Abstract
Background:
Adverse skin scarring varies by anatomical site with, for example, presternal skin showing a greater hypertrophic response when compared with eyelid; such differences have traditionally been attributed to regional variations in skin tension, thickness, and Langer’s lines. Fibroblasts are the main cell implicated in fibrosis, and they too are known to show anatomical variation in their expression, differentiation, and intercellular interactions. We, therefore, investigated whether intrinsic differences in skin fibroblasts derived from separate locations might contribute to the observed discrepancies in clinical scarring.
Methods:
Primary in vitro cultures were established using matched eyelid and presternal skin from 3 healthy donors undergoing blepharoplasty surgery. We used an in vitro collagen gel model of fibroblast-mediated tissue contraction to compare the properties of the dermal fibroblasts from each site. Cell contractile force and matrix stiffness were assessed in 3-dimensional tissue constructs using an automated high-throughput device.
Results:
Dermal fibroblasts isolated from eyelid and sternum differ both in their ability to contract a gel matrix and in their response to cytokine stimulation; despite having lower intrinsic contractile force (P < 0.01) and resting stiffness (P < 0.02), the presternal cells were more contractile (P < 0.001) following stimulation with serum, or inflammatory cytokines transforming growth factor-β (P < 0.01) and interleukin-1β (P < 0.05).
Conclusions:
The propensity to cutaneous scarring may, at least in part, result from intrinsic differences in the local fibroblasts’ ability to contract and their sensitivity to inflammatory cytokines. Improved understanding of the underlying molecular pathways should prove useful in identifying new therapeutic targets for altering surgical and other scarring.
Cutaneous healing is a complex biological response after injury, serving to reestablish the barrier function of skin, but postoperative scarring can present a significant problem with pain, pruritus, functional impairment, poor aesthetics, and psychological morbidity.1
The propensity to cutaneous scarring differs by anatomical site, with presternal skin being particularly prone to hypertrophic scarring and keloid formation.2 In contrast, periocular skin usually heals with imperceptible scars,3 and this has allowed good aesthetic results to be achieved with a “laissez faire” approach—in which a tissue defect is left to heal by secondary intention.4–7 Regional variability in scarring has been attributed to anatomic differences in the orientation of skin tension lines and in skin thickness,5,6 with wounds under tension having been shown to scar excessively.7 Studies to compare site differences in the intrinsic properties of skin are limited, but these suggest wide variation in resting tension,8,9 stress, elasticity,10 and thickness—with eyelid skin being the thinnest.11 Although understanding the role of mechanical tension in scarring has resulted in the development of stress-shielding devices to improve cutaneous scars,12 the many treatments for scarring—none of which has proved widely effective13—suggest that our understanding of the pathophysiology of scar formation remains limited. It is, however, notable that embryonic tissues heal without scarring but later loose this ability, and such changes in the propensity to scarring have focused research on molecular signaling—with key cytokines [such as vascular endothelial growth factor, transforming growth factor-β (TGFβ), platelet-derived growth factor, interleukin-1β (IL1β), and epidermal growth factor] having been implicated in paracrine signaling during scar formation.14
Fibroblasts are a key cell involved in wound repair and collagen deposition, and their behavior is modulated by cytokines, stress, and intracellular and cell–matrix interactions—perturbations of which can contribute to excessive scars.15 Fibroblasts are a diverse group of cells, and their behavior and expression profiles vary with anatomic location, for example, they show regional differences in their ability to support inflammatory cells, such as neutrophils and T-lymphocytes.16 Differentially expressed genes include those implicated in matrix synthesis, lipid metabolism, and signaling pathways controlling migration, proliferation, and eventual fate of the cells; indeed, it has been suggested that fibroblasts from different sites should be considered distinct cell types.17 This proposal has been confirmed in a subsequent study, in which expression profiles alone were used to identify, with 80% accuracy, the original anatomic locations of cultured fibroblasts.18
Prior research has generally focused on the properties of fibroblasts derived from scar tissue itself, and the intrinsic contractility, scarring, and biological diversity of healthy dermal fibroblasts from different locations have not been described. We conjectured that—when controlling for mechanical input and culture conditions—such dermal fibroblasts are likely to show differences in their contractile properties and responses to cytokine stimulation, and that this might be related to variability in the tendency to cutaneous scarring. Cutaneous fibroblasts were, therefore, isolated from matched eyelid and presternal skin, and their contractile phenotype was characterized in our functional in vitro model of scar formation.19–21
MATERIAL AND METHODS
Clinical Samples
This study adhered to the tenets of the Declaration of Helsinki and was approved by both local and regional ethics committees (Ethics approval ref 11/LO/1171). Upper eyelid skin biopsies and a 5-mm punch biopsy of presternal skin were obtained from 3 consecutive patients undergoing routine upper eyelid blepharoplasty, and these patients having given consent to the additional biopsy of presternal skin. Patients with previous eyelid or peristernal surgery were excluded.
All patients were Caucasian, and patient 1 was a female aged 47 years, patient 2 was a female aged 53 years, and patient 3 was a male aged 62 years. None had a past medical history of chronic disease, hypertrophic cutaneous scarring, or keloid formation. Incisions were closed using subcuticular 6/0 prolene that was removed after 1 week. Patients were reviewed after 3 months, and the scars were assessed using the Vancouver scar scale22 to clinically quantify the eyelid and the presternal scar formation.23,24
Isolation of Fibroblasts
Biopsy samples were wrapped in sterile gauze, moistened with normal saline, and transported to the laboratory at +8°C. The biopsies were mechanically dispersed, and the tissue fragments placed in tissue culture dishes in Dulbecco’s modified Eagle’s medium (DMEM) with high glucose (Invitrogen, Life Technologies, Paisley, UK), supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich, Gillingham, UK), 100 IU/mL penicillin, 4.5 g/L of l-glutamine, and 100 g/mL streptomycin (Invitrogen), and incubated at 37°C with 5% CO2. Following outgrowth from the explant, the fibroblast populations were trypsinized and maintained routinely in the above medium. The cells were used between passages 4 and 8 for all experiments.
Collagen Contraction Assay
These were performed according to the method described by Tovell et al.19 Briefly, fibroblasts were seeded in a 1.5 mg/mL collagen type-I matrix (First Link UK Ltd, Wolverhampton, UK) at a concentration of 7 × 104 cells/mL in complete medium. After polymerization at 37°C, the gels were detached from the edge of the well and 2 mL of culture medium added. Gel contraction was monitored daily for 7 days by digital photography, the gel areas measured using ImageJ software (http://rsb.info.nih.gov/ij/), and the contraction plotted as a percentage area normalized to the original area at day 0. For cytokine stimulation, the gels were made with 14 × 104 cells/mL in serum-free medium, with or without 5 ng/mL recombinant human TGFβ1 or 10 ng/mL IL1β (R&D Systems, Abingdon, UK)—placed in both the gel mix and the medium.
Force Measurement
A fibroblast suspension of 3.3 × 105 cells/mL in 1.5 mg/mL collagen type-I in complete medium was pipetted as 300 μL/well into the MC-8 chamber (InvivoSciences, Madison, Wis.) and incubated at 37°C for 30 minutes, to complete polymerization. Then, 350 μL of complete medium was added to each well, and the gels were incubated for 48 hours to form tissue-like matrices. The medium was changed to serum-free DMEM for 18 hours before force measurement. The MC-8 chamber was then placed on the stage of the Palpator device (InvivoSciences), which automatically stretches the matrices and measures the contractile force; to obtain stable measurements, the matrices were preconditioned by stretching 3 times before force measurements. To measure force changes following serum stimulation, the serum-free medium was replaced with DMEM supplemented with 20% serum and the forces measured every 15 minutes for 3 iterations. The samples were run as quadruplicates, and the force calculated from the average of the measurements from a minimum of 3 experiments. A custom Matlab algorithm was used to analyze the data, and calculate cell contractile forces and matrix stiffness.25
Statistical Analysis
All graphs display mean and standard error. Unless otherwise stated, all comparisons were performed using the Student’s t test, and individual significance (α-risk) values are displayed.
RESULTS
Presternal Fibroblasts Display Faster Matrix Contraction, when Compared with Matched Eyelid-derived Cells
Fibroblasts from pairs of eyelid and presternal skins from 3 different donors established well in culture, displayed the characteristic elongated morphology of fibroblasts (irrespective of origin; Fig. 1), and showed comparable growth parameters, such as doubling time (data not shown). We used a validated in vitro model of cell-mediated collagen gel contraction19–21 to assess the contraction potential of these fibroblasts in the presence of 10% FBS, which classically mimics the early wound response. As expected from their dermal origin, both sets of fibroblasts were very efficient in contracting collagen gels, reaching 70% contraction by day 7 (Fig. 2). However, the early contraction was significantly faster for presternal cells (P < 0.01)—suggesting that they possess a greater intrinsic propensity to contraction in response to serum stimulation. Similarly, review of the surgical incisions in our patients showed worse Vancouver scar scale scores (greater clinical scarring) for presternal incisions (mean 4.3), when compared with blepharoplasty incisions (mean, 1.2; P = 0.005; Mann–Whitney U test).
Fig. 1.
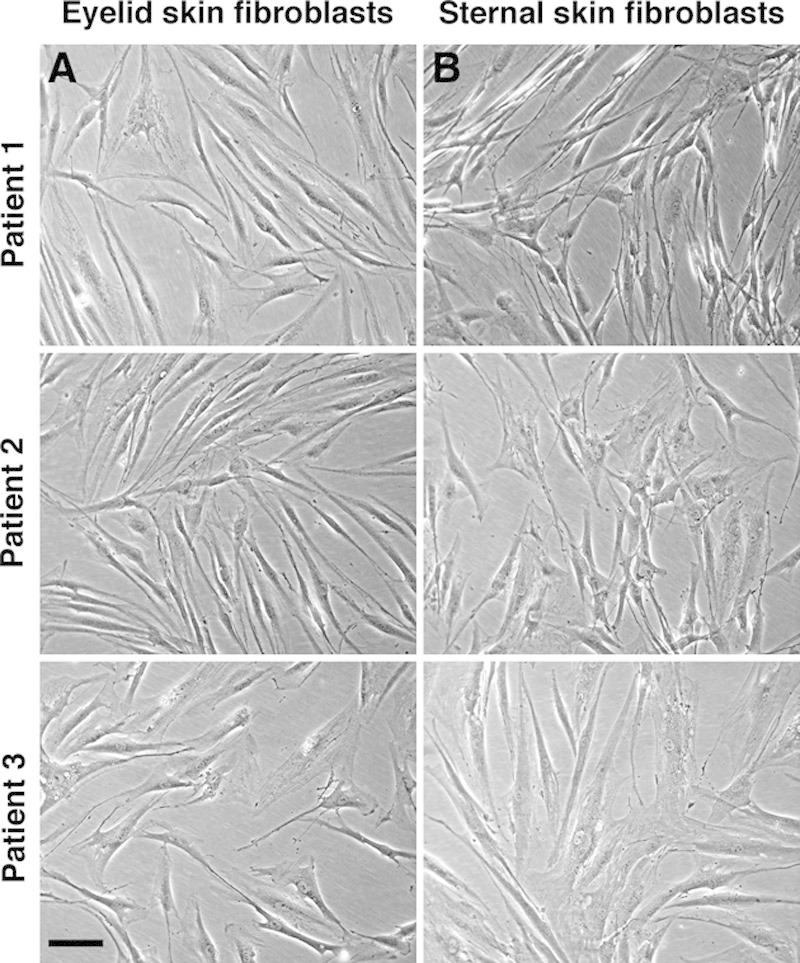
Presternal and eyelid cutaneous fibroblasts show similar morphology in standard monolayer cultures. Eyelid fibroblasts (A) display a typical elongated fibroblast shape, similar to that of their matching presternal counterpart cells (B). Bar = 100 μm.
Fig. 2.
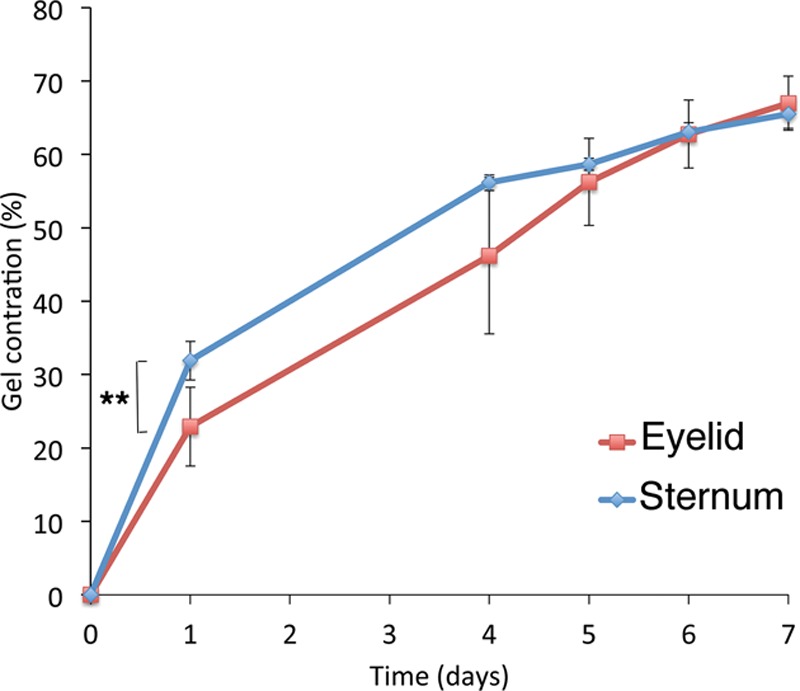
Presternal cutaneous fibroblasts display increased early contraction of a collagen matrix after serum stimulation when compared with matching eyelid cells. Fibroblasts from the eyelid (red), or from the presternal area (blue), were embedded in free-floating collagen matrices and monitored daily for 7 days. Contraction is expressed as the percentage decrease in the gel area, relative to the area at time 0. Shown is the mean result of at least 3 experiments run in triplicate; error bars indicate standard error. ** indicates significant difference, P < 0.01.
Presternal and Lid Cutaneous Fibroblasts Have Different Mechanical Properties
To investigate whether a greater cellular force might underlie the scarring propensity of presternal cutaneous fibroblasts, we used the Palpator—an automated device that measures cellular contractile force and extra-cellular matrix rigidity in engineered 3-dimensional (3D) tissue matrices. Eyelid and presternal fibroblasts were cultured in specialized 3D chambers to generate contracted tissues at tensional equilibrium; these matrices were then incubated overnight in serum-free medium, and the cellular contractile force (Fig. 3A) and matrix elasticity (Fig. 3B) were assessed before and after stimulation with 20% FBS (Fig. 3). When compared with eyelid-derived fibroblasts, presternal cells showed a significantly lower contractile force at rest (P < 0.01) and a lower matrix elastic force (P < 0.02). However, presternal fibroblasts showed a marked and significant increase in cellular force after serum stimulation (P< 0.001), whereas the increased contractile force of eyelid fibroblasts was not statistical significant. As expected,25 serum stimulation did not significantly affect the matrix elastic force from either tissue (Fig. 3B).
Fig. 3.
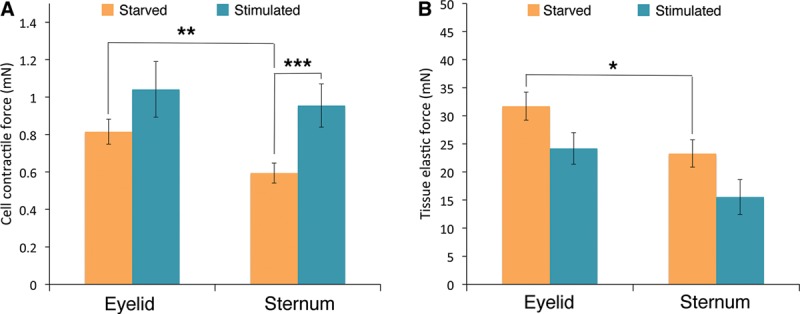
Presternal and eyelid cutaneous fibroblasts differ in their cell-force profiles: eyelid fibroblasts and presternal cells were cultured in collagen gels to generate 3D tissue-like matrices, which were then starved overnight in serum-free medium. Cellular force generation and tissue matrix rigidity were determined before (starved) and after stimulation with 20% FBS for 15 minutes (stimulated). A, Eyelid cutaneous fibroblasts display greater intrinsic baseline cellular contractile force when compared with matching presternal fibroblasts under resting conditions (**, P < 0.01), but only presternal cells showed a significant increase in contractile force following stimulation (***, P < 0.001). B, Matrices prepared with eyelid cutaneous fibroblasts displayed greater elastic force than those made with presternal cells (*, P < 0.02), with no change after serum stimulation.
Presternal Fibroblasts Display Increased Cytokine-induced Matrix Contraction
A range of inflammatory cytokines promote fibroblast contraction after wounding, and specific cytokines (such as TGFβ1 and IL1β) have been linked to tissue scarring and fibrosis. To assess whether the presternal cells’ increased propensity to serum-stimulated matrix contraction (Fig. 3) was linked to an increased sensitivity to inflammatory cytokines, we performed gel contraction assays in the presence of either TGFβ1 or IL1β. As shown in Figure 4, neither eyelid nor presternal skin cells generated significant matrix contraction in the serum-free medium. However, after stimulation with either FBS, TGFβ1, or IL1β, fibroblasts from both tissues showed a significant increase in gel contraction (Fig. 4A, B; P< 0.05). Although gel contraction in response to inflammatory cytokines was minimal for eyelid-derived fibroblasts, both TGFβ1 and IL1β elicited a strong contraction response from presternal cells (Fig. 4C; P < 0.05), with TGFβ1 being particularly effective in promoting gel contraction.
Fig. 4.
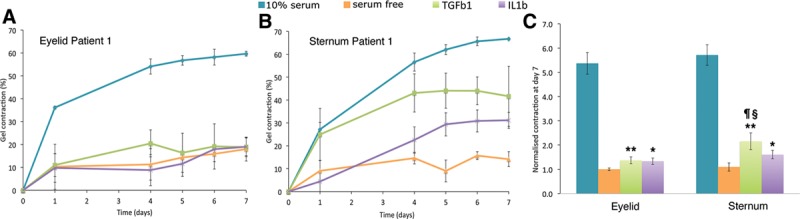
Presternal cutaneous fibroblasts show a greater contraction in response to inflammatory cytokines, when compared with those derived from matching eyelid skin. Fibroblasts were embedded in free-floating collagen matrices in a serum-free medium, supplemented with either TGFβ1 (5 ng/mL) or IL1β (10 ng/mL) and contraction monitored daily. Shown are typical contraction curves from a single patient’s eyelid skin (A) and presternal skin (B). The mean and standard error for 3 experiments done in triplicate is shown. C, Average contraction at day 7 is shown for matching eyelid and presternal fibroblast matrices for all 3 patients, normalized to the value of eyelid cells in a serum-free medium. Both sets of cells displayed a significant contractile response following cytokine stimulation, when compared with serum-free medium (*, P < 0.05; **, P < 0.01). However, presternal fibroblasts show a stronger response to TGFβ1 when compared with IL1β (¶, P < 0.05), and a greater response to TGFβ1 when compared with that of eyelid-derived cells (§, P < 0.05).
DISCUSSION
Cutaneous wound healing is a complex process, and the biological mechanisms underlying a successful physiological resolution rather than a pathological scarring outcome are still largely unknown.1 With eyelid incisions usually healing well, and scar hypertrophy and keloids being common on the chest wall, we chose to use paired eyelid and presternal tissues for our study to represent opposite ends of the clinical spectrum of cutaneous scarring.2,3,5 Because fibroblasts are the primary drivers of scarring15 and their biological properties are known to vary with their anatomical origin,14,16–18,23 we surmised that the strikingly different scarring behavior observed in eyelid and chest skin could reflect different properties of the local cutaneous fibroblasts. We used our well-characterized in vitro 3D model of tissue contraction21,26–29 to assess the contractile properties of the tissue-derived fibroblasts in classical free-floating collagen gels and further analyzed the intrinsic mechanical properties of the cells within engineered tissues mimicking physiological environments.25
In line with their scarring potential in vivo, we found that presternal fibroblasts displayed greater matrix contraction ability in vitro when compared with eyelid cells, upon stimulation with both serum and inflammatory cytokines TGFβ1 and IL1β. As we have shown previously using a range of ocular cells, including fibroblasts isolated from scarred tissue,20,21 our in vitro contraction assay using soft collagen gels is a good reflection of the potential of the cells for scarring in vivo.20,28,30 This implies that, upon tissue injury and local release of cytokines, presternal cells may have an inherent ability to generate more contracture and scarring—an observation in accord with clinical experience. Interestingly, when the cells’ properties were analyzed within more physiological engineered tissues at tensional homeostasis, resting presternal fibroblasts were found to have a lower intrinsic cellular contractile force compared with eyelid cells, generating a tissue of overall slightly lower stiffness. This suggests that contrary to the traditional belief that local tension lines are largely responsible for the abnormally high scarring potential of the chest tissue,2,5,6,31,32 local mechanical tension per se might not be the major factor underlying the fibroblasts’ propensity for scarring. Indeed, although an increase in tension is usually linked to hypertrophic scarring,15,26,27 partly through an increase in the local α-smooth muscle actin producing myofibroblast population,15 keloid scars on the other hand only have a minor contractile compartment, being largely driven by cytokine-mediated stimulation of extracellular matrix over-production.5,13–15 Despite their lower intrinsic force at rest, the presternal cells generated a stronger contractile force when stimulated with serum – consistent with the idea that increased sensitivity to serum components and cytokines upon wounding, as well as the ability to develop a greater contractile force, might underlie the propensity for presternal cutaneous scarring. This behavior is reminiscent of that of fibrotic fibroblasts isolated from the conjunctiva of patients with Floppy Eyelid Syndrome, whereby the diseased fibroblasts displayed increased collagen matrix contraction abilities,21 despite the affected tissues of origin displaying a disorganized, much less stiff, matrix.30 This suggests that whilst fibroblasts’ behavior is adaptable and intimately linked to tissue biomechanics, their propensity to scar may eventually be down to how they respond to the local stimuli upon wounding.
Overall, the results presented here support our hypothesis that, when controlling for culture and mechanical environments, there are intrinsic differences in the mechanobiology of eyelid and presternal cutaneous fibroblasts. These intrinsic differences might be an important determinant in the formation of clinically significant scarring. Differences in the resting tensions within, and rigidity of, engineered tissue matrices derived from eyelid and presternal fibroblasts might also belie the traditional belief that differences in clinical scarring are solely related to tissue tension and skin thickness. Indeed, dermal fibroblasts populations are extremely heterogeneous,14,16–18,23 and the overall mixed populations that we have isolated may comprise various subsets of fibroblasts and fibroblast-like cells, including mesenchymal stem cells.33–36 Furthermore, as dermal fibroblasts from the face originate from the neural crest, whereas fibroblasts from the ventral skin are derived from the lateral plate mesoderm,34,35 and we cannot exclude the possibility that the differences we observed between the 2 populations are linked to the different anatomical origins of the cells analyzed. Although further studies will be needed to decipher the mechanisms at play, this preliminary work suggests that local fibroblast behavior following wounding, and particularly, the response to inflammatory cytokines, may play a significant part in the scarring outcome and thus could be specifically targeted, in combination with the current measures to reduce tension, to minimize scarring.
Footnotes
Presented at the European Society of Ophthalmic Plastic and Reconstructive Surgery meeting in Budapest, September 2014, and at the British Oculoplastic Surgery Society meeting in Belfast, June 2015.
Disclosure: This work was funded by a Collin Award from the British Oculoplastic Surgery Society (Dr. Ezra). Laboratory and imaging facilities were supported by the Wellcome Trust and Fight for Sight. The Palpator was acquired through a research grant from the Medical Research Council (grant G0801049 to Dr. Bailly). Dr. Ezra and Dr. Rose acknowledge financial support from the Department of Health through the award made to the National Institute for Health Research (NIHR) Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. The Article Processing Charge was paid for by the NIHR Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology.
REFERENCES
- 1.Baker R, Urso-Baiarda F, Linge C, et al. Cutaneous scarring: a clinical review. Dermatol Res Pract. 2009;2009:625376. doi: 10.1155/2009/625376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elliot D, Cory-Pearce R, Rees GM. The behaviour of presternal scars in a fair-skinned population. Ann R Coll Surg Engl. 1985;67:238–240. [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HS, Kim SE, Evans GR, et al. The usability of the upper eyelid crease approach for correction of medial orbital wall blowout fracture. Plast Reconstr Surg. 2012;130:898–905. doi: 10.1097/PRS.0b013e318262f3d9. [DOI] [PubMed] [Google Scholar]
- 4.DaCosta J, Oworu O, Jones CA. Laissez-faire: how far can you go? Orbit. 2009;28:12–15. doi: 10.1080/01676830802417510. [DOI] [PubMed] [Google Scholar]
- 5.Brissett AE, Sherris DA. Scar contractures, hypertrophic scars, and keloids. Facial Plast Surg. 2001;17:263–272. doi: 10.1055/s-2001-18827. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa R, Akaishi S, Huang C, et al. Clinical applications of basic research that shows reducing skin tension could prevent and treat abnormal scarring: the importance of fascial/subcutaneous tensile reduction sutures and flap surgery for keloid and hypertrophic scar reconstruction. J Nippon Med Sch. 2011;78:68–76. doi: 10.1272/jnms.78.68. [DOI] [PubMed] [Google Scholar]
- 7.Evans ND, Oreffo RO, Healy E, et al. Epithelial mechanobiology, skin wound healing, and the stem cell niche. J Mech Behav Biomed Mater. 2013;28:397–409. doi: 10.1016/j.jmbbm.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Flynn C, Taberner A, Nielsen P. Mechanical characterisation of in vivo human skin using a 3D force-sensitive micro-robot and finite element analysis. Biomech Model Mechanobiol. 2011;10:27–38. doi: 10.1007/s10237-010-0216-8. [DOI] [PubMed] [Google Scholar]
- 9.Jacquet E, Josse G, Khatyr F, et al. A new experimental method for measuring skin’s natural tension. Skin Res Technol. 2008;14:1–7. doi: 10.1111/j.1600-0846.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 10.Edwards C, Marks R. Evaluation of biomechanical properties of human skin. Clin Dermatol. 1995;13:375–380. doi: 10.1016/0738-081x(95)00078-t. [DOI] [PubMed] [Google Scholar]
- 11.Ezra DG, Beaconsfield M, Collin R. Surgical anatomy of the upper eyelid: old controversies, new concepts. Exp Rev Ophthalmol. 2009;1:47–57. [Google Scholar]
- 12.Gurtner GC, Dauskardt RH, Wong VW, et al. Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann Surg. 2011;254:217–225. doi: 10.1097/SLA.0b013e318220b159. [DOI] [PubMed] [Google Scholar]
- 13.Bran GM, Goessler UR, Hormann K, et al. Keloids: current concepts of pathogenesis (review). Int J Mol Med. 2009;24:283–293. doi: 10.3892/ijmm_00000231. [DOI] [PubMed] [Google Scholar]
- 14.Ashcroft KJ, Syed F, Bayat A. Site-specific keloid fibroblasts alter the behaviour of normal skin and normal scar fibroblasts through paracrine signalling. PLoS One. 2013;8:e75600. doi: 10.1371/journal.pone.0075600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarrazy V, Billet F, Micallef L, et al. Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair Regen. 2011;19(Suppl 1):s10–s15. doi: 10.1111/j.1524-475X.2011.00708.x. [DOI] [PubMed] [Google Scholar]
- 16.Filer A, Parsonage G, Smith E, et al. Differential survival of leukocyte subsets mediated by synovial, bone marrow, and skin fibroblasts: site-specific versus activation- dependent survival of T cells and neutrophils. Arthritis Rheum. 2006;54:2096–2108. doi: 10.1002/art.21930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang HY, Chi JT, Dudoit S, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rinn JL, Bondre C, Gladstone HB, et al. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006;2:e119. doi: 10.1371/journal.pgen.0020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tovell VE, Dahlmann-Noor AH, Khaw PT, et al. Advancing the treatment of conjunctival scarring: a novel ex vivo model. Arch Ophthalmol. 2011;129:619–627. doi: 10.1001/archophthalmol.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Fitchett C, Kozdon K, et al. Independent adipogenic and contractile properties of fibroblasts in Graves’ orbitopathy: an in vitro model for the evaluation of treatments. PLoS One. 2014;9:e95586. doi: 10.1371/journal.pone.0095586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ezra DG, Ellis JS, Beaconsfield M, et al. Changes in fibroblast mechanostat set point and mechanosensitivity: an adaptive response to mechanical stress in floppy eyelid syndrome. Invest Ophthalmol Vis Sci. 2010;51:3853–3863. doi: 10.1167/iovs.09-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baryza MJ, Baryza GA. The Vancouver scar scale: an administration tool and its interrater reliability. J Burn Care Rehabil. 1995;16:535–538. doi: 10.1097/00004630-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 23.González-López JJ, González-García FJ, Sales-Sanz M, et al. Long-term cicatrization analysis in periocular incisions for oculoplastic surgery performed with cold blade and Colorado needle. Ophthal Plast Reconstr Surg. 2014;30:225–228. doi: 10.1097/IOP.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 24.Jenwitheesuk K, Surakunprapha P, Jenwitheesuk K, et al. Role of silicone derivative plus onion extract gel in presternal hypertrophic scar protection: a prospective randomized, double blinded, controlled trial. Int Wound J. 2012;9:397–402. doi: 10.1111/j.1742-481X.2011.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marquez JP, Legant W, Lam V, et al. High-throughput measurements of hydrogel tissue construct mechanics. Tissue Eng Part C Methods. 2009;15:181–190. doi: 10.1089/ten.tec.2008.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahlmann-Noor AH, Martin-Martin B, Eastwood M, et al. Dynamic protrusive cell behaviour generates force and drives early matrix contraction by fibroblasts. Exp Cell Res. 2007;313:4158–4169. doi: 10.1016/j.yexcr.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin-Martin B, Tovell V, Dahlmann-Noor AH, et al. The effect of MMP inhibitor GM6001 on early fibroblast-mediated collagen matrix contraction is correlated to a decrease in cell protrusive activity. Eur J Cell Biol. 2011;90:26–36. doi: 10.1016/j.ejcb.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tovell VE, Chau CY, Khaw PT, et al. Rac1 inhibition prevents tissue contraction and MMP mediated matrix remodeling in the conjunctiva. Invest Ophthalmol Vis Sci. 2012;53:4682–4691. doi: 10.1167/iovs.11-8577. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Ezra DG, Burton MJ, et al. Doxycycline prevents matrix remodeling and contraction by trichiasis-derived conjunctival fibroblasts. Invest Ophthalmol Vis Sci. 2013;54:4675–4682. doi: 10.1167/iovs.13-11787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ezra DG, Ellis JS, Gaughan C, et al. Changes in tarsal plate fibrillar collagens and elastic fibre phenotype in floppy eyelid syndrome. Clin Experiment Ophthalmol. 2011;39:564–571. doi: 10.1111/j.1442-9071.2011.02506.x. [DOI] [PubMed] [Google Scholar]
- 31.Wilson AM. Use of botulinum toxin type A to prevent widening of facial scars. Plast Reconstr Surg. 2006;117:1758–1766; discussion 1767. doi: 10.1097/01.prs.0000209944.45949.d1. [DOI] [PubMed] [Google Scholar]
- 32.Wilson AM. Widening of scars: foe coaxed into a friend? The Millard technique revisited. Plast Reconstr Surg. 2000;106:1488–1493. doi: 10.1097/00006534-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. J Cell Sci. 2004;117:667–675. doi: 10.1242/jcs.01005. [DOI] [PubMed] [Google Scholar]
- 34.Wong CE, Paratore C, Dours-Zimmermann MT, et al. Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J Cell Biol. 2006;175:1005–1015. doi: 10.1083/jcb.200606062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Driskell RR, Watt FM. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 2015;25:92–99. doi: 10.1016/j.tcb.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Blasi A, Martino C, Balducci L, et al. Dermal fibroblasts display similar phenotypic and differentiation capacity to fat-derived mesenchymal stem cells, but differ in anti-inflammatory and angiogenic potential. Vasc Cell. 2011;3:5. doi: 10.1186/2045-824X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


