Abstract
Background:
Prosthetic breast reconstruction is generally considered contraindicated after previous breast irradiation. As a result, patients undergoing a salvage mastectomy for recurrent breast cancer or “risk-reducing” mastectomies after previous conservative surgery and radiotherapy (CS + RT) are usually offered autologous breast reconstruction. However, not all such patients are suitable candidates for a major flap reconstruction. The purpose of this study is to review our results of immediate 2-stage prosthetic breast reconstruction after CS + RT.
Methods:
A retrospective review was undertaken for 671 consecutive patients with prosthetic-only breast reconstruction performed by a single surgeon over a 12.5-year period. Twenty-two patients who qualified for the criteria were audited. Outcomes examined include complications, loss of tissue expander or implant, revisional surgery, and aesthetic result.
Results:
Twenty-two patients underwent 33 mastectomies and immediate 2-stage breast reconstructions after previous CS + RT (15 for recurrent cancer and seven “risk-reduction”) and 11 contralateral risk-reducing mastectomies. One patient died due to extensive metastatic disease. There was no reconstruction failure. The average breast implant size was 491.7 g (range 220 -685g). Seroma was the most common complication and occurred in 3 of 22 patients (13.6%) after stage 1 and 3 of 21 patients (14.3%) after stage 2 reconstruction. The revisional surgery rate was 28.6%. Aesthetic result was rated as excellent in 9.5%, good in 76.2%, and fair in 14.3%.
Conclusions:
For selected patients, immediate 2-stage prosthetic breast reconstruction can be performed successfully after a salvage mastectomy subsequent to a recurrence after CS + RT.
Prosthetic-only breast reconstructions are generally considered contraindicated after previous breast irradiation.1,2 For example, patients who have been diagnosed to have recurrent breast cancer after previous conservative surgery and radiotherapy (CS + RT) requiring a salvage mastectomy are usually treated without a reconstruction or offered autologous breast reconstruction. Patients who develop an isolated in-breast recurrence are also at an added risk of future metastatic disease, and hence, complex surgery such as flap reconstructions may not be appropriate for this group of patients.3,4
Some patients previously treated with CS + RT may elect to undergo a “risk-reducing” mastectomy without recurrence, particularly when other family members are subsequently diagnosed with breast cancer or a breast cancer gene mutation is discovered. However, not all patients are eligible or willing to sacrificed another part of their body as a donor area because of previous abdominal surgery or cost and they may forgo the documented benefits of breast construction.5,6 Although the utilization of 2-stage prosthetic immediate breast reconstruction is on the increase,7 there is very little literature on prosthetic breast reconstruction in this group of patients. The purpose of this study is to review our results of immediate 2-stage prosthetic breast reconstruction after mastectomy subsequent to previous CS + RT.
PATIENTS AND METHODS
A retrospective review was undertaken of all breast reconstructions performed by a single surgeon (T.L.) between June 1998 and December 2010. A total of 671 patients who underwent all forms of prosthetic-only breast reconstruction were identified and their files audited. Twenty-two of the 671 patients (3.3%) fulfilled the criteria of having had immediate 2-stage prosthetic breast reconstruction after mastectomy for an in-breast recurrence of cancer (n = 15) or risk-reducing mastectomy subsequent to prior CS + RT (n = 7); they form the basis of this report. Of the 15 patients who underwent mastectomy for recurrent breast cancer, 4 underwent a contralateral risk-reducing mastectomy; 7 patients had bilateral risk-reducing mastectomies, so a total of 11 patients allowed a comparison of the irradiated and nonirradiated side as their own control group.
All these 22 patients received whole-breast irradiation to a dose of 50 Gy by 6 MV photons over 5–6.5 weeks in 1.8 or 2.0 Gy fractions. Boosts were given at the primary tumor site using electron therapy or photon beam therapy to a dose of 10 Gy in 5 fractions. In general, the boost dose was prescribed to the 80–90% electron isodose line where the target volume depth (measured by breast ultrasound or a planning computed tomography scan) was incorporated within this isodose line.
Factors examined in this study were the rate of complications and in particular reconstruction failure, revision surgery, and aesthetic result. The aesthetic result is assessed using a 4-point scale by the operating surgeon (T.L.) where “excellent” refers to a good aesthetic result that is also symmetrical to the contralateral breast, which may or may not have undergone augmentation, reduction, mastopexy, or mastectomy with reconstruction. A patient is considered to have a “good” result if the reconstruction is good but not symmetric to the contralateral breast. When the reconstruction is considered not good, then it is assessed as “fair,” and a “poor” result usually results from a failure.
The average time from salvage mastectomy and insertion of the expander to stage 2 implant insertion for 21 eligible patients was 13.2 months (range 5–33 months). The average follow-up time from the date of stage 2 breast reconstruction was 39.7 months for 18 patients who were available for follow-up. Three patients were lost to follow-up after their initial postoperative follow-up appointments 2 weeks after surgery because of residence in rural areas, and 1 patient died of metastatic disease. Twenty implants were manufactured by Allergan, Inc. (Irvine, Calif.) and 1 by Mentor Corporation (Santa Barbara, Calif.).
One patient who had RT to the chest area for Hodgkin’s lymphoma at 15 years of age before developing bilateral breast cancer at 42 years of age and undergoing bilateral mastectomies and immediate 2-stage prosthetic breast reconstruction was excluded given the low dose of radiation in this setting.
This study has been approved by the Western Sydney Local Health Network Human Research Ethics Committee.
RESULTS
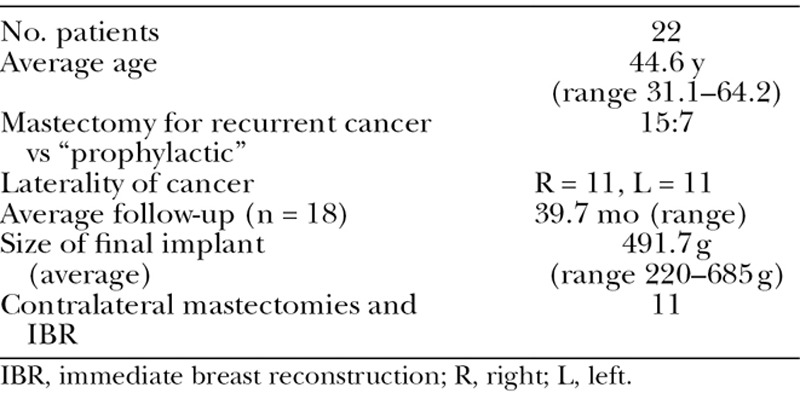
Twenty-two patients underwent 33 immediate 2-stage prosthetic breast reconstructions (22 ipsilateral with prior RT and 11 contralateral with no prior RT). The average age of the patients was 44.6 years (range 31.1–64.2 years; Table 1). Eleven patients (50%) had left-sided breast cancer and the other 11 (50%) had right-sided. Thirteen of 22 patients underwent prior adjuvant chemotherapy (CT), and 3 of them required CT after completion mastectomy and insertion of a tissue expander. Five patients who did not undergo previous adjuvant CT had postmastectomy CT as part of their treatment for their recurrence.
Table 1.
Patients’ Clinical and Demographic Data
There were no reconstruction failures in the 33 immediate 2-stage prosthetic breast reconstructions. However, 1 patient developed progressive local recurrence 6 months after her mastectomy and reconstruction requiring an extensive chest wall resection and split skin graft. Her tissue expander was removed at the same time of the excision, and she died not long after.
Another patient with an in-breast recurrence requested a contralateral augmentation some months after the tissue expander insertion. To safely achieve symmetry on her irradiated side with greater expansion, it was decided to add a latissimus dorsi myocutaneous (LD) flap to the tissue expander before eventual stage 2 reconstruction. The remaining patients went on to stage 2 reconstruction with removal of their tissue expanders and insertion of a silicone gel implant as planned.
Of the 21 patients who completed stage 2 prosthetic breast reconstruction, the average anatomical silicone gel breast implant size was 491.7 g (range 220–685 g).
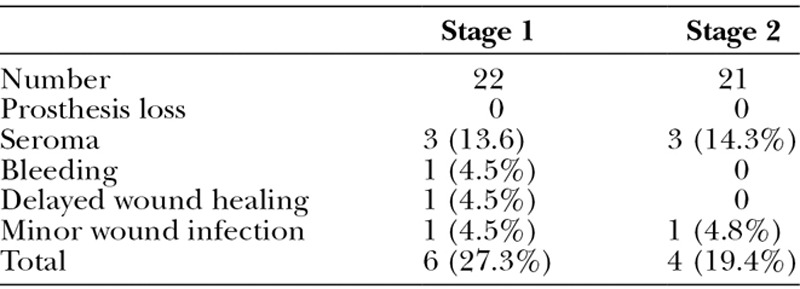
Seroma was the most common complication. After stage 1 insertion of a tissue expander, 3 patients (13.6%) developed a seroma requiring repeated weekly ultrasound-guided aspirations until resolution. One patient (4.5%) developed postoperative bleeding necessitating return to theatre for hemostasis. Another patient developed delayed wound edge healing and underwent debridement and suture of the wound, whereas the third patient had minor wound infection requiring oral antibiotics only. Both these patients recovered well and proceeded to stage 2 reconstruction.
After stage 2 reconstruction when the expander was exchanged for the silicone implant, seroma again was the commonest complication. Three patients out of 21 (14.3%) developed seroma, but they were not the same as the previous 3 who developed seroma after stage 1 reconstruction. Another patient developed a minor wound infection that was treated by oral antibiotics (Table 2).
Table 2.
Complications
In terms of revisional surgery, 1 patient elected to receive an LD flap 6 months after stage 2 reconstruction, which was attributed to general tightness of the irradiated chest wall tissue. Five other patients underwent revisional surgeries: 1 patient at 4 months postsurgery to correct asymmetry after a 685-g Mentor CPG (Mentor Worldwide LLC, Santa Barbara, Calif.) implant reconstruction with a contralateral mastopexy. It was replaced by a smaller Allergan anatomically shaped 455-g implant. Two other patients underwent dermis-fat grafts and fat transfer to contour areas superior to the implants, 1 at 5 months and the other one at 2 years post-operatively. One patient had the implant pocket lowered 6 months after stage 2 for asymmetry. Another patient underwent revision of the implant 4 years later because of weight gain on the contralateral side and a larger implant was inserted. The total rate of revision is thus 28.6%.
Aesthetic results were recorded for 21 patients as excellent in 2 patients (9.5%), 16 good (76.2%), and 3 fair (14.3%).
DISCUSSION
Since the 1980s, CS + RT has become the mainstream treatment for suitable newly diagnosed breast cancer as opposed to a formal mastectomy.8–12 However, when a small percentage of these patients develop local recurrence, they usually go on to a completion mastectomy.4,13–17 The dilemma for these patients and their reconstructive surgeons is that the chest wall (including part or all of the pectoralis muscles) and skin of the breast have been previously irradiated.
Conventional teaching would consider prosthesis-alone breast reconstruction to be contra- indicated because of concerns about wound breakdown, capsular contracture, skin tightness, and fibrosis of the pectoralis muscle restricting stretching after insertion of an expander. As a result, these patients are generally offered autologous forms of breast reconstruction techniques such as pedicle or free transverse rectus abdominis myocutaneous or more recently deep inferior epigastric perforator flaps, or at least a latissimus dorsi myocutaneous flap in addition to an implant.
In their study on the prognosis after breast cancer recurrence after CS + RT and salvage mastectomy, Francis et al4 found that the 5-year rate of freedom from second relapse was 46%, and the overall 5-year survival rate was only 59%. As a result, it is important to investigate these patients with body computed tomography scans, bone scans, or positron emission tomography–computed tomography scans before embarking on major autologous breast reconstructions. However, many patients are very reluctant to undergo more disfiguring surgery with scarring in the abdomen or their backs when they are already concerned about losing their breast after a recurrence, which of course is psychologically devastating. The option of immediate 2-stage prosthetic breast reconstruction would also be appropriate for patients who have had a previous abdominoplasty or multiple abdominal surgeries that would preclude them having a transverse rectus abdominis myocutaneous or deep inferior epigastric perforator flap. Moreover, in view of the relatively poor prognosis, it may be prudent to consider a less onerous reconstructive option, if it has an equivalent or less morbidity risk such as a prosthetic reconstruction. This less complicated approach, with a shorter operation without an autologous donor site, provides the patients an additional choice to have their breast forms reconstructed without compromising another part of their body.
Lam et al18 published a systematic review of immediate 2-stage prosthetic breast reconstruction in 715 patients who underwent adjuvant RT after insertion of a tissue expander or an implant and found an average reconstruction failure rate of 18.6% (range 0–45%). However, these were all performed on primary breast cancer patients who have not had a previous CS + RT. Only 1 of the 12 studies included in the systematic review contained a separate group of 7 patients who underwent previous CS + RT, which were not included in the analysis for the systematic review.19 The authors reported that “4 of the 7 breasts reconstructed after previous radiation and breast conservation ultimately required latissimus flaps, 1 electively and 3 secondarily to correct problems with their initial postmastectomy reconstructions.” It should be pointed out that these patients had a saline-filled implant after tissue expansion, whereas all the patients in this study received silicone gel implants.
Few studies have touched upon this subject, and they, similar to the one quoted above, often included breast reconstruction cases of mixed groups and/or in small numbers.20–23 Overall, they tended to report unfavorable results. More recently, Hirsch et al24 reported in 2012 on the outcomes of 66 immediate tissue expander/implant breast reconstructions (7 different surgeons) for salvage mastectomies after CS + RT. Overall, they had 70% complication rate, and only approximately 60% of their patients successfully completed 2 stages of reconstruction. The largest series of 121 similar patients was reported by Cordeiro et al,25 also in 2012. They reported a 29.8% early complication rate of which 18.2% was of mastectomy flap necrosis and 2.5% seroma rate. Tissue expanders were lost in 1.7% and implant in 2.5% giving a combined prosthesis failure rate of 4.2%. However, only 48 patients were available for longer term follow-up of more than 12 months. They concluded that “carefully selected” patients with salvage mastectomy after previous CS + RT can successfully complete 2-stage immediate prosthetic breast reconstruction, and that the rate of early complications in this group is higher but acceptable.
In this study, a total of 22 patients among 477 immediate 2-staged prosthetic breast reconstructions constituted less than 5% of our population. Apart from the single patient who developed progressive local disease and had an extended excision of the whole chest wall requiring split skin graft reconstruction and removal of her tissue expander, all of the remaining 21 breast reconstructions were successful. The average weight of the final implant of just below 500 g indicated that they were not small reconstructions only, especially with 3 patients reaching the maximum available size of 685 g for a moderate height Allergan implant. Although our numbers are relatively small, we can safely say that our results are comparable with any series of immediate 2-stage prosthetic breast reconstruction after mastectomy in primary breast cancer patients with no previous RT. Many of these series have been reported in the literature showing a 4–40% failure rate, especially after adjuvant RT.18
Our study is similar to Cordeiro et al’s25 in that all cases were performed by 1 surgeon, whereas Hirsch et al24’s included cases from 7 surgeons with an average of less than 10 cases each. The high complication rate in their cohort may reflect the experience of some of their surgeons. The most common early complication in Cordeiro et al’s25 report is that of mastectomy flap necrosis of 18%. This is despite the fact that their “mastectomy flaps were tailored to minimize skin excess.” This was rather different from our findings as seroma was our most common complication, with 13.6% after stage 1 and again 14.3% after stage 2, but there was no skin flap necrosis. We believe this could be because of the difference in the amount of skin flap being spared and our high index of suspicion utilizing ultrasound scan to detect seromas and performing weekly ultrasound-guided drainage if a seroma is found. Otherwise, if a seroma is left untreated, it will add stress to the circulation of a skin flap. In addition, we insert all expanders empty and do not inflate them for 4 weeks to again minimize the stress on the mastectomy flaps. In contrast, Cordeiro et al25 generally inflated the tissue expanders up to 50% of its expected volume and further inflate them after 2 weeks.
Two patients had “minor wound infection” for which a minor infection is defined as infection settled by oral antibiotics only. Our experience is that previously irradiated skin often shows some erythema after surgery, and it may not be caused by infection. However, we cannot afford the risk and patients often end up having an extra course of oral antibiotics.
As stated above, 6 patients underwent further surgery giving a 28.6% revisional rate. We believe that capsular contracture is too subjective to be accurate in a retrospective review, but no patient underwent revisional surgery because of stated capsular contracture, although the patient who elected to receive an additional LD flap attributing to general tightness of the irradiated chest wall tissue may be considered to have had capsular contracture. Three patients underwent revision for asymmetry. One of them had contralateral mastopexy at stage 2 breast reconstruction. Ideally, a contralateral mastopexy is probably best done with stage 1 reconstruction, so that its final size and shape will be available for sizing for the final implant at stage 2. Another patient had the implant pocket lowered 6 months after stage 2 for asymmetry. The third patient underwent revision to a larger implant after 4 years because of weight gain. This is probably the main disadvantage for prosthetic breast reconstructions in that the implant volume remains the same, whereas the contralateral natural breast will continue to undergo progressive ageing and ptosis or weight changes. As a result, bilateral mastectomies and breast implant reconstructions are probably ideal as both implants sizes would remain constant.
The remaining 2 patients had dermis-fat grafts and fat transfer to contour the upper pole, 1 at 5 months and the other at 2 years postoperatively. This is another disadvantage of implants as the full-height implants often result in too much fullness superiorly, but the shorter medium-height or the short-height implants may result in some hollowing of the infraclavicular fossa as most breast surgeons extend their mastectomy to this area. Increasingly, most reconstructive surgeons are advocating fat-transfer or “lipofilling” to any irregularities and even just to “rejuvenate” the mastectomy skin flaps all round.26 In addition to the above 6 patients, 1 elected to augment the contralateral side and as a result had an LD flap added before stage 2 to allow larger reconstruction than the original breast envelope provides.
Surveying a number of reports on immediate 2-stage breast reconstructions, all cosmetic assessments are carried out by the authors or their fellow surgeons. Some use a 2-point assessment of acceptable or unacceptable cosmetic result27 or excellent/good versus fair/poor.28 Others adopt a 3-point assessment of good/fair/poor grading29,30 or very good to excellent/good/poor to fair.31 We, therefore, propose to combine the above into a 4-grade system that covers all and define each grade to make it more comparable:
Excellent—good reconstruction and symmetric to contralateral breast (Fig. 1).
Good—good reconstruction but asymmetric to contralateral breast (Fig. 2).
Fair—fair reconstruction and/or asymmetric breasts.
Poor—reconstruction failure.
Fig. 1.
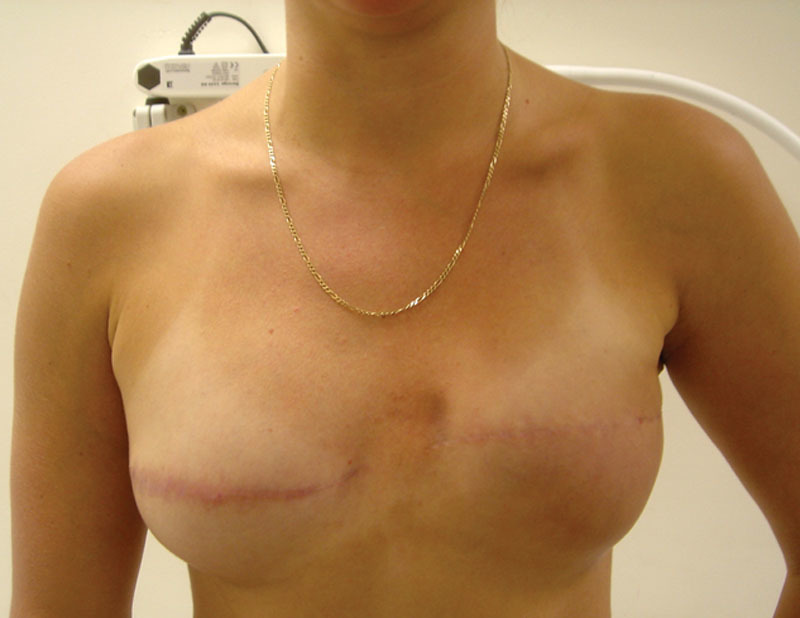
Thirty-year-old woman 7 years after bilateral mastectomies and 2-stage immediate breast reconstruction for recurrent left breast cancer after prior conservative surgery and radiotherapy.
Fig. 2.
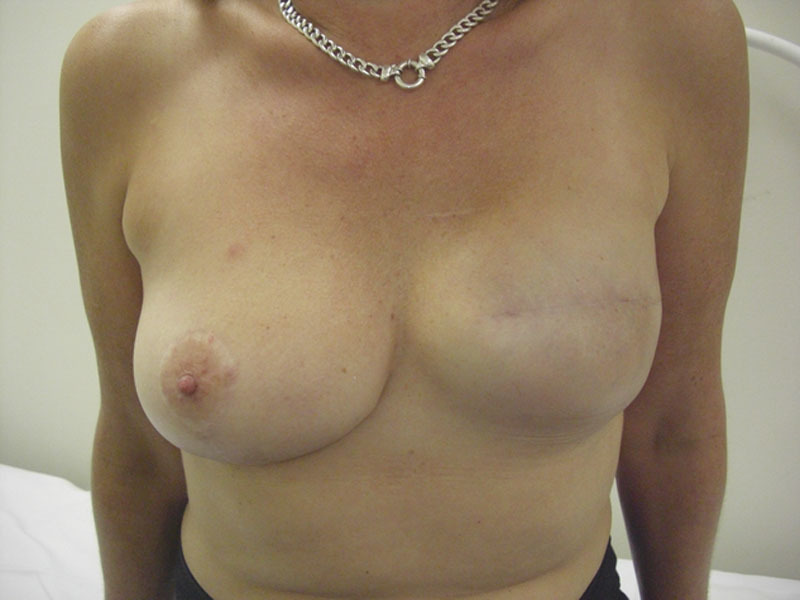
Forty-two-year-old patient 5 years after right mastectomy and immediate breast reconstruction for recurrent breast cancer after prior conservative surgery and radiotherapy.
As indicated above, one would expect all bilateral implant reconstructions to be assessed to be “ excellent.” However, even in nonirradiated patients, often there is asymmetry because of anatomical variations or surgical techniques. For previously irradiated skin, we find that the soft tissue quality is different between the breast cancer side and the risk-reducing side. Apart from the obvious difference because of irradiation, we believe that the oncological surgeons tend not to be as aggressive in excising soft tissue on the risk-reducing side. As a result, when same-size implants are used, the previously irradiated side tends to appear to have tighter soft tissue around the implant making it look smaller than the contralateral side. That is why only 2 of the 11 bilateral reconstructions were judged to be excellent and the rest ‘good’. All the contralateral risk- reducing mastectomy and breast reconstructions healed without complications and were ‘good’ aesthetically themselves but our assessment is based on the irradiated side as the primary focus.
In summary, we present a series of 21 consecutive patients who all successfully completed immediate 2-stage prosthetic breast reconstruction after salvage or risk-reducing mastectomies with no failures in the presence of no further tumor recurrence. We believe that this adds to the armamentarium of options of breast reconstruction for patients who are undergoing a salvage mastectomy after previous CS + RT.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by Dr. Lam.
REFERENCES
- 1.Kronowitz SJ, Robb GL. Breast reconstruction with postmastectomy radiation therapy: current issues. Plast Reconstr Surg. 2004;114:950–960. doi: 10.1097/01.prs.0000133200.99826.7f. [DOI] [PubMed] [Google Scholar]
- 2.Agha-Mohammadi S, De La Cruz C, Hurwitz DJ. Breast reconstruction with alloplastic implants. J Surg Oncol. 2006;94:471–478. doi: 10.1002/jso.20484. [DOI] [PubMed] [Google Scholar]
- 3.Boyages J, Langlands A. Postmastectomy radiation therapy: better late than never. Aust N Z J Surg. 1998;68:550–553. doi: 10.1111/j.1445-2197.1998.tb02098.x. [DOI] [PubMed] [Google Scholar]
- 4.Francis M, Cakir B, Ung O, et al. Prognosis after breast recurrence following conservative surgery and radiotherapy in patients with node-negative breast cancer. Br J Surg. 1999;86:1556–1562. doi: 10.1046/j.1365-2168.1999.01252.x. [DOI] [PubMed] [Google Scholar]
- 5.Stevens LA, McGrath MH, Druss RG, et al. The psychological impact of immediate breast reconstruction for women with early breast cancer. Plast Reconstr Surg. 1984;73:619–628. doi: 10.1097/00006534-198404000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Harcourt DM, Rumsey NJ, Ambler NR, et al. The psychological effect of mastectomy with or without breast reconstruction: a prospective, multicenter study. Plast Reconstr Surg. 2003;111:1060–1068. doi: 10.1097/01.PRS.0000046249.33122.76. [DOI] [PubMed] [Google Scholar]
- 7.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131:15–23. doi: 10.1097/PRS.0b013e3182729cde. [DOI] [PubMed] [Google Scholar]
- 8.Veronesi U, Banfi A, Salvadori B, et al. Breast conservation is the treatment of choice in small breast cancer: long-term results of a randomized trial. Eur J Cancer. 1990;26:668–670. doi: 10.1016/0277-5379(90)90113-8. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 10.Blichert-Toft M, Brincker H, Andersen JA, et al. A Danish randomized trial comparing breast-preserving therapy with mastectomy in mammary carcinoma. Preliminary results. Acta Oncol. 1988;27:671–677. doi: 10.3109/02841868809091767. [DOI] [PubMed] [Google Scholar]
- 11.Taylor R, Stubbs JM, Langlands AO, et al. Predictors of mastectomy for women with breast cancer in the Greater Western Region of Sydney. Breast J. 1999;5:116–121. doi: 10.1046/j.1524-4741.1999.00135.x. [DOI] [PubMed] [Google Scholar]
- 12.Boyages J, Bosch C, Langlands AO, et al. Breast conservation: long-term Australian data. Int J Radiat Oncol Biol Phys. 1992;24:253–260. doi: 10.1016/0360-3016(92)90680-g. [DOI] [PubMed] [Google Scholar]
- 13.Haffty BG, Goldberg NB, Fischer D, et al. Conservative surgery and radiation therapy in breast carcinoma: local recurrence and prognostic implications. Int J Radiat Oncol Biol Phys. 1989;17:727–732. doi: 10.1016/0360-3016(89)90058-8. [DOI] [PubMed] [Google Scholar]
- 14.Fowble B, Solin LJ, Schultz DJ, et al. Breast recurrence following conservative surgery and radiation: patterns of failure, prognosis, and pathologic findings from mastectomy specimens with implications for treatment. Int J Radiat Oncol Biol Phys. 1990;19:833–842. doi: 10.1016/0360-3016(90)90002-2. [DOI] [PubMed] [Google Scholar]
- 15.Osborne MP, Borgen PI, Wong GY, et al. Salvage mastectomy for local and regional recurrence after breast-conserving operation and radiation therapy. Surg Gynecol Obstet. 1992;174:189–194. [PubMed] [Google Scholar]
- 16.Chaudary MA, Nagadowska M, Smith P, et al. Local recurrence after breast conservation treatment: outcome following salvage mastectomy. The Breast. 1998;7:33–8. [Google Scholar]
- 17.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer mortality: meta-analysis of individual patient data on 10,801 women in 17 randomised trials. Early Breast Cancer Trialist’s Collaborative Group (EBCTCG). Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam TC, Hsieh F, Boyages J. The effects of postmastectomy adjuvant radiotherapy on immediate two-stage prosthetic breast reconstruction: a systematic review. Plast Reconstr Surg. 2013;132:511–518. doi: 10.1097/PRS.0b013e31829acc41. [DOI] [PubMed] [Google Scholar]
- 19.Spear SL, Onyewu C. Staged breast reconstruction with saline-filled implants in the irradiated breast: recent trends and therapeutic implications. Plast Reconstr Surg. 2000;105:930–942. doi: 10.1097/00006534-200003000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Pearl RM, Wisnicki J. Breast reconstruction following lumpectomy and irradiation. Plast Reconstr Surg. 1985;76:83–86. doi: 10.1097/00006534-198507000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Forman DL, Chiu J, Restifo RJ, et al. Breast reconstruction in previously irradiated patients using tissue expanders and implants: a potentially unfavorable result. Ann Plast Surg. 1998;40:360–363; discussion 363. doi: 10.1097/00000637-199804000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Percec I, Bucky LP. Successful prosthetic breast reconstruction after radiation therapy. Ann Plast Surg. 2008;60:527–531. doi: 10.1097/SAP.0b013e318172f5fc. [DOI] [PubMed] [Google Scholar]
- 23.Persichetti P, Cagli B, Simone P, et al. Implant breast reconstruction after salvage mastectomy in previously irradiated patients. Ann Plast Surg. 2009;62:350–354. doi: 10.1097/SAP.0b013e318184aac8. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch EM, Seth AK, Dumanian GA, et al. Outcomes of tissue expander/implant breast reconstruction in the setting of prereconstruction radiation. Plast Reconstr Surg. 2012;129:354–361. doi: 10.1097/PRS.0b013e31823ae8b1. [DOI] [PubMed] [Google Scholar]
- 25.Cordeiro PG, Snell L, Heerdt A, et al. Immediate tissue expander/implast breast reconstruction after salvage mastectomy for cancer recurrence following lumpectomy/irradiation. Plast Reconstr Surg. 2012;129:341–350. doi: 10.1097/PRS.0b013e318205f203. [DOI] [PubMed] [Google Scholar]
- 26.Choi M, Small K, Levovitz C, et al. The volumetric analysis of fat graft survival in breast reconstruction. Plast Reconstr Surg. 2013;131:185–191. doi: 10.1097/PRS.0b013e3182789b13. [DOI] [PubMed] [Google Scholar]
- 27.Jhaveri JD, Rush SC, Kostroff K, et al. Clinical outcomes of postmastectomy radiation therapy after immediate breast reconstruction. Int J Radiat Oncol Biol Phys. 2008;72:859–865. doi: 10.1016/j.ijrobp.2008.01.055. [DOI] [PubMed] [Google Scholar]
- 28.Anderson PR, Freedman G, Nicolaou N, et al. Postmastectomy chest wall radiation to a temporary tissue expander or permanent breast implant–is there a difference in complication rates? Int J Radiat Oncol Biol Phys. 2009;74:81–85. doi: 10.1016/j.ijrobp.2008.06.1940. [DOI] [PubMed] [Google Scholar]
- 29.Tallet AV, Salem N, Moutardier V, et al. Radiotherapy and immediate two-stage breast reconstruction with a tissue expander and implant: complications and esthetic results. Int J Radiat Oncol Biol Phys. 2003;57:136–142. doi: 10.1016/s0360-3016(03)00526-1. [DOI] [PubMed] [Google Scholar]
- 30.Nava MB, Pennati AE, Lozza L, et al. Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plast Reconstr Surg. 2011;128:353–359. doi: 10.1097/PRS.0b013e31821e6c10. [DOI] [PubMed] [Google Scholar]
- 31.Cordeiro PG, Pusic AL, Disa JJ, et al. Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg. 2004;113:877–881. doi: 10.1097/01.prs.0000105689.84930.e5. [DOI] [PubMed] [Google Scholar]


