Abstract
Background:
Acellular dermal matrices (ADMs) are often used to improve lower-pole contour, as well as allow for single-stage reconstruction, but numerous studies have shown an increased complication rate using ADM. As such, our group has developed a minimal-ADM-use technique to lower complications while effectively recreating lower-pole contour.
Methods:
A total of 380 postmastectomy prosthesis-based breast reconstructions were performed in 265 patients by a single surgeon. One hundred eight reconstructions were performed using the traditional ADM technique, with a large piece of ADM along the entire inferior and lateral borders. Two hundred twenty-five reconstructions were performed with the minimal-use technique, patching only the lateral area of the reconstruction. Thirty-five reconstructions were performed without the use of any ADM for high-risk reconstructions, most often in morbidly obese patients.
Results:
Comparing the traditional technique with the minimal-use technique, the seroma rate dropped from 3% to 0%. The rate of infection and reconstruction loss fell from 9% to 1%. Upon greatly reducing or eliminating the use of ADM use in obese patients, the seroma rate decreased from 15.4% to 5.7%, and the reconstruction loss rate decreased from 38% to 9%.
Conclusions:
This article describes a new surgical approach to minimize the amount of ADM necessary to create an aesthetically pleasing breast reconstruction. We believe that this approach helps avoid the complications of seroma, infection, and loss of the reconstruction. In certain obese patients, total avoidance of ADM may be the better choice.
Eighty percent of mastectomy reconstruction is implant based, using tissue expansion as the initial method to reestablish the skin envelope. An addition to this treatment method was the use of acellular dermal matrix (ADM) derived from human cadavers (eg, AlloDerm; Life-Cell Corp., Branchburg, N.J.) for inferolateral pole coverage. AlloDerm is still widely used, although a number of other ADMs have been created, as well. ADM helps avoid elevating additional muscle or fascia, decreasing donor-site morbidity. It is also felt to improve lower-pole contour, as well as allow for single-stage reconstruction with a permanent prosthesis.1–6
These benefits are not without cost. Numerous studies have shown an increased complication rate using ADM.7–10 Before incorporation into tissues, it is a true prosthetic and a liability in regard to avoiding complications. The traditional technique of ADM placement also requires a lot of tailor tacking in an effort to reproduce a 3-dimensional structure with this 2-dimensional material. This can be difficult to teach and learn and may introduce degrees of asymmetry between the 2 sides in inexperienced hands. In addition, often times, more than 1 piece of the standard 8 × 16 cm ADM is needed per side of reconstruction, at almost $4000 a piece, further adding a significant cost to the reconstructive procedure.
Our group has recently demonstrated that that minimizing the surface area of ADM used correlated with a reduction in seroma, infection, and overall complication rates (Liu AS, Kutz RH, and Guo L, 2012, unpublished article). As such, we developed a minimal-ADM-use technique in prosthesis-based breast reconstruction that can be applied to a majority of our patients. This technique can be used with all types ADMs, as well as the newer nonbiologic mesh scaffolds, as it is based on the same surgical principles. It allows us to harness the benefits of these matrices, achieving good inferior pole contour while minimizing the associated complications. Also its simplicity improves the ease of instruction, standardization, and reproducibility at a large teaching institution. Here, we present our surgical technique for implant-based reconstruction using a minimal amount of ADM.
PATIENTS AND METHODS
After obtaining approval from our institutional review board, we performed a retrospective review of all minimal-use-ADM-assisted postmastectomy prosthesis-based immediate reconstructions performed by the senior author at the Brigham and Women’s Hospital between July 2006 and October 2011. This included both single-stage and tissue expander-based reconstructions. Aseptic AlloDerm was the main source of ADM used in these reconstructions.
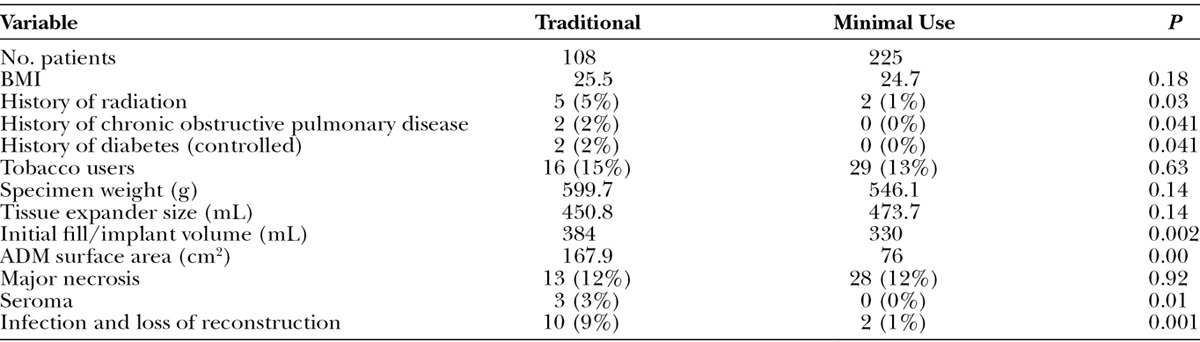
A total of 380 postmastectomy prosthesis-based breast reconstructions were performed in 265 patients. Twelve reconstructions were removed from the study, as they were delayed reconstructions or the patient refused the possibility of ADM use (2 cases). One hundred eight reconstructions were performed using the traditional ADM sling technique (Fig. 1A), with a large piece of ADM along the entire inferior and lateral borders of the reconstruction (group 1). From 2009 and on, 225 reconstructions were performed with the minimal-use technique of ADM (Fig. 1B), wherever feasible, patching only the lateral area of the reconstruction (group 2). Also during this time, 35 reconstructions were performed without the use of any ADM, as these were considered high-risk reconstructions. Patients in this group were most often morbidly obese with excessive lateral chest wall tissue redundancy or had particular comorbidities that would impair wound healing or ADM incorporation. Of note, 28 of the reconstructions in group 1 were performed after 2008. This occurred either because the patient had an unusually high origination of the pectoralis muscle with respect to the inframammary fold or because the muscle and its overlying soft tissue at the level of the inframammary fold sustained excess skeletonization during the mastectomy. These groups were well-matched in terms of chemotherapy, radiation therapy, and smoking status (Table 1).
Fig. 1.
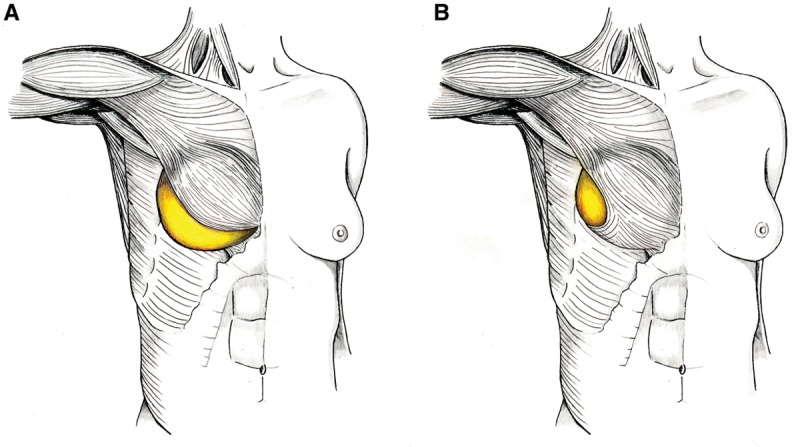
A, Traditional method of ADM placement: A large sling of ADM is inset between the released lateral and inferior borders of the pectoralis major and the chest wall. B, Minimal-use technique of ADM placement: A small patch of ADM is inset between only the lateral border of the pectoralis major and the chest wall. The pectoralis is more widely undermined, but not released, to accommodate the prosthesis.
Table 1.
Traditional vs Minimal-use ADM Technique
A separate analysis was also performed examining outcomes of obese patients (body mass index [BMI] > 30) before (13 patients) and after (53 patients) the institution of the new approach. Since the beginning of 2009, most obese patients with excess lateral tissue redundancy were reconstructed without using any ADM (the 35 previously discussed reconstructions). In the 18 obese patients in whom ADM was used, it was only minimally employed, most often because the patient had an unusually high pectoralis origin, relative to the inframammary fold (IMF).
Patients’ medical records were reviewed for complications that occurred in the first 3-month period following mastectomy and initial stage of breast reconstruction. Only reconstructions with at least 3 months of follow-up were included in the study. Patient outcomes after exchange for a permanent prosthesis were not studied. The complications that were studied include major mastectomy flap necroses (defined as those requiring excision and reclosure), seromas that required aspiration, and infections significant enough to lead to prosthesis removal.
Demographic information was collected regarding perioperative chemotherapy and radiation therapy, patient’s BMI, mastectomy specimen weight, volume of initial intraoperative fill, ADM sheet surface area (calculated by multiplication of the recorded length and width used), smoking, and medical comorbidities. Initial intraoperative fill, or initial fill, was defined as the final volume instilled into the prosthesis at the end of the initial reconstruction procedure immediately following mastectomy. In most cases, the prosthesis at the initial reconstruction was a tissue expander, but in 56 cases (26 in group 1 and 30 in group 2—percentage not statistically significant), an implant was placed in a 1-stage reconstruction immediately following mastectomy. In these cases, the initial intraoperative fill is the same as the final implant size if silicone implants were used or the final fill volume if saline implants were used. A smoker was defined as someone who had smoked in the 6 months leading up to surgery or in the 3-month period following surgery. Medical comorbidities of interest consist of diabetes mellitus and chronic obstructive pulmonary disease—that is, conditions that may predispose patient to infections or impair oxygen delivery to the tissue. The statistical analysis was performed using Microsoft Excel with chi-square and two-tailed t tests used.
Surgical Approach
For those patients, mostly morbidly obese, especially those with much lateral tissue redundancy, we opted to forego the use of ADM and use serratus and pectoralis minor muscle/fascia instead to form the lateral border of the breast pocket. For the majority of our more normal weighted patients, once the mastectomy is complete, the viability of the skin, the overall muscle integrity, and, in particular, the integrity of the pectoralis major at the inferior and medial origin (as this must remain intact) are inspected. From the lateral border of the pectoralis, a wide subpectoral plane is created, first superiorly. Aggressive medial elevation of the muscle is then begun, stopping just after exposing the fat medial to the muscle while maintaining the muscle attachment to the overlying soft tissue. A similar approach is taken inferiorly, elevating the pectoralis to a level just below the IMF as marked on the skin. The overall attachment of the pectoralis remains intact, enabled by the overlying soft tissue and investing fascia. The lateral opening underneath the pectoralis begins at the IMF and curves laterally and superiorly toward the axilla. A curvilinear line lateral to the pectoralis is marked on the chest wall, mimicking a natural lateral breast contour. Along with the imaginary stretched out pectoralis major by a tissue expander, this amounts to a teardrop shaped lateral opening to the eventual breast pocket with the apex pointing to the axilla.
The length and width of ADM needed (11 × 6 cm on average) is marked. One piece of rectangular 8 × 16 cm thick ADM is often sufficient for any bilateral reconstruction. The sheet is bisected diagonally, and each piece is cut into a “teardrop” shape (Fig. 2A). The inferolateral margin of the ADM (along the previous marking) is sewn in with 2-0 Vicryl. A 19F round drain is placed is set along the inferior and lateral aspect of the pocket, exiting from the axilla.
Fig. 2.
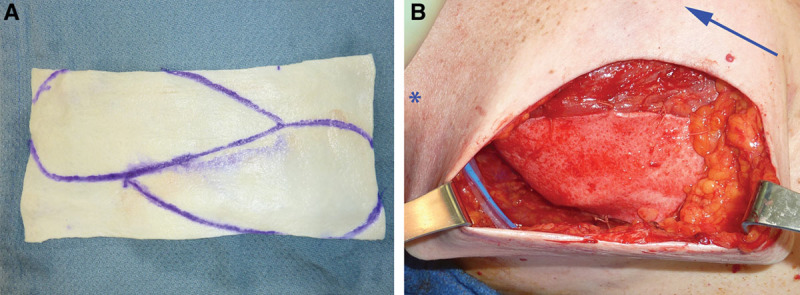
A, 8 × 16 cm sheet of thick AlloDerm, marked for use. B, Intraoperative lateral view of the minimal-use technique with the ADM inset and the tissue expander filled (asterisk designates axilla and arrow, situated medially, points to the head of the patient).
The pocket is irrigated with bacitracin, and the empty tissue expander (Natrelle; Allergan, Irvine, Calif.) is placed in the subpectoral pocket, as inferior and medial as possible. A permanent prosthesis may also be used. The anterior edge of the ADM is sutured to the lateral pectoralis border, and the tissue expander is filled until either the ADM is taut (Fig. 2B) or the breast skin envelope can be closed with zero tension. The breast skin edge is also trimmed as needed and closed with 3-0 and 4-0 Monocryl and dressed with Dermabond. Perioperative intravenous antibiotics are given, and these were continued postoperatively in their oral formulation for 2–3 days after the closed suction drains were removed (normally within 2 weeks postoperatively).
RESULTS
Traditional Technique vs Minimal-use Technique
In group 1, the average BMI was 25.5 vs 24.7 in group 2 with mastectomy weights being 600 vs 545 g, respectively. This was not significantly different. The tissue expander volume was 450.8 in group 1 and 473.7 in group 2. The initial fill volume did drop significantly from 384 to 330 mL (P = 0.002). Also, the amount of ADM used decreased from 170 to 75 cm2 per reconstruction (P < 0.05). Comparing group 1 and group 2, the seroma rate dropped significantly from 3% to 0% (P = 0.01) and the incidence of major skin necrosis remained stable at 12.4%, from 12.0% previously. Despite this, the rate of major infection and tissue expander removal fell from 9% to 1% (P < 0.05). It should be noted that of the 10 patients in group 1 who lost their reconstruction, only 3 of those patients had received radiation therapy (not statistically significant). Of note, 1 patient in group 2 whose tissue expander was removed was due to a rare complication of pyoderma gangrenosum over the chest postoperatively (Table 1).
Restriction of ADM Use in Obese Patients
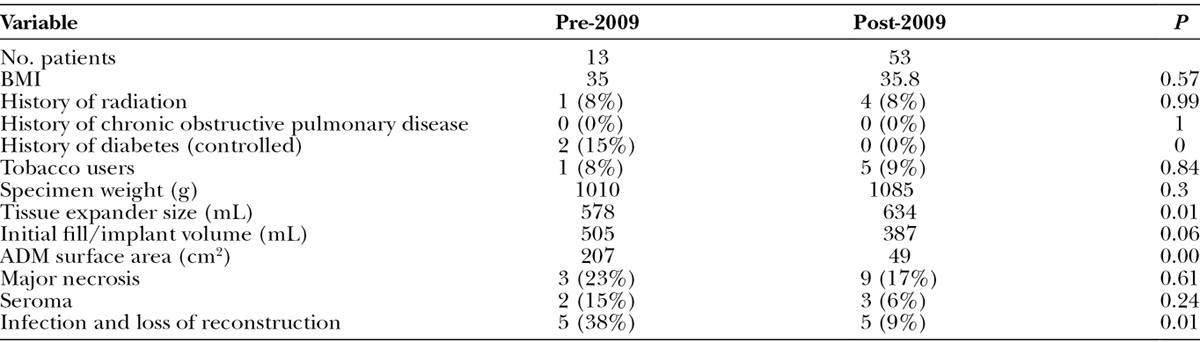
This analysis also examined the outcomes of obese patients (BMI > 30) after systematically reducing the amount of ADM used in breast reconstructions, and moreover, eliminating ADM in patients whose resultant mastectomy pockets would be excessive with large amounts of dead space laterally (Table 2). The BMI of the 2 cohorts of patients before and after instituting this practice pattern (January 2009) remained stable at 34.97 and 35.82, respectively. Average mastectomy weights were 1010 and 1085 g, respectively. Tissue expander sizes did increase significantly, and initial fill volume showed a trend toward decreased volumes [578 vs 634 mL (P = 0.01), 505 vs 387 mL (P = 0.06)]. The incidence of tobacco use in these 2 cohorts was high (38% pre-2009 and 54% since-2009). All complication measures decreased with institution of this practice pattern. Seroma rate decreased from 15.4% (2 of 13 patients) to 5.7% (3 of 53 patients), major necrosis rates decreased from 23.1% to 16.9% (P = 0.61), and infections leading to expander removal decreased from 38% to 9% (P < 0.01). As expected, surface area of ADM use decreased significantly from an average of 206 to 49.5 cm2 per reconstruction (P < 0.05).
Table 2.
Restriction of ADM Use in Obese Patients
DISCUSSION
No prospective randomized controlled trials are currently available that definitively show whether the use of ADM increases complication rates in implant-based postmastectomy reconstructions. Some retrospective studies demonstrate no statistically significant difference with the addition of ADM in breast reconstructions in terms of infection and seroma formation.4,11,12 Others have found that infection, seroma, mastectomy flap necrosis rates, and overall complication rates were significantly higher in breast reconstructions using ADM.7–10 Many of these studies had relatively smaller sample sizes, and even though a trend toward worse rates of infection was seen, they did not become statistically significant, likely due to the sample size. Further, many of these studies referenced here were earlier in the field’s experience. As such, the overall higher rates of complications may have masked the true effect that ADM may have on outcomes.
Success of the reconstruction is dependent on incorporation of the ADM into the surrounding tissue.9,10 ADM is a human collagen scaffold that is gradually penetrated by host tissue ingrowth and eventually replaced with host cells.5,6,13–16 As such, it is reasonable to think that at the time of placement and in the months prior to incorporation, ADM should be considered a foreign body and, like any foreign body implanted in patients, is a surgical liability. A smaller piece of ADM, more likely congruent with the overlying vascularized tissues, should become incorporated easier than a larger piece of ADM and thus should become resistant to infection earlier. More recently, though AlloDerm is still widely used, other dermal matrices and synthetic meshes have been introduced. These products still suffer the same general liability, trading an additional foreign body in the surgical site in lieu of the vascularized coverage of the pectoralis.
We looked for a surgical method to help decrease the liability of ADM, maximizing vascularized tissue coverage while not compromising aesthetic results (Fig. 3). Serratus fascia elevation can achieve the goal of inferolateral coverage, but it does not allow for the same degree of intraoperative fill. Further, in the process of mastectomy, the serratus fascia is often damaged, thus precluding its use. A large amount of ADM is not necessary to rapidly achieve lower-pole fullness.17 The approach described in this article is possible if the pectoralis major muscle is left intact by the mastectomy surgeon, especially with preservation of the overlying soft tissue around the periphery. This allows aggressive elevation of the pectoral muscle off the chest wall, without totally detaching inferiorly and medially as in the traditional approach to create a complete inferomedial sling to hold the implant in a natural position with a natural-appearing inframammary fold. By doing so, the amount of ADM needed to complete support for the prosthesis decreases significantly, mostly limited to the lateral aspect. This has a number of ramifications. First of all, the tissue under the incision is vascularized muscle as opposed to ADM, which is frequently encountered with the traditional placement in prosthesis-based reconstruction. In this study, we did not see a decrease in mastectomy flap necrosis, and yet, the rate of tissue expander loss did decrease significantly. We believe that this is due to the presence of good muscle coverage under the incision. This also has the advantage of good muscle coverage under the incision at the time of implant exchange and nipple creation.
Fig. 3.
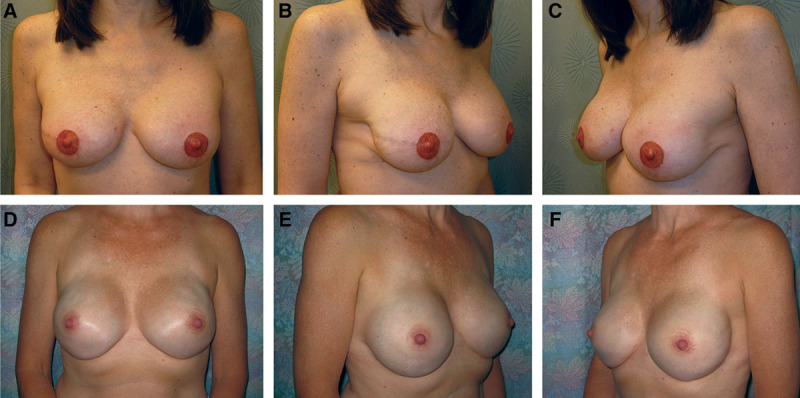
A–C, Postoperative appearance of a skin-sparing mastectomy patient reconstructed with the minimal-use technique and tissue expanders, followed by implant exchange, nipple reconstruction, and tattooing. D–F, Postoperative appearance of a nipple-sparing mastectomy patient reconstructed with the minimal-use technique and immediate permanent prosthesis placement. This illustrates the ability to rapidly achieve lower-pole fullness.
A second benefit of limiting the amount of ADM is that the rate of seromas decreased significantly, as well. This is intuitive as the surface area of the “seroma-genic” ADM decreases so does the incidence of postoperative seroma.7 Minimizing postoperative fluid collection allows placement of fewer drains and earlier removal of those drains, which, along with decreased need for seroma aspiration, contribute to lower prosthesis infection and loss. As such, the incidence of infection and prosthesis loss also decreased significantly with our new approach.
Our surgical technique decreased the amount of ADM from 170 to 75 cm2 per reconstruction. The cost benefit of this is significant. One standard 8 × 16 cm sheet of ADM is sufficient for a complete bilateral reconstruction.
Our study also examined the effect of limiting or eliminating ADM use in obese patients. We found this to be an important change in our practice. Seroma and infection rates all decreased significantly. Further, tissue expander removal and reconstruction loss, which is discouraging for the surgeon and catastrophic for the patient, decreased significantly. We believe this is due to the recognition that, for ADM to become incorporated, it must be in contact with vascularized tissues. The mastectomy pocket of obese patients is often excessively large. This tissue redundancy leads to poor contact with the ADM, and if a large surface area of ADM is within the pocket, large areas can remain unincorporated. Limiting the amount of ADM limits this liability. Ironically, many would argue that a full ADM sling would be particularly beneficial in obese patients as those patients often need lower-pole support more than relatively normal weighted patients. Although this may be true to some extent, we believe that the benefits of significantly decreasing seroma, infection, and prosthesis loss far outweigh the marginally increased cosmetic outcome in, particularly, the obese patient population. No gross difference in cosmetic outcome was evident with the omission of ADM.
One of the drawbacks of this technique is its dependency on the ablative breast surgeons. As mentioned, preservation of the investing fascia and soft tissue, particularly around the inframammary fold, is essential to the feasibility of our surgical approach. This has allowed us to detach the pectoralis major muscle without fully detaching its soft-tissue attachment to the beast envelope, therefore, still enabling full expansion of the lower pole. Not infrequently, however, mastectomy surgeons can be overly aggressive in skeletonizing the overlying tissue and destroying the inframammary fold, rendering our technique not applicable. We have been fortunate to have most of our mastectomy surgeons observing the anatomical landmarks of the breasts during mastectomy. But this luxury can obviously lack with other mastectomy surgeons. Therefore, the technique described here may not be easily used in those situations.
CONCLUSIONS
This article describes a new surgical approach to minimize the amount of ADM necessary to create an aesthetically pleasing breast reconstruction. Although it is widely speculated that ADM usage has a steep learning curve, we believe that this simple and consistent approach to its use has constituted a large part of our experience in decreasing its dreaded postoperative complications, such as seroma, infection, and loss of the reconstruction. And in the cases of certain obese patients, total avoidance of ADM may end up serving them better in the end.
ACKNOWLEDGMENTS
The authors recognize Anaeze Offodile and Ryan Cauley for their generous assistance in creating the figure drawings.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by Dr. Guo.
REFERENCES
- 1.Namnoum JD. Expander/implant reconstruction with AlloDerm: recent experience. Plast Reconstr Surg. 2009;124:387–394. doi: 10.1097/PRS.0b013e3181aee95b. [DOI] [PubMed] [Google Scholar]
- 2.Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg. 2005;55:232–239. doi: 10.1097/01.sap.0000168527.52472.3c. [DOI] [PubMed] [Google Scholar]
- 3.Spear SL, Parikh PM, Reisin E, et al. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg. 2008;32:418–425. doi: 10.1007/s00266-008-9128-8. [DOI] [PubMed] [Google Scholar]
- 4.Sbitany H, Sandeen SN, Amalfi AN, et al. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg. 2009;124:1735–1740. doi: 10.1097/PRS.0b013e3181bf803d. [DOI] [PubMed] [Google Scholar]
- 5.Zienowicz RJ, Karacaoglu E. Implant-based breast reconstruction with allograft. Plast Reconstr Surg. 2007;120:373–381. doi: 10.1097/01.prs.0000267340.31742.1. [DOI] [PubMed] [Google Scholar]
- 6.Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg. 2006;57:1–5. doi: 10.1097/01.sap.0000214873.13102.9f. [DOI] [PubMed] [Google Scholar]
- 7.Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg. 2010;125:429–436. doi: 10.1097/PRS.0b013e3181c82d90. [DOI] [PubMed] [Google Scholar]
- 8.Antony AK, McCarthy CM, Cordeiro PG, et al. Acellular human dermis implantation in 153 immediate two-stage tissue expander breast reconstructions: determining the incidence and significant predictors of complications. Plast Reconstr Surg. 2010;125:1606–1614. doi: 10.1097/PRS.0b013e3181d4fb2a. [DOI] [PubMed] [Google Scholar]
- 9.Lanier ST, Wang ED, Chen JJ, et al. The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg. 2010;64:674–678. doi: 10.1097/SAP.0b013e3181dba892. [DOI] [PubMed] [Google Scholar]
- 10.Liu AS, Kao HK, Reish RG, et al. Postoperative complications in prosthesis-based breast reconstruction using acellular dermal matrix. Plast Reconstr Surg. 2011;127:1755–1762. doi: 10.1097/PRS.0b013e31820cf233. [DOI] [PubMed] [Google Scholar]
- 11.Preminger BA, McCarthy CM, Hu QY, et al. The influence of AlloDerm on expander dynamics and complications in the setting of immediate tissue expander/implant reconstruction: a matched-cohort study. Ann Plast Surg. 2008;60:510–513. doi: 10.1097/SAP.0b013e31816f2836. [DOI] [PubMed] [Google Scholar]
- 12.Nahabedian MY. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg. 2009;124:1743–1753. doi: 10.1097/PRS.0b013e3181bf8087. [DOI] [PubMed] [Google Scholar]
- 13.Becker S, Saint-Cyr M, Wong C, et al. AlloDerm versus DermaMatrix in immediate expander-based breast reconstruction: a preliminary comparison of complication profiles and material compliance. Plast Reconstr Surg. 2009;123:1–6; discussion 107. doi: 10.1097/PRS.0b013e3181904bff. [DOI] [PubMed] [Google Scholar]
- 14.Gamboa-Bobadilla GM. Implant breast reconstruction using acellular dermal matrix. Ann Plast Surg. 2006;56:22–25. doi: 10.1097/01.sap.0000185460.31188.c1. [DOI] [PubMed] [Google Scholar]
- 15.Bindingnavele V, Gaon M, Ota KS, et al. Use of acellular cadaveric dermis and tissue expansion in postmastectomy breast reconstruction. J Plast Reconstr Aesthet Surg. 2007;60:1214–1218. doi: 10.1016/j.bjps.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Newman MI, Swartz KA, Samson MC, et al. The true incidence of near-term postoperative complications in prosthetic breast reconstruction utilizing human acellular dermal matrices: a meta-analysis. Aesthetic Plast Surg. 2011;35:100–106. doi: 10.1007/s00266-010-9631-6. [DOI] [PubMed] [Google Scholar]
- 17.Chepla KJ, Dagget JR, Soltanian HT. The partial AlloDerm sling: reducing allograft costs associated with breast reconstruction. J Plast Reconstr Aesthet Surg. 2012;65:924–930. doi: 10.1016/j.bjps.2012.02.016. [DOI] [PubMed] [Google Scholar]


