Abstract
Background
The incidence of lentigo maligna (LM) may be increasing, but no population-based epidemiologic studies have been performed.
Objective
To determine the incidence of LM in Olmsted County, Minnesota, as well as overall and recurrence-free survival.
Methods
Using the Rochester Epidemiology Project, we identified all adult residents of Olmsted County, Minnesota, with a first lifetime diagnosis of LM between 1970 and 2007. Medical records were reviewed to determine demographic, clinical, and surgical data, and incidence and survival were calculated.
Results
Among 145 patients identified, median (range) age at diagnosis of LM was 70 (33-97) years. Treatment changed over time, with Mohs micrographic surgery becoming available after 1986. No patients died of LM; 5 had local recurrence. Estimated local recurrence-free survival at 5, 10, 15, and 20 years after diagnosis was 98%, 96%, 92%, and 92%, respectively. Overall age- and sex-adjusted incidence of LM among adults was 6.3 per 100,000 person-years, increasing from 2.2 between 1970 and 1989 to 13.7 between 2004 and 2007.
Limitations
Retrospective study; homogeneous population.
Conclusion
The incidence of LM increased significantly among residents of Olmsted County, Minnesota, over an extended time frame, with incidence being significantly higher among men than women and increasing with age.
Keywords: excision, incidence, lentigo maligna, lentigo maligna melanoma, melanoma, melanoma in situ, Mohs micrographic surgery, Rochester Epidemiology Project
Introduction
Lentigo maligna (LM) is a form of malignant melanoma in situ seen predominantly on chronically sun-damaged skin. It is found most commonly on the head and neck of elderly white persons, with a peak incidence in the seventh or eighth decade. Although it is generally accepted that the incidence of this melanocytic tumor is increasing in the United States as a result of an aging population that has suffered significant photodamage as a group, no population-based epidemiologic studies have been published to support this belief.
Most of the epidemiologic data concerning LM is from the 1980s. Newell and colleagues (1) accessed the Surveillance, Epidemiology, and End Results database from the 1970s and early 1980s to show that the age-adjusted incidence of LM melanoma (an invasive form of LM) in the United States was between 0.6 and 0.8 cases per 100,000 population, with a male : female ratio of 1.3:1. The in situ LM was not separated from the invasive LM melanoma in this study. An Australian study suggested a slightly higher incidence of LM—1.3 per 100,000—in that relatively more sun-exposed country in the southern hemisphere (2). The incidence of LM in Australia also seems to be increasing over time (3). Both of these studies, however, are from the early 1980s. Additionally, some authors have hypothesized that these incidence values may be an underrepresentation because many in situ melanomas are treated in the outpatient setting and are not reported (4).
LM is a precursor to LM melanoma, a more invasive and potentially serious form of skin malignancy. The rate of progression of LM to LM melanoma has been reported at 4.7%, 15%, and 5% to 50% in 3 separate studies (5-7). Therefore, it is important to determine the incidence rate of LM. To update and better define the incidence of LM in the United States, and to describe the demographic composition of patients with this disease, we undertook an epidemiologic study in a Midwestern county.
Methods
The study was approved by the respective institutional review boards of Olmsted Medical Center and Mayo Clinic in Rochester, Minnesota. Only records of patients who had previously provided consent to use their data for research purposes were studied.
The Rochester Epidemiology Project (REP) is a medical record linkage system that captures medical data on patients receiving care from almost all sources available to the local population of Olmsted County, Minnesota (8). The system provides a platform for conducting population-based analytical studies because all care provided to Olmsted County residents is available for study.
The REP databases were searched to identify potential cases of LM by using the appropriate ICD-9 (International Classification of Diseases, Ninth Revision) and HICDA (Hospital Adaptation of the International Classification of Diseases) codes. Any patients determined to have invasive LM melanoma were excluded. Diagnosis of LM was established by confirming the consensus dermatopathology diagnosis determined from skin biopsy specimens after re-review by a board-certified dermatopathologist. Date of diagnosis was defined as the date of biopsy. Patients of any age who were residents of Olmsted County at the time of a confirmed first lifetime diagnosis of LM, with the date of diagnosis between 1970 and 2007, were included in the study. The medical records of all included patients were then reviewed for patient demographics, tumor characteristics, clinical course, and outcomes.
Statistical Analysis
Continuous features were summarized with mean (SD) or median (range); categorical features were summarized with frequency counts and percentages. Changes in features by year of diagnosis were evaluated using Kruskal-Wallis tests and Cochran-Armitage trend tests. Overall survival and local recurrence-free survival were estimated using the Kaplan-Meier method. The duration of follow-up for overall survival was calculated from the date of diagnosis to the date of death or last follow-up; the duration of follow-up for local recurrence-free survival was calculated from the date of diagnosis to the date of local recurrence or last follow-up. Comparisons of overall survival among patient groups of interest were evaluated using log-rank tests.
Incidence rates per 100,000 person-years were calculated using incident cases of LM as the numerator and age- and sex-specific estimates of the adult population of Olmsted County, Minnesota, as the denominator. The population at risk was estimated using census data from 1970, 1980, 1990, and 2000, with linear interpolation for intercensal years. Incidence rates were age- and sex-adjusted to the structure of the 2000 US white population. The relationships between the incidence of LM and age at diagnosis, sex, and calendar year of diagnosis were assessed by fitting generalized linear models using the SAS procedure GENMOD (SAS Institute Inc). Incident cases were grouped into 5 age intervals (18-49, 50-59, 60-69, 70-79, and ≥80 years) and 4 calendar-year intervals (1970-1989, 1990-1999, 2000-2003, and 2004-2007). The models fit the natural logarithm of crude incidence rates as a linear function of age at diagnosis, sex, and calendar year of diagnosis, with a Poisson distribution used to model the error structure. The significance of the linear trends for the features of interest and interaction terms among these features were assessed using likelihood ratio statistics.
All analyses were performed using the SAS software package (version 9.2). All reported P values were 2-sided, and P<.05 was considered statistically significant.
Results
After search of the REP, 145 patients with at least 1 incident lesion were included in the study. Patient, disease, and treatment characteristics are summarized in Table 1. Median (range) age of the patients was 70 (33-97) years. Of 142 patients with data available, no patient had a history of photosensitivity disorder, skin cancer syndrome, other host immunosuppression, or solid organ transplant. Two patients (1%) had 2 incident lesions: 1 had lesions on the cheek and scalp (both on the right side), and 1 had lesions on the leg (right) and abdomen (left). The features of treatment, surgical margins, and type of closure were the same for both lesions in each patient, so the features of the largest lesion only are summarized in Table 1. The face was the most common site of the lesion (48%), followed by the trunk (21%) and extremities (17%). The cheeks were involved in 26% of all patients and were the most common site of LM on the face. A comparison of select features by year of diagnosis is shown in Table 2.
Table 1. Patient and Disease Characteristics.
| Characteristic | Valuea (N=145) |
|---|---|
| Age at diagnosis, y | 70 (33-97) |
| Age at diagnosis, y | |
| 18-49 | 19 (13) |
| 50-59 | 20 (14) |
| 60-69 | 30 (21) |
| 70-79 | 51 (35) |
| ≥80 | 25 (17) |
| Men | 93 (64) |
| White (n=132) | 132 (100) |
| Year of diagnosis | |
| 1970-1989 | 21 (14) |
| 1990-1999 | 40 (28) |
| 2000-2003 | 34 (23) |
| 2004-2007 | 50 (34) |
| History of skin cancer (n=140) | |
| No | 90 (64) |
| NMSC | 35 (25) |
| NMSC and other primary MM | 10 (7) |
| Other primary MM | 5 (4) |
| History of hematologic malignancy (n=142) | |
| No | 141 (99) |
| NHL | 1 (1) |
| Site of LM, specific | |
| Cheek | 38 (26) |
| Neck | 11 (8) |
| Forearm | 11 (8) |
| Nose | 10 (7) |
| Temple | 10 (7) |
| Forehead | 9 (6) |
| Chest | 8 (6) |
| Leg | 8 (6) |
| Middle/lower back | 8 (6) |
| Upper back | 8 (6) |
| Arm | 6 (4) |
| Ear | 6 (4) |
| Shoulder | 6 (4) |
| Scalp | 3 (2) |
| Lip | 2 (1) |
| Abdomen | 1 (1) |
| Site of LM, general | |
| Face | 69 (48) |
| Trunk | 31 (21) |
| Extremities | 25 (17) |
| Neck | 11 (8) |
| Ear | 6 (4) |
| Scalp | 3 (2) |
| Side of body (n=136) | |
| Right | 71 (52) |
| Left | 56 (41) |
| Central | 9 (7) |
| Treatment (n=143) | |
| Excision | 109 (76) |
| Mohs micrographic surgery | 26 (18) |
| None | 7 (5) |
| Cryoablation | 1 (1) |
| Surface area, cm2 | |
| Preoperative (n=112) | 1.52 (0.01-56.25) |
| Postoperative (n=94) | 6.23 (0.36-52.50) |
| Surgical margins, cm (n=88) | 0.50 (0.10-1.50) |
| Closure (n=129) | |
| Primary | 97 (75) |
| Rotation flap | 10 (8) |
| Advancement flap | 6 (5) |
| FTSG | 6 (5) |
| Second intention | 4 (3) |
| IPF | 4 (3) |
| Interpolation flap | 1 (1) |
| Primary and second intention (same lesion) | 1 (1) |
Abbreviations: FTSG, full-thickness skin graft; IPF, island pedicle flap; LM, lentigo maligna; MM, malignant melanoma; NHL, non-Hodkin lymphoma; NMSC, nonmelanoma skin cancer.
Values are No. of patients (%) or median (range).
Table 2. Comparison of Select Features by Year of Diagnosis.
| Feature | Year of LM Diagnosisa | ||||
|---|---|---|---|---|---|
|
|
|||||
| 1970-1989 (n=21) | 1990-1999 (n=40) | 2000-2003 (n=34) | 2004-2007 (n=50) | P Value | |
| Age at diagnosis, y | 63 (37-94) | 71 (33-94) | 70 (38-92) | 71 (41-97) | .78 |
| Surface area, cm2 | |||||
| Preoperative (n=112) | 1.69 (1.20-5.76) | 1.32 (0.09-21.32) | 1.32 (0.04-56.25) | 1.96 (0.01-23.65) | .35 |
| Postoperative (n=93) | 4.50 (1.40-12.00) | 6.16 (1.10-22.09) | 6.75 (1.00-36.00) | 6.25 (0.36-52.50) | .54 |
| Men | 16 (76) | 23 (58) | 24 (71) | 30 (60) | .47 |
| Site | .90 | ||||
| Face | 8 (38) | 22 (55) | 17 (50) | 22 (44) | |
| Trunk | 7 (33) | 7 (18) | 6 (18) | 11 (22) | |
| Extremities | 2 (10) | 7 (18) | 6 (18) | 10 (20) | |
| Neck | 3 (14) | 3 (8) | 2 (6) | 3 (6) | |
| Ear | 0 | 1 (2) | 2 (6) | 3 (6) | |
| Scalp | 1 (5) | 0 | 1 (3) | 1 (2) | |
| Treatment (n=143) | (n=20) | (n=39) | <.001 | ||
| Excision | 13 (65) | 33 (85) | 26 (76) | 37 (74) | |
| MMS | 0 | 6 (15) | 7 (21) | 13 (26) | |
| None | 6 (30) | 0 | 1 (3) | 0 | |
| Cryoablation | 1 (5) | 0 | 0 | 0 | |
Abbreviations: LM, lentigo maligna; MMS, Mohs micrographic surgery.
Values are No. of patients (%) or median (range).
The overall age- and sex-adjusted incidence of LM among adults was 6.3 (95% CI, 5.3-7.4) per 100,000 person-years. Incidence rates overall by age at diagnosis and sex are summarized in Table 3 and illustrated in Figure 1. Incidence rates by calendar year of diagnosis are summarized in Table 3 and illustrated in Figure 2. Only 3 patients (2%) were younger than 40 years at diagnosis (the youngest was 33 years old) and only 5 patients (3%) had a diagnosis between 1970 and 1979. As such, the first age group included patients aged 18 to 49 years and the first calendar year group included patients with diagnosis between 1970 and 1989.
Table 3. Incidence of Lentigo Maligna by Age and Sex, Overall and by Calendar Year of Diagnosis.
| Age category, y | Women | Men | Overall | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| No | Ratea | No | Ratea | No. | Ratea | |
| All Years | ||||||
| 18-49 | 10 | 1.0 | 9 | 1.0 | 19 | 1.0 |
| 50-59 | 8 | 4.0 | 12 | 6.3 | 20 | 5.1 |
| 60-69 | 11 | 7.8 | 19 | 15.5 | 30 | 11.4 |
| 70-79 | 13 | 12.3 | 38 | 51.9 | 51 | 28.5 |
| ≥80 | 10 | 12.6 | 15 | 43.3 | 25 | 21.9 |
| All | 52 | 3.8b | 93 | 9.9b | 145 | 6.3c |
| 1970-1989 | ||||||
| 18-49 | 1 | 0.2 | 3 | 0.7 | 4 | 0.4 |
| 50-59 | 1 | 1.3 | 5 | 6.7 | 6 | 3.9 |
| 60-69 | 1 | 1.6 | 1 | 2.0 | 2 | 1.8 |
| 70-79 | 1 | 2.1 | 5 | 17.1 | 6 | 7.8 |
| ≥80 | 1 | 3.2 | 2 | 15.7 | 3 | 6.8 |
| All | 5 | 0.8b | 16 | 4.0b | 21 | 2.2c |
| 1990-1999 | ||||||
| 18-49 | 4 | 1.4 | 1 | 0.4 | 5 | 0.9 |
| 50-59 | 4 | 7.3 | 2 | 3.7 | 6 | 5.5 |
| 60-69 | 2 | 5.2 | 4 | 11.5 | 6 | 8.2 |
| 70-79 | 3 | 10.1 | 11 | 51.8 | 14 | 27.5 |
| ≥80 | 4 | 16.4 | 5 | 50.0 | 9 | 26.2 |
| All | 17 | 4.2b | 23 | 9.1b | 40 | 6.1c |
| 2000-2003 | ||||||
| 18-49 | 1 | 0.8 | 3 | 2.5 | 4 | 1.7 |
| 50-59 | 0 | 0 | 1 | 3.6 | 1 | 1.8 |
| 60-69 | 4 | 21.9 | 7 | 41.2 | 11 | 31.2 |
| 70-79 | 3 | 22.3 | 11 | 103.7 | 14 | 58.2 |
| ≥80 | 2 | 17.6 | 2 | 37.1 | 4 | 23.8 |
| All | 10 | 5.6b | 24 | 17.4b | 34 | 10.8c |
| 2004-2007 | ||||||
| 18-49 | 4 | 3.2 | 2 | 1.6 | 6 | 2.5 |
| 50-59 | 3 | 8.5 | 4 | 12.1 | 7 | 10.2 |
| 60-69 | 4 | 18.3 | 7 | 35.2 | 11 | 26.3 |
| 70-79 | 6 | 41.1 | 11 | 91.3 | 17 | 63.8 |
| ≥80 | 3 | 24.2 | 6 | 91.3 | 9 | 47.5 |
| All | 20 | 10.0b | 30 | 19.2b | 50 | 13.7c |
Incidence per 100,000 person-years.
Incidence per 100,000 person-years, age-adjusted to 2000 US white population.
Incidence per 100,000 person-years, age- and sex-adjusted to 2000 US white population.
Figure 1.
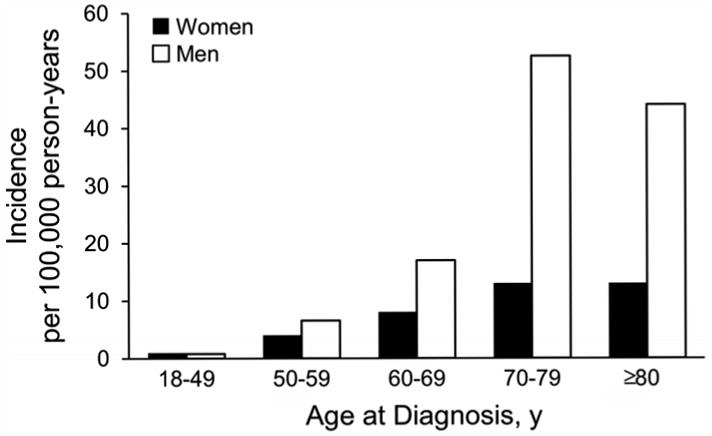
Incidence of lentigo maligna by age at diagnosis and sex.
Figure 2.
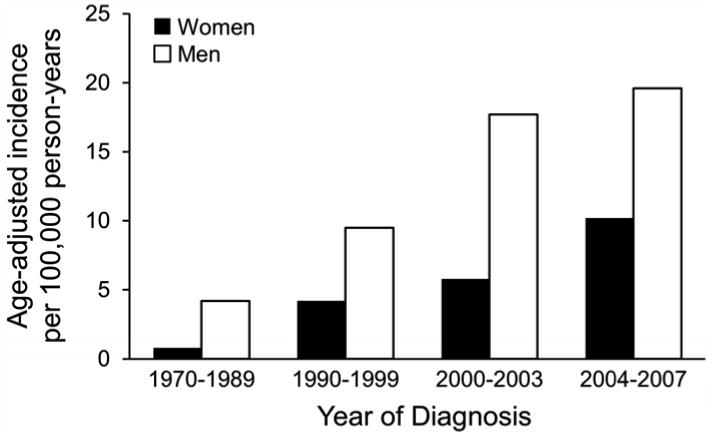
Incidence of lentigo maligna by calendar year of diagnosis and sex.
Incidence of LM increased significantly with age (P<.001) and by year of diagnosis (P<.001) and was higher for men than for women (P<.001). In addition, there was evidence that the increase in incidence with age was different for men and women (P=.005). Although there was no difference in the overall incidence of LM between men and women aged 18 to 49 years (1.0 per 100,000 person-years), the incidence was significantly higher among men than among women aged 50 years and older, with the highest being among men aged 70 to 79 years (51.9 per 100,000). Age-adjusted incidence per 100,000 person-years across the entire period of study was 9.9 (95% CI, 7.8-12.0) for men compared with 3.8 (95% CI, 2.7-4.8) for women. Incidence rates per 100,000 person-years increased from 1.0 (95% CI, 0.6-1.5) for patients 18 to 49 years old at diagnosis to 21.9 (95% CI, 14.2-32.4) for patients aged 80 years or older at diagnosis. Age- and sex-adjusted incidence per 100,000 person-years increased from 2.2 (95% CI, 1.2-3.2) between 1970 and 1989 to 13.7 (95% CI, 9.8-17.5) between 2004 and 2007.
At last follow-up, 42 patients (29%) had died at a mean of 7.4 years after diagnosis (median, 6.2 years; range, 0.5-33.0 years): 39 (27%) died of other causes and 3 (2%) died of unknown causes. No patient died of LM. Among the 103 patients (71%) still alive at last follow-up, the mean duration of follow-up was 9.5 years (median, 8.1 years; range, 2.4-32.4 years). Estimated overall survival rates (95% CI; number still at risk) at 5, 10, 15, and 20 years after diagnosis were 88% (82%-93%; 103), 70% (61%-79%; 45), 59% (49%-72%; 20), and 52% (40%-68%; 10), respectively. Estimated overall survival rates (95% CI; number still at risk) 5 years after diagnosis for patients treated with excision or Mohs micrographic surgery were 89% (83%-95%; 76) and 83% (70%-100%; 18), respectively (P=.99).
One patient was missing information regarding local recurrence. Among the other 144, local recurrence developed in 5 patients (3%) at 0.4, 2.8, 4.5, 9.2, and 13.3 years after diagnosis, respectively. Estimated local recurrence-free survival rates (95% CI; number still at risk) at 5, 10, 15, and 20 years after diagnosis were 98% (95%-100%; 99), 96% (91%-100%; 43), 92% (84%-100%; 17), and 92% (84%-100%; 9), respectively. Too few local recurrences were observed (<10) to compare local recurrence-free survival by year of diagnosis and type of treatment. No patient had distant metastases from LM during follow-up.
Discussion
This population-based study demonstrates a significant increase in the incidence of LM in the past 40 years. The findings in our study support those of previous reports showing a peak incidence of LM occurring in an older population (9). However, our data suggest an even higher incidence of LM among older persons than previously reported. Furthermore, a study by Swetter et al (10) reported a 52% increase in the incidence rate among both sexes aged 45 to 64 years between 1990 and 2000. During the same study period, we found a higher incidence of LM and also a steady increase in the incidence rate among men and women between 50 and 70 years of age. For another perspective, the overall incidence of LM in our study was 6.3 per 100,000 person-years, whereas in 4 European population-based studies, the incidence of new patients with basal cell carcinoma ranged from 77 to 158 per 100,000 person-years (11).
The risk of LM has been reported to be far greater among the fair-skinned population and among those with a history of nonmelanoma skin cancer (12). Since all of the patients in our study who reported race were white, no comparison of the incidence of LM among different skin colors was undertaken. A substantial portion of our study population (25%) had a history of nonmelanoma cancer, which lends additional support to this being a risk factor for the development of LM.
The face was the most common lesion site, followed by the trunk and extremities. Of all locations on the face, the cheeks were the most common site of LM. The areas most commonly affected by LM or LM melanoma have been reported to be the head and neck (13,14), whereas our study showed the neck to be the third most commonly affected area between 1970 and 1989 and the fourth between 1990 and 2007. LM still had a predilection for the head, more specifically the cheeks. However, the extremities have been an increasingly common site for LM. This increased localization of LM on limbs could be a result of increased exposure of extremities to ultraviolet light or greater awareness and concern among the public about skin cancers, possibly prompting visits to physicians, full body examinations, and the subsequent discovery of abnormal skin lesions.
The type of treatment for LM changed significantly over time in this study. Mohs micrographic surgery became a more common treatment after 1986, due to availability of this procedure in the study region at that time. Recurrence of LM was noted in only 3.5% of the patients, with a wide variability in time between the index visit and recurrence of LM. Too few local recurrences were observed to compare local recurrence-free survival by year of diagnosis and type of treatment. Importantly, no patient had development of distant metastases during follow-up and no patients died of LM melanoma. The estimated overall survival rates mentioned above are not as important with regard to LM itself, given that there were no deaths from LM. However, these data emphasize that treating these patients is worth the postoperative morbidity after surgery, since no metastatic disease or death resulted from LM and the local recurrence rate was too small to discern a difference between treatment with Mohs micrographic surgery and excision.
Our study has several limitations. The resources provided by the REP allow researchers to generate validated and accurate estimates of population-based incidences of disease. Nonetheless, this type of research has some inherent limitations, such as the possibility of incomplete reporting of incident cases of LM in the medical record over time. However, because the final pathologic diagnosis of all specimens is included in the REP, it is unlikely that any cases were missed. Some residents may have sought care for LM outside of Olmsted County, Minnesota. In addition, the county's population is primarily white and highly educated compared with the population of the United States as a whole. Thus, data collected in our study may not be representative of patients with LM in other parts of the United States or in other countries.
Conclusion
This study demonstrates a significant increase in the incidence of LM among residents of Olmsted County, Minnesota, over an extended time frame. The incidence is significantly higher among men than women and increases with age. Mohs micrographic surgery became a more common treatment after 1986 due to the availability of this procedure in the study region. Because there were no disease-related deaths, there was no difference in survival based on the treatment modality used. The treatment of LM in this population was very effective, with a 100% disease-specific survival rate.
Acknowledgments
Funding sources: None.
Abbreviations
- LM
lentigo maligna
- REP
Rochester Epidemiology Project
Footnotes
Conflict of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Newell GR, Sider JG, Bergfelt L, Kripke ML. Incidence of cutaneous melanoma in the United States by histology with special reference to the face. Cancer Res. 1988 Sep 1;48(17):5036–41. [PubMed] [Google Scholar]
- 2.Holman CD, Mulroney CD, Armstrong BK. Epidemiology of pre-invasive and invasive malignant melanoma in Western Australia. Int J Cancer. 1980 Mar 15;25(3):317–23. doi: 10.1002/ijc.2910250303. [DOI] [PubMed] [Google Scholar]
- 3.Little JH, Holt J, Davis N. Changing epidemiology of malignant melanoma in Queensland. Med J Aust. 1980 Jan 26;1(2):66–9. [PubMed] [Google Scholar]
- 4.Smalberger GJ, Siegel DM, Khachemoune A. Lentigo maligna. Dermatol Ther. 2008 Nov-Dec;21(6):439–46. doi: 10.1111/j.1529-8019.2008.00244.x. [DOI] [PubMed] [Google Scholar]
- 5.Weinstock MA, Sober AJ. The risk of progression of lentigo maligna to lentigo maligna melanoma. Br J Dermatol. 1987 Mar;116(3):303–10. doi: 10.1111/j.1365-2133.1987.tb05843.x. [DOI] [PubMed] [Google Scholar]
- 6.Penneys NS. Microinvasive lentigo maligna melanoma. J Am Acad Dermatol. 1987 Oct;17(4):675–80. doi: 10.1016/s0190-9622(87)70254-0. [DOI] [PubMed] [Google Scholar]
- 7.Samaniego E, Redondo P. Lentigo maligna. Actas Dermosifiliogr. 2012 Jul 31; doi: 10.1016/j.ad.2012.05.006. [Epub ahead of print] English, Spanish. [DOI] [PubMed] [Google Scholar]
- 8.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996 Mar;71(3):266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 9.Cohen LM. Lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol. 1995 Dec;33(6):923–36. doi: 10.1016/0190-9622(95)90282-1. Erratum in: J Am Acad Dermatol. 1997 Jun;36(6 Pt 1):913. [DOI] [PubMed] [Google Scholar]
- 10.Swetter SM, Boldrick JC, Jung SY, Egbert BM, Harvell JD. Increasing incidence of lentigo maligna melanoma subtypes: northern California and national trends 1990-2000. J Invest Dermatol. 2005 Oct;125(4):685–91. doi: 10.1111/j.0022-202X.2005.23852.x. [DOI] [PubMed] [Google Scholar]
- 11.de Vries E, Micallef R, Brewster DH, Gibbs JH, Flohil SC, Saksela O, et al. EPIDERM Group. Population-based estimates of the occurrence of multiple vs first primary basal cell carcinomas in 4 European regions. Arch Dermatol. 2012 Mar;148(3):347–54. doi: 10.1001/archdermatol.2011.2244. [DOI] [PubMed] [Google Scholar]
- 12.Gaudy-Marqueste C, Madjlessi N, Guillot B, Avril MF, Grob JJ. Risk factors in elderly people for lentigo maligna compared with other melanomas: a double case-control study. Arch Dermatol. 2009 Apr;145(4):418–23. doi: 10.1001/archdermatol.2009.1. [DOI] [PubMed] [Google Scholar]
- 13.Whiteman DC, Stickley M, Watt P, Hughes MC, Davis MB, Green AC. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006 Jul 1;24(19):3172–7. doi: 10.1200/JCO.2006.06.1325. [DOI] [PubMed] [Google Scholar]
- 14.Cohen LM, McCall MW, Hodge SJ, Freedman JD, Callen JP, Zax RH. Successful treatment of lentigo maligna and lentigo maligna melanoma with Mohs' micrographic surgery aided by rush permanent sections. Cancer. 1994 Jun 15;73(12):2964–70. doi: 10.1002/1097-0142(19940615)73:12<2964::aid-cncr2820731213>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]


