Deka AC, Deka BC. Treatment of keloids by 90Sr-90Y beta-rays. Strahlenther Onkol Organ Dtsch Röntgenges Al. 1991;167:397–402. [PubMed] [Google Scholar]
- 49.Doornbos JF, Stoffel TJ, Hass AC, et al. The role of kilovoltage irradiation in the treatment of keloids. Int J Radiat Oncol Biol Phys. 1990;18(4):833–839. doi: 10.1016/0360-3016(90)90405-9. [DOI] [PubMed] [Google Scholar]
- 50.Deka BC, Deka AC, Avadhani JS, et al. Treatment of keloids with strontium 90 beta rays. Indian J Cancer. 1987;24:15–21. [PubMed] [Google Scholar]
- 51.Inalsingh CH. An experience in treating five hundred and one patients with keloids. Johns Hopkins Med J. 1974;134:284–290. [PubMed] [Google Scholar]
- 52.King GD, Salzman FA. Keloid scars. Analysis of 89 patients. Surg Clin North Am. 1970;50:595–598. doi: 10.1016/s0039-6109(16)39136-8. [DOI] [PubMed] [Google Scholar]
- 53.Rio E, Bardet E, Peuvrel P, et al. Perioperative interstitial brachytherapy for recurrent keloid scars. Plast Reconstr Surg. 2009;124:180e–181e. doi: 10.1097/PRS.0b013e3181a83b7e. [DOI] [PubMed] [Google Scholar]
- 54.Luo S, Benathan M, Raffoul W, et al. Abnormal balance between proliferation and apoptotic cell death in fibroblasts derived from keloid lesions. Plast Reconstr Surg. 2001;107:87–96. doi: 10.1097/00006534-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 55.Kal HB, Veen RE. Biologically effective doses of postoperative radiotherapy in the prevention of keloids. Dose-effect relationship. Strahlenther Onkol. 2005;181:717–723. doi: 10.1007/s00066-005-1407-6. [DOI] [PubMed] [Google Scholar]
- 56.Draaijers LJ, Botman YA, Tempelman FR, et al. Skin elasticity meter or subjective evaluation in scars: a reliability assessment. Burns. 2004;30:109–114. doi: 10.1016/j.burns.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 57.Draaijers LJ, Tempelman FRH, Botman YAM, et al. Colour evaluation in scars: tristimulus colorimeter, narrow-band simple reflectance meter or subjective evaluation? Burns J Int Soc Burn Inj. 2004;30:103–107. doi: 10.1016/j.burns.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 58.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 59.Kenney JA., Jr Pigmentary disorders in black skin. Clin Dermatol. 1989;7:1–10. doi: 10.1016/0738-081x(89)90052-7. [DOI] [PubMed] [Google Scholar]
- 60.Dinh Q, Veness M, Richards S. Role of adjuvant radiotherapy in recurrent earlobe keloids. Australas J Dermatol. 2004;45:162–166. doi: 10.1111/j.1440-0960.2004.00079.x. [DOI] [PubMed] [Google Scholar]
- 61.Li W, Wang Y, Wang X, et al. A keloid edge precut, preradiotherapy method in large keloid skin graft treatment. Dermatol Surg. 2014;40:52–57. doi: 10.1111/dsu.12374. [DOI] [PubMed] [Google Scholar]
- 62.van Leeuwen MC, van der Wal MB, Bulstra AE, et al. Intralesional cryotherapy for treatment of keloid scars: a prospective study. Plast Reconstr Surg. 2015;135:508–509. doi: 10.1097/PRS.0000000000000911. [DOI] [PubMed] [Google Scholar]


