Abstract
This review provides evidence that antenatal hypoxia, which represents a significant and worldwide problem, causes prenatal programming of the lung. A general overview of lung development is provided along with some background regarding transcriptional and signaling systems of the lung. The review illustrates that antenatal hypoxic stress can induce a continuum of responses depending on the species examined. Fetuses and newborns of certain species and specific human populations are well acclimated to antenatal hypoxia. However, antenatal hypoxia causes pulmonary vascular disease in fetuses and newborns of most mammalian species and humans. Disease can range from mild pulmonary hypertension, to severe vascular remodeling and dangerous elevations in pressure. The timing, length, and magnitude of the intrauterine hypoxic stress are important to disease development, however there is also a genetic-environmental relationship that is not yet completely understood. Determining the origins of pulmonary vascular remodeling and pulmonary hypertension and their associated effects is a challenging task, but is necessary in order to develop targeted therapies for pulmonary hypertension in the newborn due to antenatal hypoxia that can both treat the symptoms and curtail or reverse disease progression.
Keywords: Hypoxia, pulmonary hypertension, sheep, fetal programming, newborns
INTRODUCTION
A mother's uterus protects the unborn child and provides nutritional support. Unfortunately, but of great importance, prenatal stresses can alter uterine function and program the fetus causing disease after birth. The lung is particularly vulnerable since it undergoes a marked transition with birth. The lung switches from low blood flow and not exchanging air to receiving large blood flow and respiring. Respiratory abnormalities due to bronchopulmonary dysplasia, diaphragmatic hernia, antenatal hypoxia and other complications associated with pregnancy are extremely important health problems afflicting ~2% of newborns in the United States [1-3]. Neonatal lung diseases are difficult to treat, often resulting in long-term complications or death.
The respiratory system exhibits significant developmental plasticity, making it especially sensitive to hypoxemia and other stresses during the prenatal and neonatal periods. Maternal prenatal hypoxemia due to living at high altitudes, placental insufficiency, and smoking are relatively common examples. There are as many as 140 million permanent residents at altitudes >2,500 m [4]. As of 2008 ~ 13% of pregnant women in the United States, carrying just over 500,000 unborn children, smoked during the last 3 months of gestation [2]. This is really a worldwide problem, however, as roughly 9% of all women smoke [5]. Together these place a significant number of newborns at risk of developing pulmonary hypertension [6-8], high altitude pulmonary edema [4], as well as pulmonary hypertension later in life [9].
Antenatal hypoxia, a relatively common prenatal stress, is not only associated with high altitude living and smoking but also with various other prenatal disorders including maternal anemia or placental insufficiency. The resultant fetal hypoxemia can cause respiratory distress and pulmonary hypertension (PH) in newborns [10, 11]. The high pulmonary vascular resistance (PVR) associated with antenatal hypoxia causes backpressure on the right heart leading to cardiac hypertrophy and insufficiency. The devastating results of PH on right ventricular (RV) function eventually lead to heart failure, which is a common characteristic of all sub-classes of pulmonary arterial hypertension. The lack of definitive treatment options coupled with treatments that only temporarily alleviate the symptoms result in a slow but progressive disease. This debilitating disease of infants is unacceptable and illustrates why we need to vigorously investigate all aspects of pulmonary hypertension of the newborn, especially in the setting of easily identifiable risk factors, including antenatal hypoxia. These investigations hold promise of discovering more definitive treatment options that can slow or even reverse the disease.
Focusing on specific etiological risk factors, high altitude living is especially relevant, since it affects large populations and the stress does not have as many confounding issues as smoking, maternal malnutrition, or placental insufficiency. Living in elevations greater than 2,500 m is common and pregnancy at such altitudes causes antenatal stress to the unborn fetus. Many of the resident populations live in the Himalayas and its surrounding ranges, the Andes of Central and South America, portions of the Rocky Mountains in the United States, as well as other high altitude areas around the world [12]. Of particular interest is why infants from one population adapt to the high altitude environment while those from another population do not. For example, native Tibetan and Peruvian populations appear to have adapted to the high altitude environment [12-14]. The lung structural and functional development of the infants is altered in order to enhance blood oxygenation, although these infants can still have cardio-pulmonary complications after birth. In comparison, infants born to Han Chinese who have recently moved to Tibet and Europeans that move to the high Andes are more likely to have low birth weight and lung dysfunction, including elevated pulmonary pressures and reduced blood oxygenation [8, 14, 15].
In this review, we examine lung development to provide a frame of reference, and then focus on those elements that are likely to be vulnerable to low oxygen tension in utero. We examine adapted residents and review functional and genetic evidence from animal as well as human studies that works to explain divergence in the ability of infants born at high altitude to acclimatize. We then appraise our clinical understanding of the disease and the current treatment regimens as well as several relevant emerging therapeutic strategies.
THE MAMMALIAN LUNG: DEVELOPMENTAL PROGRESSION AND FUNCTION
Airway and vasculature structure
Developmental progression of the human lung is well described and outlined in a number of fine reviews over the past five decades [16-23] and in several books [24-26]. The progression of human lung development is summarized in Table 1 and then comparisons to other species are made in Table 2.
Table 1.
Developmental Stages of the Human Lung
| Developmental Stage | Gestational Age (approximate) | Processes |
|---|---|---|
| Embryonic period / Organogenesis | Week 1 through 7 | - Lungs appear at ~day 26 - Early lung lobes form - Dichotomous divisions form the bronchial tree (branching morphogenesis) [27] - Pulmonary arteries [28-31] and veins [32] form via angiogenesis, vasculogenesis or both. - Epithelial-mesenchymal “cross-talk” is important [33-35] |
| Pseudoglandular | Week 5 through 17 | - Bronchial tree forms to terminal bronchiole via branching morphogenesis [36] - Epithelial-based acinus/alveolus forms via cell differentiation [37, 38] - Arterial and venous system develop in parallel to the airways [31, 32] - Capillary plexus forms connecting arteries and veins [39, 40] - Epithelial-mesenchymal “cross-talk” is important [33-35] |
| Canalicular | Week 16 through 26 | - Respiratory epithelial growth and differentiation - Respiratory bronchiole formation - Further development of acinus/alveolus - Lung parenchyma “canalization” by capillaries [24, 26] -Air-blood barrier is formed |
| Saccular | Week 24 through term | - Distal saccule formation (lung size increases) - Alteration of capillary network (inter-air-space wall capillaries increase in number) - Interstitial elastic fibers are laid and differentiation of cells further advances -Surfactant production by type II epithelial cells begins - Larger vessels muscularize [41] |
| Alveolarization | Week 36 through 18 months post-natally | - Alveoli form via septation of the saccules (~90% post-natally in humans) [24, 25, 42] |
| Microvascular Maturation | Starts shortly after birth and continues through the first few years of life | - Capillary network morphs from bi-layered to single-layered through capillary fusion [24, 25, 43] |
Table 2.
Length of Gestation and Developmental Stages of the Lung
| Species | Length of Gestation (days) | Pseudoglandular Stage* | Canalicular Stage* | Saccular Stage* | Alveolar Stage* | References |
|---|---|---|---|---|---|---|
| Mouse | 19-21 | 50 to 85% | 85 to 90% | 90% to postnatal day 5 | Begins postnatal day 4 | [48] |
| Rat | 21-23 | 50 to 85% | 85 to 100% | 95% to postnatal day 4 | Begins postnatal day 4 | [20, 49] |
| Rabbit | 28-35 | 30 to 80% | 75 to 90% | 85 to 100% | 90 to 100% | [20] |
| Guinea Pig | 58-75 | 50 to 60% | 60 to 75% | 75 to 100% | 80 to 100% | [50] |
| Pig | 101-130 | Completed by 50% | 45 to 80% | 75 to 100% | 90% to postnatal day 14 | [51] |
| Sheep | 145-150 | Completed by 60% | 50 to 80% | 80 to 90% | 85% to day 14 | [20] |
| Human | 270 | Completed by 50% | 50 to 75% | 60 to 100% | 100% to several months postnatally | [20] |
| Cow | 280-290 | Completed by 45% | 45 to 65% | 65 to 80% | 80 to 90% | [52] |
Percentages refer to the portion of gestation that has completed at the beginning and end of each respective developmental stage
In brief, human lung development begins in the embryonic period during the 1st week of gestation up through week 7. This is followed by 3, somewhat overlapping, fetal stages of lung development. The pseudoglandular stage is between weeks 5 to 17, the canalicular stage between weeks 16 and 26, and the saccular stage from week 24 to term. Prior to birth, the alveolarization stage begins around week 36, and continues for as long as 2-3 years after birth [24, 25, 44, 45]. Finally, microvascular maturation begins shortly after birth and continues for the first few years of life, with less defined boundaries compared to the other stages [44, 46, 47]. These aforementioned stages are heavily based on airway formation and development, and although vascular development has been significantly less studied, it is just as integral to lung development.
The lung is distinct among all of the organs because it develops two vascular systems. The bronchial circulation arises from the thoracic aorta and provides oxygenation of various lung tissues while the pulmonary circulation transports deoxygenated blood from the right ventricle to the alveoli for blood oxygenation and then returns the oxygenated blood to the left heart. Vascular development of the lung is broken into three distinct processes. First, there is proximal angiogenesis, which is the formation of vessels from pre-existing ones. Second, there is distal vasculogenesis, which is the de novo formation of vessels from angioblasts or endothelial precursor cells within peripheral blood lakes of the mesenchyme. Lastly, there is proximal-distal vessel fusion during the pseudoglandular period [40, 53]. More recently, variations of this theory have emerged from different investigative groups. These differing views support either an all-vasculogenesis theory [54] or an all-angiogenesis theory [55], the latter of which heavily relies on vascular remodeling. Regardless of the actual mechanism, it is evident that vascular development starts early in fetal life, continues after birth and is heavily based on epithelial-mesenchymal cell interactions, often referred to as “cross-talk” [33-35, 53].
Developmental stages of the lung
During the embryonic period, the lungs appear at approximately day 26 of gestation as a ventral out-pouching of the foregut. The newly formed lung bud elongates, dichotomizes and invades the surrounding mesenchyme. The airway tree is slowly formed with continuous dichotomous divisions, and during this period the entire bronchial tree forms down to the terminal bronchiole [36]. The vascular connections are also established at this early stage of development. The main pulmonary trunk through to the intrapulmonary arteries is formed, either through angiogenesis, vasculogenesis or a combination of these processes [29, 30]. The vascular development at this stage parallels the airway tree, with supernumerary arteries for alveoli situated on the airway walls [31].The venous system develops in-between airway branches within connective tissue septa [32]. A capillary plexus also starts to form [39] and becomes a connecting bridge between pulmonary arteries and veins during this period [40]. The bronchial circulatory network of the lungs begins to form as well, which eventually connects to the pulmonary veins of the left atrium [56, 57]. Ultimately, the bronchial, arterial, and venous structural pattern is very similar to the adult lung by the end of the pseudoglandular stage.
The canalicular stage follows, and is characterized by respiratory epithelium growth, and formation of respiratory bronchioles and pulmonary acini, which are the gas-exchange units. The distal lung capillary bed increases dramatically, due to the “canalization” of the lung parenchyma by capillaries. The capillaries also come closer to the epithelial layer of the expanding air-spaces, and the cuboidal epithelium starts to flatten and differentiate into type I and type II airway epithelial cells. This marks a shift in the focus of lung development to the more functional elements needed for gas exchange [24, 45].
The saccular stage is the final prenatal stage of lung development, and is associated with the formation of saccules distally to the terminal bronchioles, significantly increasing the surface of the lung parenchyma through dichotomization. These saccules eventually morph into alveolar ducts and sacs during the alveolar stage [24, 25]. The interstitial tissue between air spaces is compressed and the capillary network is altered, increasing the number of capillaries within the inter-air-space walls. Elastic fibers are laid within this thinning interstitial tissue providing structural support, and differentiation further advances with maturation of type I and II alveolar cells, and increase in ciliated, Clara, basal and neuroendocrine cells. Finally, the larger vessels of the pulmonary vasculature muscularize [41].
A few weeks before birth and lasting close to 2-3 years after birth, the alveolar stage begins. More than 90% of the alveoli are formed post-natally with many in the first 6 months of life. This stage is characterized by changes of the terminal airway saccules such that they give rise to the alveolar ducts, alveolar sacs and single alveoli [42]. Microvascular maturation overlaps heavily with alveolar development, and transforms the capillary network of the lung parenchyma into its adult form. During this period the bi-layered capillary network will become the typical single-layered capillary network found in the more mature lung. The two main mechanisms through which this is accomplished include capillary fusions [43] and preferential growth [24]. The cellular growth and organization of the pulmonary vasculature is integral to its formation and function (Fig. 1). Moreover vessel wall structure is based on the external diameter of the artery and the vascular generation where it is located. Pre-capillary microvessels and capillaries have only an endothelial barrier with the pneumocytes at the alveoli (~10 μm ex ternal diameter). In slightly larger vessels, the walls become muscularized, with non-muscular and partially muscular vessels found at up to 150 μm external diameter. The fully muscularized vessels, and thicker arteries have an elastic lamina that creates a more pronounced infrastructure and helps delineate the endothelial and smooth muscle layers [58]. Contacts between neighboring cells and matrix help determine cell phenotype and are important in responses to growth factors and regulators of vasomotor tone [25, 45].
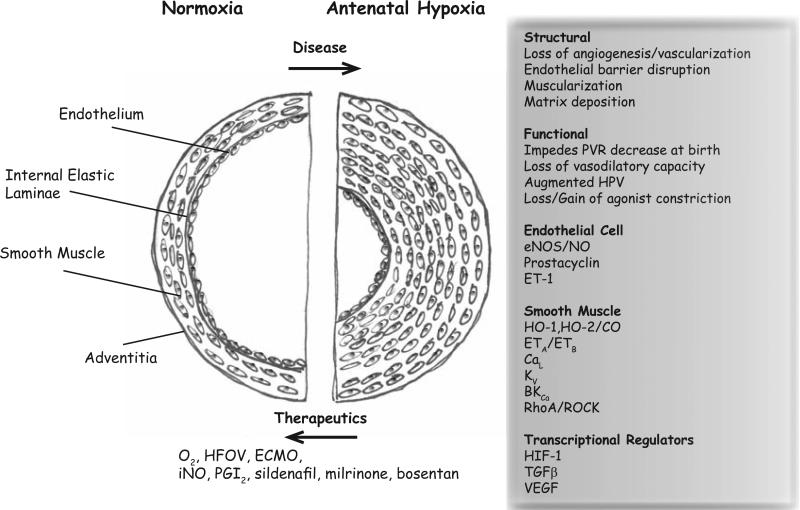
Fig. (1). The pulmonary arterial wall in normal and antenatal hypoxia diseased lung.
In a normal lung the vessel wall and smooth muscle layer is thin. The endothelium lines the lumen of the artery and in distal arteries there is no smooth muscle or elastic lamina. With antenatal hypoxia there can be thickening of the smooth muscle layer that impinges on the arterial lumen along with alterations in myocyte reactivity, as well as disruption of endothelial cell structure with loss of barrier function. These changes are manifested through a number of disruptions involving transcriptional regulators, signaling pathways, and ion channels. The figure summarizes the major components that are discussed in this review.
The growth and organization of the pulmonary vasculature is coupled to its form and function. The endothelial cells are vital to vasculogenesis. They coalesce into tubes and form the vessels, creating junctional complexes with one another by fusing their membranes into a contiguous structure. This endothelial barrier is critical in all vessels, but particularly important in the microvessels and capillaries of the lung where the endothelial cell is buttressed against the pneumocyte of the alveoli. This provides the final barrier between the blood and the alveoli and the reduced membrane width facilitates gas diffusion. During development the interactions between endothelial and mural cells are very important because they promote vessel wall formation. The vessels that feed the terminal bronchioles and larger airways become muscularized. Mature larger diameter vessels, ~ 75 μm and larger [58], have an elastic lamina that creates a more pronounced infrastructure and helps delineate the endothelial and smooth muscle layers. The endothelial cells comprise the intima and are on the luminal side of the elastic lamina. Smooth muscle myocytes make up the medial layer, which is on the outer surface of the elastic lamina. Fibroblasts are on the outer surface of the vessel, making up the adventitia. The endothelium, as mentioned, forms a tightly coupled syncytium and the myocytes of the medial layer are also connected via gap junctions. The endothelial and smooth muscle cells couple to one another through myoendothelial junctions that traverse the internal elastic lamina layer. There are also additional contacts between neighboring cells within the adventitia. Lastly, all of the cells are tethered into the matrix, which helps define cell phenotype and is important in determining the responses to growth factors and regulators of vasomotor tone [25, 26].
Vascular reactivity in utero
Vascular reactivity is fundamental to the ability of the lung to regulate its blood flow. Prior to birth the lung does not have any role for gas exchange. Because the lung does not serve its primary purpose it is not beneficial for the fetus to expend effort on providing high perfusion to this organ. The unique ability of the lungs’ vascular bed to constrict in response to hypoxia and dilate in response to normoxia or hyperoxia helps the lung to limit its perfusion before birth. After birth, constriction in response to hypoxia facilitates blood oxygenation by shunting blood away from low oxygen-tension alveoli and towards ones rich in oxygen. As early as the 100th day of gestation sheep fetuses will exhibit increased PVR mediated by pulmonary vasoconstriction in response to low oxygen tension, and this response becomes progressively greater later in pregnancy and after birth [59, 60]. Conversely, an increase in the oxygen tension of 5 mmHg can cause pulmonary arterial dilation, increased pulmonary blood flow and decreased PVR [61].
Several mechanisms keep fetal PVR high. These include low oxygen tension, low basal vasodilator production, and increased vasoconstrictor production in combination with enhanced myogenic tone [25, 62]. Moreover the fetal circulation is also characterized by progressive changes in vasore-activity. For example, we find that ovine fetuses have reduced constriction to membrane depolarization with high potassium [63] or serotonin [64, 65] as compared to adults. Also in the ovine fetus, vasoreactivity increases in late gestation (136-146 days) compared to early gestation (94-101 days), with increased hyperoxia-induced vasodilation and pulmonary vascular blood flow [66]. In the human fetus there are similar increases in hyperoxia-induced vasodilation during the final weeks of gestation, weeks 31 through 36 [67]. This enhanced vasodilatory capacity to elevated PO2 is likely due to increases in the function and expression of oxygen sensing mechanisms, including an increase in large conductance voltage- and calcium-activated potassium (BKCa) channels [68], and is consistent with functional maturation of the pulmonary circulation in addition to structural growth and development.
Our knowledge regarding the mechanisms associated with pulmonary arterial contraction is extensive. Unfortunately, most of what we know is derived from studies performed in arteries and cells isolated from adult animals. These examinations show that many stimulatory agonists act through functional G-protein coupled receptors (GPCRs). Some of the most potent vasoconstrictors including endothelin-1 (ET-1), serotonin (5-HT) and prostaglandin F2α (PGF2α) act through Gq-coupled receptors. The signaling pathways these agonists activate are intricate and well organized (see Fig. 2). Gq-coupled receptors cause contraction through phospholipase C (PLC) and receptor tyrosine kinase pathways. In turn, these pathways act to modulate the function of Ca2+-dependent and independent signaling pathways primarily through myo-inositol 1,4,5-trisphosphate (InsP3), Diacylglycerol (DAG) and the Ras homolog gene family, member A (RhoA) and Rho-associated, coiled-coil containing protein kinase (ROCK) signaling pathway. Ultimately, the pathways converge to mediate their effects through phosphorylation and activity of the 20 kDa myosin light chain (MLC20).
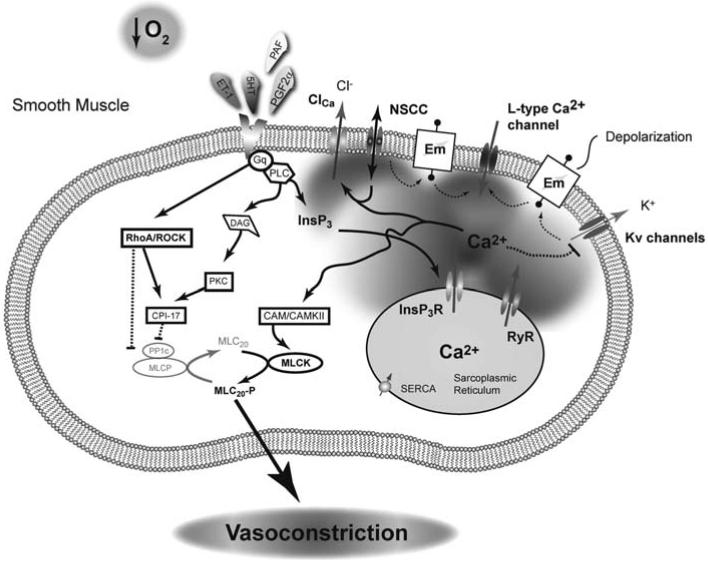
Fig. (2). Pulmonary vasoconstriction in the fetus is a highly coordinated process.
A combination of low oxygen tension and humoral mediators constrict the lung in-utero. The mechanisms associated with hypoxic-induced pulmonary vasoconstriction remain controversial, but a combination of activation of L-type Ca2+ channels, ryanodine receptors, rho-kinase, non-selective cation channels, and inhibition of K+ channels are each important in contraction of pulmonary arteries from adult animals. Pulmonary vascular resistance of the fetal lung is thought to be maintained at a high level due to increased vasoactive agonists, including elevated ET-1 levels. The high ET-1, released from the vascular endothelium, works through a Gq coupled receptor to activate a number of intracellular signaling pathways that lead to smooth muscle cell contraction through simultaneous activation of MLCK and inhibition of MLCP. Solid line with arrow: Activation pathway, Dashed line with bar: Inhibition pathway.
Membrane depolarization and Ca2+ dependent contraction
Membrane depolarization is a fundamental feature of GPCR receptor activation, which is intimate to extracellular Ca2+ entry. Based on studies performed in pulmonary arteries and myocytes isolated from adult animals there is still debate over the exact mechanisms involved in membrane depolarization, and even less is known regarding this process in fetal tissues and cells. However, the general consensus is that membrane depolarization is due to coordinated regulation of several ion channels. Receptor activation inhibits voltage-gated K+-channels (Kv) through elevations in cytosolic Ca2+, and this causes membrane depolarization [69, 70]. Other evidence indicates receptor stimulation will activate Ca2+-dependent chloride conductances (ClCa) [71-73]. ClCa activity may depend on coordinated activation of non-selective cation channels following depletion of the sarcoplasmic reticulum Ca2+ [72] or possibly through Ca2+ sparks, which are due to localized Ca2+ increases mediated by ryanodine receptor (RyR) activation [74]. Together these K+, Cl−, and non-selective cation conductances regulate the membrane potential, and in turn Ca2+ influx through L-type Ca2+ channel as well as non-selective cation channel pathways.
The cytosolic Ca2+ increase following receptor activation and coordinated membrane depolarization is attributable to interplay of activation and inactivation of Ca2+ permeable ion channels on the sarcoplasmic reticulum and plasma membrane. The sarcoplasmic reticulum of smooth muscle cells includes InsP3 receptors (InsP3R) and RyRs, which release Ca2+ into the cytosol when stimulated by GPCR signaling pathways. The spatial arrangement of InsP3Rs and RyRs on the sarcoplasmic reticulum and the temporal aspects of Ca2+ release are important to smooth muscle cell excitability [75, 76]. Our understanding of Ca2+ signaling in fetal pulmonary arterial myocytes is still limited, but studies performed on fetal sheep pulmonary vascular myocytes illustrate that platelet activating factor (PAF) causes InsP3 generation and Ca2+ responses [77, 78]. This is substantiated by our own studies showing that ATP, phenylephrine and serotonin all cause Ca2+ responses in pulmonary arterial myocytes from fetal sheep [64, 79]. Even still, ET-1-dependent activation of G protein coupled receptors can also induce RyR-generated Ca2+ sparks [80], illustrating the complexity of Ca2+ signaling within pulmonary vascular myocytes.
Studies performed in fetal sheep and additional models by our laboratory and by others illustrate fetal pulmonary arterial smooth muscles have several Ca2+ entry pathways. L-type Ca2+ channels are the best understood and characterized Ca2+ permeable ion channel. These channels are activated by membrane depolarization due to GPCR stimulation or acute hypoxia. We find L-type Ca2+ channels provide for roughly one-quarter of the contraction due to serotonin [64] and potassium-induced membrane depolarization in sheep fetal pulmonary arteries [63]. L-type Ca2+ channel blockers in piglets also dramatically reduce pulmonary arterial pressures and increase flow in vivo [81]. Although L-type Ca2+ channel activity is central to pulmonary arterial contractility, data shows that non-selective cation channels are also important to fetal pulmonary arterial Ca2+ signaling and arterial reactivity [64, 82]. In particular, we found that inhibition of non-selective cation channels with SKF96365 blocked about one-half of the serotonin-mediated contraction above the portion sensitive to the L-type Ca2+ channel inhibitor nifedipine [64]. This pharmacological evidence is imprecise, but suggests that canonical transient receptor potential channels (TRPC) are important for this Ca2+ influx pathway [83]. Indeed, TRPC1, TRPC3, TRPC5 and TRPC6 are expressed in fetal sheep pulmonary arteries [82]. These data illustrate that voltage-dependent and non-selective cation channels coordinate to regulate Ca2+ signaling and arterial contraction. Nevertheless, detailed studies are required to resolve the mechanistic changes that occur during fetal development, their importance during the transition period at birth, as well as the impact of antenatal hypoxia. Together the Ca2+ influx across the plasma membrane combines with Ca2+ release from the sarcoplasmic reticulum to cause global Ca2+ responses. The magnitude of this Ca2+ response governs the degree of myosin light chain kinase (MLCK) activation and in coordination with sensitization mechanisms this regulates the strength of pulmonary arterial contraction [84].
Ca2+ independent contraction
Sensitization of the myosin light chain to increases in cytosolic Ca2+ is a fundamental feature of arterial contractility. We routinely observe this phenomenon in fetal sheep pulmonary arteries. For example, roughly one-quarter of the contractile response to 5-HT remains in sheep fetal pulmonary arteries after we remove extracellular Ca2+ [64]. Sensitization is achieved largely through a convergence in signaling that reduces myosin light chain phosphatase (MLCP) activity. This allows for increased MLC20 phosphorylation by MLCK, which enhances contraction. These sensitization responses are largely due to RhoA/ROCK pathways as is illustrated in our recent studies that show RhoA/ROCK contributes substantially to depolarization mediated pulmonary arterial contraction in fetal sheep [63]. The importance of sensitization is substantiated by other recent studies establishing that inhibition of RhoA/ROCK reduces pulmonary vascular resistance in fetal lambs [85, 86]. Together these studies demonstrate the significance of Rho-kinase and point to its therapeutic potential for treatment of pulmonary vascular disease in the newborn.
In addition to Rho-kinase, studies performed in newborn piglets and adult humans and rats show that Protein kinase C (PKC) also causes pulmonary artery (PA) contraction [87, 88]. Following receptor stimulation, DAG produced through PLC cleavage activates Protein Kinase C (PKC) and then the 17 kDa PKC-potentiated inhibitory protein of PP1 (CPI-17). This signaling cascade acts to reduce the function of protein phosphatase 1C (PP1C), a catalytic protein that promotes myosin light chain phosphatase activity. We were unable to find any details regarding a role of PKC in fetal pulmonary arterial contractility, although the data from the newborn piglet suggests there is a reduction in one particular PKC isoform (PKC zeta) within two weeks following birth [87]. Notably, PKC function and the influence of antenatal hypoxia have been extensively studied in the fetal sheep cerebral vasculature [89, 90], where the function of various isoforms changes substantially. Although the pulmonary and cerebral vasculatures develop at different rates and are distinct functionally these data illustrate that examinations of PKC in the fetal pulmonary vasculature are an open area for investigation.
Pulmonary vascular transition at birth
During the transition from intra-uterine to extra-uterine life, significant changes occur. Most importantly, PVR decreases dramatically immediately after birth due to increased vessel lumen diameter and decreased wall thickness. These changes are partly mediated by cytoskeleton remodeling that occurs in both endothelial and pulmonary artery smooth muscle cells as seen in newborn piglets [91]. There is also a contribution of fetal lung fluid-removal by the alveolar epithelium as observed in newborn rabbits [92]. Interestingly, the exact mechanism that initiates pulmonary vasodilation or how the pulmonary artery endothelium or smooth muscle cells are targeted is not entirely known. The prevailing theory is that initial lung expansion and subsequent rhythmic distention as well as increased oxygen tension, trigger a series of events that result in pulmonary arterial vasodilation (Fig. 3). Endothelial cell maturation and nitric oxide (NO) production are also important, including the release of vasodilatory substances (NO, PGI2) and the inhibition of vasoconstrictive signals [93-95]. There is also the potential for release and activation of other endothelium derived hyperpolarization factors (EDHF) including carbon monoxide and activation of endothelial K+ channels that can hyperpolarize the smooth muscle through myo-endothelial gap junctions [96-100]. The close relationship between elevated oxygen tension and increased NO production [101], such as occurs when the newborn takes its first breath, will facilitate pulmonary arterial vasodilation at birth.
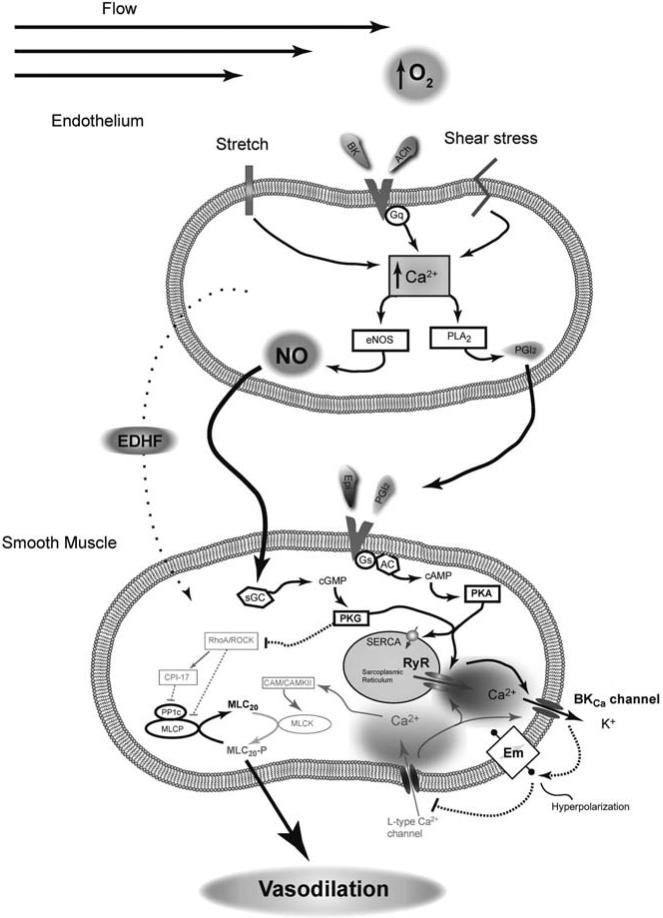
Fig. (3). Pulmonary vasodilation at birth is orchestrated.
Mechanical forces due to breathing, combined with increases in vascular flow and blood oxygenation act to dilate vessels of the lung. Vasodilatory substances, shear stress and membrane stretch work together to increase prostacyclin (PGI2), nitric oxide (NO) production, and other pathways defined broadly as endothelial derived hyperpolarizing factors (EDHF) that have not been fully examined in the fetus. Signaling molecules released from the endothelium act in concert with epinephrine (Epi) and other neuro-humoral substances to increase Protein Kinase A and G activity (PKA and PKG). These kinases phosphorylate a wide array of different substrates to impinge on vascular contraction. As discussed, antenatal hypoxia depresses a number of these vasodilatory signals which leads to maintenance of vasoconstriction. Solid line with arrow: Activation pathway, Dashed line with bar: Inhibition pathway.
Oxygenation and NO are critical components to vessel dilation [93, 102-106] and the attendant reductions in pulmonary vascular resistance that occur during the transition from intra-uterine to extra-uterine life [107]. Studies performed primarily by Dr. David Cornfield show that RyRs couple to BKca in the fetal pulmonary vasculature and are activated by NO and oxygen signaling [68, 108-111]. The portion of the signaling cascade leading from RyR activation to vessel dilation and the decrease in pulmonary vascular resistance shown in (Fig. 3) is based on this series of studies [68, 70, 108-112]. This pathway is backed by experiments performed in the cerebral vasculature [113-116]. Collectively, these studies show that RyR activation stimulates BKCa channels. Activation of the BKCa channels promotes vasodilation through membrane hyperpolarization, inhibition of L-type Ca2+ channels, and depression of cytosolic Ca2+, which results in vasodilation [117].
Nitric oxide also causes vasodilation through other pathways. Notably, Rho-kinase regulation by protein kinase G (PKG) downstream from nitric oxide is a fundamental regulator of vasomotor tone [118]. Indeed suppression of Rho-kinase activity contributes to PKG mediated vasorelaxation in vessels from fetal sheep [118], indicating its importance during the transition at birth.
IMPACT OF ANTENATAL HYPOXIA ON PULMONARY FUNCTION
The respiratory system exhibits significant developmental plasticity throughout the early stages of life. This makes the lung vulnerable to threatening factors such as maternal nutritional deficiencies, placental insufficiency, smoking, and gestation at high altitude. The timing of these extrinsic stressors is important when considering the ultimate effect on lung function [119]. One common theme among these stressors, including living at high altitude, is that they can cause intrauterine growth retardation. In turn, this can have profound and long-lived consequences into adulthood.
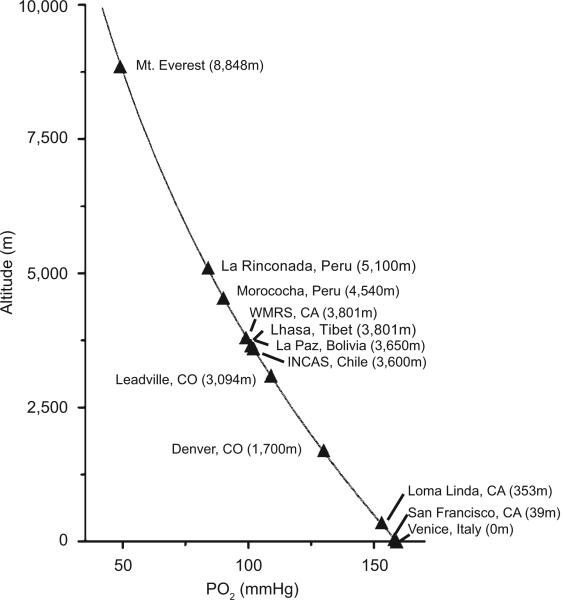
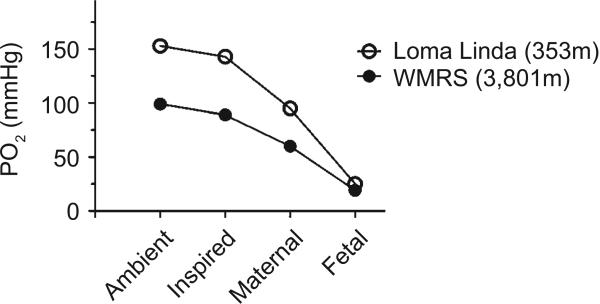
There is a fundamental relationship between altitude and O2 in the ambient air. Although the O2 percentage in the air remains constant at 20.9%, the partial pressure of oxygen (PO2) falls with increasing altitude. The relationship between ambient PO2 and altitude, which is governed by Boyle's law, is presented in (Fig. 4). However the inter-relationships between the inspired PO2, the mother's alveolar PO2 that determines arterial PO2, and the ability to deliver oxygen to the fetus through the placenta are more important, as shown in (Fig. 5). Based on the oxyhemoglobin saturation curve of the adult, once the mother travels to high altitudes both maternal and fetal arterial partial pressure of oxygen (PaO2) falls, such that O2 delivery to the fetal tissues becomes increasingly compromised as altitude increases.
Fig. (4). High-altitude travel is accompanied by reduced ambient PO2.
Human infants are born at altitudes ranging from sea level to about 5,100 m. We highlight work from two high altitude field stations that are at elevations similar to high-altitude cities in Tibet and Bolivia. This graph is based on Boyle's law, where the partial pressure of a gas is inversely related to the altitude.
Fig. (5). High-altitude decreases maternal and fetal arterial PO2.
Values were obtained for sheep at altitudes simulating the ambient PO2 for the Barcroft facilities at the White Mountain Research Station [WMRS]. The maternal PaO2 is considerably higher than that of the fetus at low altitude and more greatly influenced by exposure to high altitude.
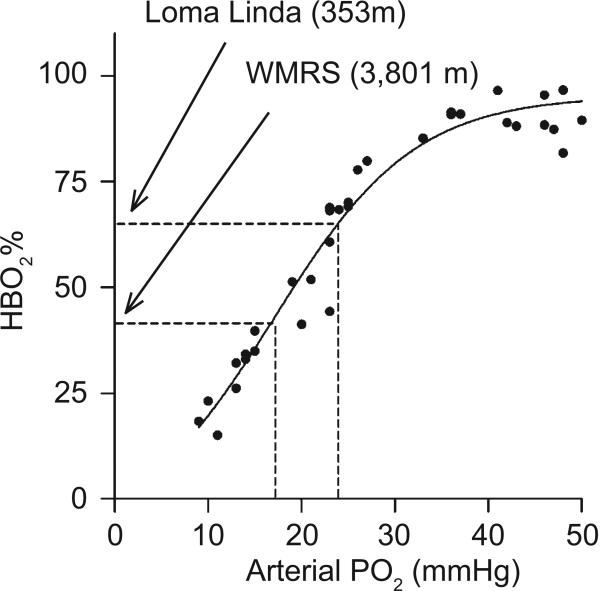
Previous studies from our group demonstrate that the PaO2 for fetal sheep near sea level is roughly 23 mmHg, which is substantially less than the maternal PaO2 that is close to 100 mmHg [120]. Despite the marked drop in the PaO2 across the placenta, fetal hemoglobin is roughly 70% saturated with oxygen, as illustrated in (Fig. 6). Despite this low oxygen tension relative to the adult, fetal oxygen delivery is facilitated and lung growth and development requirements are met because fetal hemoglobin has a higher oxygen affinity than adult hemoglobin [121].
Fig. (6). Relationship between fetal brachial artery PO2 and oxyhemoglobin saturation (HbO2) from fetal sheep.
Blood samples were collected from chronically instrumented fetuses while the ewe was breathing an FiO2 of 0.12 to 0.21, or within three hours after cesarean section followed by mechanical ventilation with FiO2 adjusted to achieve PaO2 ranging between 25 and 50 mmHg. All lambs had PaCO2 and pH within normal range. Dashed lines denote brachial PaO2 and HbO2 levels for fetal lambs near sea level (Loma Linda) or at high altitude (Barcroft facilities at the White Mountain Research Station [WMRS]). Measurements were made in fetal lambs that were 127 to 130 days gestation.
Fetal hemoglobin is comprised of two alpha and two gamma chains, as opposed to the two alpha and two beta chains of adult hemoglobin. Slight structural differences in the gamma subunits shift the oxyhemoglobin dissociation curve to the left relative to the adult, which facilitates the transfer of oxygen from maternal to fetal blood at the placental interface. The unloading of oxygen from fetal hemoglobin in the fetal tissues is aided by relatively low O2 tensions in the tissues [121]. Because of these lower arterial and tissue oxygen tensions the fetus is acutely sensitive to reductions in maternal PaO2.
Decreases in environmental oxygen tension translate into even further reduced PaO2, as illustrated in (Fig. 6). For example, reducing inspired PO2 to simulate the altitude of the Barcroft facilities at White Mountain Research Station (WMRS) in California (3,801 m) causes maternal PaO2 to equilibrate to ~60 mmHg while the fetal PaO2 decreases from 23 to 19 mmHg [120]. Importantly, although the magnitude of the decrease in maternal arterial PO2 is greater than that of the fetus, fetal oxyhemoglobin saturations are substantially more compromised. As shown in (Fig. 6), even at sea level fetal hemoglobin saturations are in the steep portion of the hemoglobin saturation curve. This means that small declines in PaO2 cause precipitous drops in hemoglobin saturation. Travel from sea level to WMRS causes maternal oxyhemoglobin saturations to decrease from ~98% to ~85% while fetal oxyhemoglobin saturations decrease from about ~70% to 40%. Despite the fall in oxyhemoglobin saturations, the arterial oxygen content in the fetus does not fall significantly because hemoglobin concentrations increase. However, the PO2 in the fetal tissues is still lower at high altitude [120], which can have detrimental effects as will be described in this section.
Animal models for antenatal hypoxia
Some of the commonly used mammalian models of antenatal and perinatal hypoxia include cows [122], sheep [63, 118, 123, 124], rats [125], mice [126], piglets [127, 128], guinea pigs [129], and one group often uses llamas [124]. The etiology of PH in each model is unique. Finding consistencies and differences in the molecular mechanisms responsible for inducing PH or acclimating to the rarified environment is likely to unveil seminal pathways important to disease progression. But still, we cannot generalize the findings from one model to another, and this impacts our ability to extrapolate and translate laboratory studies to treatment of newborn patients.
Diversity in acclimatization
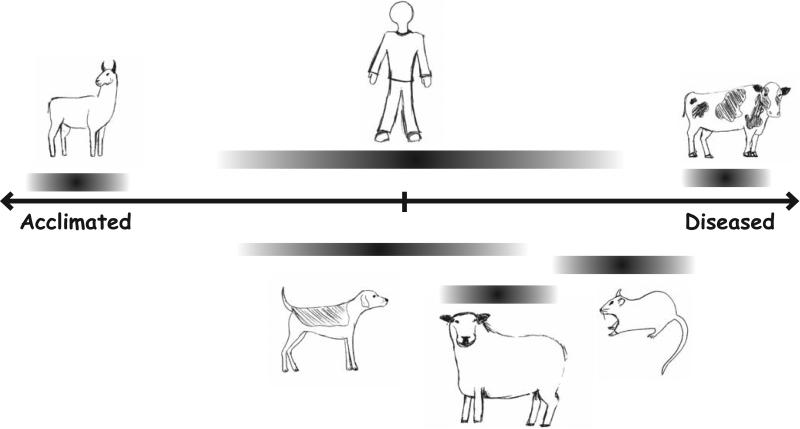
The ability of antenatal hypoxia to induce varied responses is embodied by the dramatic differences between adapted and afflicted species, which parallels human populations that respond differently to antenatal high altitude stress (Fig. 7). This ranges from species that live and thrive at high altitudes, such as llamas and yaks, to low altitude animals somewhat less tolerant to high altitude, such as sheep, or those with an exacerbated response to high altitude, such as cows, mice, and Fawn-Hooded (FH) rats. Newborn and adult animals from responsive species have substantial structural and functional transformations within 2 weeks of being placed in low oxygen chambers [125, 127, 130-132].
Fig. (7). Acclimatization continuum for perinatal hypoxia-induced lung structure and function responses amongst commonly studied species.
As discussed in the text, there is not only a wide degree of variance between individual species but also there can be variability within species. Llamas and cows are on opposite ends of the spectrum and based on the published studies they have lower variability than rats, dogs, or humans. The responses of newborn sheep are less severe than rodents or cows but the dysfunctions are similar to those found in human infants. The length and placement of the bars provides a subjective ranking based on the amalgamation of information provided in the text. We considered the influence of antenatal hypoxia on: decrease of lung diffusion capacity, elevation in pulmonary artery pressures and subsequent hypertrophy of the right ventricle, muscularization of the small vessels and overall vascular remodeling, increased HPV response, increases or decreases in the reactivity of the vessels to agonists as well as endothelial mediated vessel dilation, evidence of intrauterine growth restriction, and survival.
The most comprehensive comparative study thus far was by Tucker et al. (1975), where the muscularization of the pulmonary arteries was found to correlate highly with the degree of pulmonary hypertension. Unfortunately, these studies were performed in adolescent and adult animals and thus have limited bearing for discussions of the impact of antenatal hypoxia [132]. Animal species that have lived at high altitude for many generations including llamas [124] and yaks [133] are adapted to birth in the rarified environment. The physiological adaptations can be specific to each species, but ultimately these animals do not exhibit the pulmonary vascular remodeling or elevated pulmonary arterial pressure responses that are indicative of pulmonary vascular disease at altitude. However, one of the best in vivo bio-markers of animals that adapt well to high altitude is an improvement in pulmonary gas exchange, as evidenced by increased carbon monoxide diffusing capacity (DLCO). Such an increase in DLCO has been observed in guinea pigs [134], and foxhounds [135], as well as in Tibetans native to high altitude [14]. The DLCO is a marker for high altitude acclimatization because it is a rather simple clinical test that provides a surrogate measure for the surface area available for gas exchange. Augmented DLCO in these animal models and in human populations would correlate with increased gas exchange surface area and more efficient oxygen uptake and carbon dioxide removal at the alveoli. This ability to more effectively exchange gasses would be particularly helpful at high altitude, where the partial pressure of oxygen is reduced.
Most humans as well as sheep and pigs do not acclimate very well to high altitude living. Humans in particular exhibit a wide range of responses ranging from little or no PH to those who have pulmonary pressures nearly as high as the systemic vasculature. Although little is known regarding the extent of pulmonary vascular remodeling in human infants following antenatal hypoxia, affected infants can exhibit elevated pulmonary vascular pressures and growth retardation [8, 10-12, 14, 136, 137].
In contrast to animal models that are diseased, yaks are uniquely adapted to high altitude. Yaks have a blunted vasoconstrictor response, suggesting improved lung blood flow and oxygenation under hypoxic conditions [133]. However, the adaptations are complex because their pulmonary arteries are smaller and their endothelial cells have attenuated endothelium-dependent vasodilator responses to acetylcholine (ACh). There are additional structural changes that may help prevent pulmonary hypertension including longer, wider and rounder pulmonary arterial endothelial cells, thinner-walled pulmonary vessels and reduced amount of pulmonary arterial smooth muscle cells, especially in vessels ≤ 100 microns. Although it has been suspected that similar adaptations may occur in humans who are native to high altitudes the data is, as yet, not overwhelming [138].
Antenatal hypoxia and airway development
In many species, high altitude hypoxia stimulates lung growth and retards thoracic wall development (dysanaptic growth) [139]. This process is balanced, whereby at moderate levels of hypoxia thoracic wall growth retardation may predominate, while at severe levels of hypoxia lung growth dominates [140]. Although gestational hypoxia can retard intrauterine growth of high altitude residents, Tibetan children living at altitude exhibit accelerated lung growth, with improved lung function, diffusing capacity and ventilation as compared to lowlanders [14]. These pulmonary changes are also accompanied by decreased blood viscosity and hemoglobin levels, and higher arterial oxygen saturation with rest and exercise and less loss of aerobic performance with increasing altitude [14]. These adaptations to antenatal high altitude/hypoxic gestation, birth and living may represent a coupling of environmental and genetic factors [141] and confer an advantage from a gas exchange perspective.
In utero hypoxia that simulates an elevation of ~ 3,800 m (maternal FiO2 of 0.13) may alter alveolar development in Sprague-Dawley (SD) rats. In this animal model, alveoli are formed by septation mainly during the post-natal period resulting in larger mean alveolus volume and decreased total number of alveoli, similarly to humans [142]. These studies suggest there is a critical period of septation after birth, within the first 3 weeks of life, after which hypoxia does not alter septation and alveoli number, but may increase alveolar size [142, 143]. The latter is more consistent with the effect of high altitude hypoxia on human alveoli. Exposure of mice to hypoxia from birth to 2 weeks old, similarly impaired lung alveolarization and caused hypoxia-induced vascular remodeling and reduced lung compliance [144].
Guinea pigs and foxhounds have enhanced lung function when raised at high altitude. Weanling guinea pigs raised at high (3,800 m) compared to intermediate (1,250 m) altitudes had elevated lung volumes, increased alveolar-capillary surface areas and alveolar septal tissue volumes, with smaller alveolar duct volumes and harmonic thickness of the diffusion barrier [145]. These structural changes corresponded to the increased diffusing capacity of carbon monoxide (DLCO) mentioned earlier [134]. In a slightly different study, acclimatization of foxhound dogs for 5 months to high altitude (3,800 m) during development improved DLCO, without impacting ventilation/perfusion mismatching at 1 month, 2 years and 2.5 years after return to low altitude [135, 146], providing evidence for long-term perinatal programming of lung function.
Antenatal hypoxia and pulmonary vascular development
Many newborns that suffer significant antenatal hypoxia adapt poorly to breathing air, with abnormal modulation of pulmonary vascular myocyte and fibroblast growth and function. There is increased pulmonary vessel muscularization, as well as collagen and elastin deposition that decrease internal vessel diameter and peripheral arterial density (Fig. 1). Moreover, the antenatal hypoxic stress responsible for vessel malformation pre-disposes individuals to exaggerated responses with subsequent hypoxic insults, such as occurs when someone travels repeatedly between low and high altitudes [91, 139]. For example, when preterm and neonatal SD rats were exposed to hypoxia 3 days before and after birth, re-exposure to hypoxia at 2 weeks of age resulted in elevated right ventricular systolic pressures, RV hypertrophy, vascular pruning, and decreased radial alveolar counts compared to rats not exposed to perinatal hypoxia [147]. Subjecting these rats to hypoxic stress at 3-months old still caused pulmonary artery pressure increases, and vascular remodeling that altered pulmonary artery wall tensile stress. Initially pulmonary artery wall stresses increased, only to return to control levels in a few days after the vessel walls thickened and this correlated with changes in the elastic lamina ultrastructure [148]. Similar findings occurred in chronically hypoxic fetal sheep where the pregnant ewes were housed at 3,800 m during gestation. In these fetal sheep, the medial wall thickness was increased predominately in the distal portions of the pulmonary artery tree compared to controls [123, 149, 150]. Functionally, newborn sheep (~10 days old) born on the Andean altiplano (3,600 m) as well as WMRS (3,801 m), have PH and exacerbated hypoxic-induced pulmonary vasoconstriction [151, 224]. Although robust segmental analysis procedures to examine changes in lung structure have not been performed on either fetal or newborn sheep [152, 153], these results suggest that perinatal hypoxia causes substantial structural remodeling.
Fawn-Hooded rats are a unique strain, as newborn pups develop accelerated and severe PH when exposed to moderate hypoxemia and have spontaneous hypertension due to genetic problems with platelet aggregation [154-156]. The pulmonary hypertension response is exemplified by a series of studies that compared the effects of altitude on lung development of FH and SD rats. FH rats raised in Denver at ~1,600 m versus simulated sea-level during their gestation resulted in a decreased lung size and RV hypertrophy in the FH while the SD rats were unaffected. This was accompanied by alveolar simplification where the lungs from FH rats had larger and fewer air-spaces and thickened interstitium, without a significant difference in the level of epithelial cell maturation. These effects were not readily reversible because pulmonary arterial density was reduced in the FH rats later in life [157]. Although the malformations in FH rats are genetic and affect more than just the pulmonary vasculature [155, 158], they may be akin to human infants who appear diseased at altitude, but would be uniquely different from acclimated humans, and other species, where lung function can be enhanced. Thus, perinatal hypoxia causes a continuum of responses ranging from pathological states to enhanced functionality within human populations and among different animal species, something that also occurs in adult populations [159].
Antenatal and neonatal programming of the pulmonary vascular structures by living in hypoxic environments has been suggested for well over 40 years. In 1971 Goldberg and colleagues [160] showed that the relative medial wall thickness of small pulmonary arteries, 50-150 μm in diameter, was increased in newborn SD rats exposed to hypoxia. Expressed as the ratio of arterial media to total external diameter, this was significantly greater in pups whose mothers were maintained in a hypoxic environment (FiO2 of 0.13, ~3,800 m equivalent altitude) during their pregnancy as compared to room air (FiO2 of 0.21) or a hyperoxic environment (FiO2 of 0.40). This difference persisted at least 13 weeks after birth and was more pronounced in arteries under 80 μm, which are critical to PVR. Their finding supports the idea that the pulmonary vasculature undergoes development and remodeling in the fetus and newborn, and normally ceases in the young adult. From a developmental standpoint, more recent evidence suggests that branching morphogenesis, a key component of lung development, is also dependent on the environmental oxygen tension [161].
Antenatal hypoxia and effect on growth factors and transcriptional regulators
There are a wide array of growth factors and transcriptional regulators that precisely orchestrate lung development. While we do not fully understand how all of the players work together to grow a functional lung, we do know that antenatal hypoxia impinges on the function of some of them. Some of the growth factors affected by hypoxia include Hypoxia Inducible Factor (HIF) [162] and Vascular Endothelial Growth Factor (VEGF) [163], which will be discussed in a bit more detail. Other factors that are also likely to be significant include Fibroblast Growth Factor that is important during the pseudoglandular stage [164-166], Retinoic Acid whose signaling is crucial in early lung development and likely important to alveolarization [167], Bone Morphogenetic Protein (BMP) and BMP receptor 2 that have received substantial attention for their importance in familial pulmonary arterial hypertension [168], as well as TGF-β signaling that are both important to branching morphogenesis and alveolarization [169-171] (Fig. 1).
Hypoxia Inducible Factor
HIF is a transcription factor with three relevant isoforms that is regulated by oxygen availability, and subsequently modulates the expression of multiple genes. It's a heterodimer consisting of HIF-α and HIF-β subunits, with the former being increasingly stabilized and having higher transcriptional activity in hypoxic environments. Studies show that HIF-1α levels are elevated in human fetal lung while in utero [172]. The HIF system is therefore key to pulmonary development [162]. −/− HIF-1α knockout mice do not reach full term, with enlarged vascular structures and impaired lung morphogenesis [173]. Moreover, HIF-1α deficient (+/−) adult mice are somewhat protected from hypoxia. In these mice the increase in pulmonary arterial pressure and RV hypertrophy is attenuated compared to wild-type mice, which is mainly due to reduced pulmonary vascular remodeling [174]. Similar results were noted in HIF-2α deficient heterozygote mice [175].
Vascular Endothelial Growth Factor
VEGF and its receptor are critical mediators in pulmonary vascular remodeling under hypoxic conditions, and are dependent on HIF-1 activation [176]. In particular, hypoxia induces a more stable HIF-1α subunit, which after forming a heterodimer with HIF-1β becomes active HIF-1. The latter translocates to the nucleus where it regulates the transcription of many genes. This includes induction of VEGF and VEGF-receptor 1 [177, 178]. The importance of VEGF to lung development is supported by studies showing that inhibiting VEGF-receptor activity with Su-5416 in newborn rats, impairs pulmonary vascular growth, reduces arterial density, causes RV hypertrophy, and increases pulmonary artery wall thickness [179]. Related to this, VEGF-receptors are primarily responsible for endothelial cell differentiation and maintenance, as well as vascular organization and permeability in addition to other functions [140]. Thus, there is a direct link between tissue hypoxia, HIF-1 and VEGF, with evidence of significant alterations in vascular morphogenesis in response to hypoxic insults.
Nitric Oxide
NO signaling is one potential site of acclimatization to high altitude. In adult SD rats with hypoxia-induced PH, arterial endothelial Ca2+ metabolism becomes impaired resulting in endothelial NO synthase (eNOS) inactivity, decreased NO production and diminished endothelium-dependent relaxation [180]. However, in resident Tibetan populations NO production is increased through eNOS up-regulation, a response that may be part of the acclimatization process [181]. This compares with microvascular endothelial cells from fetal sheep cultured at a PO2 of 30-40 mmHg to mimic high altitude (>10,000 m equivalent altitude) [182]. Similarly, cultured human pulmonary arterial endothelial cells have significantly decreased eNOS activity, mRNA and protein expression, as well as mRNA half-life in the presence of hypoxia (PO2 of ~23 mmHg, >12,000 m equivalent altitude) effects that were reversed by Rho-kinase inhibition [183]. Yet, post-natal hypoxia equivalent to 5,800 m caused complex changes in eNOS expression in newborn piglets [184]. Hypoxic exposure in the first 3 days after birth reduced eNOS density. However, exposing naïve animals on days 3-6 after birth increased eNOS density whereas later exposure, on days 14-17, did not affect eNOS density [185]. The interaction between eNOS and heat-shock protein 90 (Hsp90) was examined in these hypoxic newborn piglets, where Hsp90 binding to eNOS downregulated its activity [186]. Chronic hypoxia can therefore restrict NO production through multiple pathways causing depression of eNOS expression as well as through altered interaction with binding partners that regulate function, and the extent of these influences may depend on the altitude.
Secondarily, decreased NO levels are associated with high altitude pulmonary edema (HAPE) susceptibility in humans. This further implicates the importance of NO signaling and acclimatization to high altitude [187]. Related to this, the pathogenesis of exacerbated vascular growth during the fetal and neonatal period and development of PH in FH rats exposed to chronic hypoxia also appears to be related to eNOS deficiency [188].
Carbon Monoxide
Carbon monoxide (CO) signaling is similar to nitric oxide in that this gas causes vasorelaxation [189-191]. As with nitric oxide, high altitude acclimatization alters CO production, though we know very little regarding the effects of antenatal hypoxia on this process. Carbon monoxide is produced through the activity of heme oxygenase (HO-1 and HO-2) and is thought to modulate a heme moiety that is attached to the BKCa channels. CO enhances the coupling between ryanodine receptor-generated Ca2+ signals and BKCa channels, which would accentuate vasodilation [190]. Long-term CO exposure, however, promotes vascular wall remodeling through increased production of growth factors and causes down regulation of NO production, which could impair vasodilation and promote vasoconstriction. Notably, adult llamas from the Andean altiplano have enhanced inducible heme oxygenase (HO-1) generated CO while sheep have reduced HO-1 expression and CO mediated vasodilation [99, 124]. These data illustrate that HO-1 and CO generation and function cause divergence between acclimated species and those at risk. This is certainly an important issue because HO-derived CO is likely to be important to pulmonary vascular tone as well as remodeling, and yet its importance in antenatal hypoxia-induced responses is presently unresolved.
Prostacyclin
PGI2 is released from the endothelium and is important to vessel dilation at birth [95, 192-194]. Unfortunately, we do not know much about the effects of antenatal hypoxia on its function, though hypoxia impedes its production in pulmonary vessels from fetal sheep [195]. In addition, newborn piglets exposed to simulated high altitude (ambient PO2 of 60-72 mmHg, ~6,200-7,500 m equivalent altitude) for 3 days had depressed PGI2 production and arachidonic acid mediated vasodilation [196]. However, work performed in adult mice shows that prostacyclin provides protection from chronic hypoxic stress by dilating vessels and attenuating vascular smooth muscle cell proliferation. This is illustrated with prostacyclin receptor knockout mice that exhibit a significantly greater degree of PH after exposure to hypoxia [197]. This finding also is relevant to the treatment of pulmonary arterial hypertension in humans. In these patients, remodeled pulmonary arterial smooth muscle cells may exhibit decreased PGI2 synthase expression [198]. This evidence suggests that chronic hypoxia can reduce prostacyclin function, at least in the newborn and adult, which can impact both pulmonary vascular remodeling and reactivity.
Endothelin-1
ET-1 is a critical signaling molecule in the fetus, and as discussed, helps to maintain high vascular tone before birth. This potent vasoconstrictor, as the name implies, is predominantly secreted by endothelial cells. ET-1 mediated vascular reactivity of pulmonary arteries is preserved in fetal sheep exposed to antenatal hypoxia (3,800 m) [118]. Although antenatal hypoxia may not disrupt ET-1 efficacy in the fetal sheep lung, chronic postnatal hypoxia does alter ET-1 signaling in newborn rats and sheep. Based on one series of studies in Wistar-Kyoto rats exposed to 10% O2 for 21 days (~5,800 m) ET-1 is believed to be associated with hypoxic-induced PH through pulmonary vascular remodeling, via mitogenic effects on vascular smooth muscle cells [199]. In newborn sheep born on the Andean Altiplano (3,600 m), but returned to low altitude, ET-1 potency was markedly enhanced and the efficacy was increased [100]. Moreover, hypoxia causes local ET-1 increases in the lung, and increases in pulmonary vascular pressure can elevate ET-1 levels in the serum. In piglets, chronic postnatal hypoxia (~10% O2, equivalent to ~5,800 m) causes persistently high circulating ET-1 levels, increased ETA-receptor density and binding throughout the entire pulmonary vasculature, without the normally seen transient post-natal ETB expression increase, a scenario that may impair vasodilation [200] (Fig. 3). Similarly, pulmonary arteries of chronically hypoxic piglets had as high as a 3-fold increase in contractile response to ET-1, a lack of ETB-NO-mediated vasodilatory response at 3 days and co-constriction with adjacent bronchi [201]. These findings suggest antenatal hypoxia is likely to cause complex changes in ET-1 signaling that may contribute to pulmonary vascular disease in the newborn.
Rho-Kinase
RhoA/Rho-kinase is important to vascular smooth muscle contraction and is activated in parallel with calcium signaling pathways by a variety of vasoreactive compounds including 5-HT, norepinephrine, and ET-1 [202] (Fig. 2). There are many steps to this signaling cascade and therefore multiple points at which antenatal hypoxia could alter its function. In fetal sheep, antenatal hypoxia causes selective upregulation of ROCK II expression and activity and is important to protein kinase G dependent vasorelaxation [118]. Based on our own recent studies performed in fetal sheep we know that Rho-kinase is also important to contraction due to membrane depolarization, but that antenatal hypoxia (3,800 m) does not alter its function [63]. In a chronic hypoxia neonatal SD rat model of PH (FiO2 of 0.13, ~3,800 m), RhoA/Rho-kinase activity and expression were increased. In rats with PH, Rho-kinase inhibition with Fasudil or Y-27632 reduced the elevated PVR, and yet the animals were un-responsive to inhaled NO or a systemic NO donor. In these animals, Rho-kinase inhibition failed to reverse RV dysfunction, although this was not necessarily unexpected since reversal of RV remodeling may require substantially more time once PVR is normalized [203].
Mast cells and high altitude pulmonary edema
Although the above signaling molecules and their pathways have been studied to a great extent, a growing body of literature focuses on potential inducers of these molecules and their linkages to immunological components. This follows a recent trend showing that immunological dysfunctions are critical to the pathogenesis of multiple diseases not previously thought to have a significant immunological basis. The role of mast cells to vascular remodeling via VEGF related pathways is of particular interest. For example, mast cells produce IL-8 and VEGF during asthma mediated pulmonary vascular remodeling in humans [204]. Mast cells are also important in high altitude related pathology and hypoxic conditions as highlighted by their increased numbers found in HAPE human victims post-mortem [205]. Asthma and high altitude both involve reducing the alveolar oxygen tension, which implicate mast cells as one common mediator in the process of pulmonary vascular growth and differentiation under hypoxic conditions, especially considering that mast cells release VEGF.
Endothelial barrier function
Most frequently when we consider the influences of high altitude the focus is on the direct effects of the rarified air on the ability to exchange gasses and the impact on vascular structure and reactivity with regards to blood flow. However, we also need to consider that the vascular structure is also critical to keeping the alveoli dry and the blood within the vessels. A characteristic example is acute respiratory distress syndrome, where the endothelial barrier is disrupted, resulting in fluid leakage from vessels to the alveoli. We actually know very little regarding the influences of antenatal hypoxia on the endothelial barrier function. However, chronic hypoxia can increase the endothelial permeability in porcine pulmonary arerial endothelial cell preparations. The increased permeability is important because the vascular leak allows growth factors and other serum products to move from the vascular lumen to the smooth muscle cell layer and into the alveoli [206]. Increased vascular permeability provides a pathway not only for causing pulmonary edema but also for the access of factors that cause vascular remodeling. Importantly, infants that are born at high altitude are at greater risk of developing high altitude pulmonary edema [141]. This suggests that children born at high altitude may have compromised endothelial cell function such that they are unable to properly maintain the endothelial barrier.
Antenatal hypoxia and transition at birth
In addition to vascular remodeling and reactivity to vasoactive substances after birth, antenatal hypoxia also affects the reduction in PVR with birth. Normally, when the newborn breathes at the time of birth there is a significant increase in PaO2, which increases NO, decreases ETA receptor expression and removes the hypoxic stimulus for hypoxic pulmonary vasoconstriction (HPV), which together reduce PVR [107]. Perinatal hypoxia impedes this process as illustrated in hypoxic newborn piglets where the PVR decrease at birth is attenuated [207]. This is compounded by an enhanced HPV response in antenatal hypoxia-exposed sheep born at 3,600 m [100, 151] or 3,801 m [224]. In the newborn lamb increases in PGI2 production counteract the negative effects of hypoxia [194], while newborn sheep born on the Andean altiplano have enhanced expression of eNOS, phosphodiesterase-5, and BKCa channels [100]. However, on balance, the factors that restrict pulmonary circulatory function at birth likely prevail. The resultant elevated PVR causes the fetal circulation to persist, induces a right to left shunt, slows closure of the ductus arteriosus, and leads to PH of the newborn [45].
Hypoxic induced pulmonary vasoconstriction and antenatal hypoxia
The ability of the pulmonary vasculature to constrict in response to acute hypoxia is an intrinsic process that is thought to shunt blood away from unventilated alveoli. This process maintains the ventilation to perfusion ratio, which ultimately improves gas exchange at ventilated alveoli and ensures oxygen uptake and carbon dioxide elimination. The intracellular processes that control the HPV response have remained somewhat elusive even though this has been the focus of intense research for well over a decade. There are excellent recent reviews as well as book chapters that cover the cellular mechanisms associated with hypoxic induced pulmonary vasoconstriction [208-210].
In brief, a decrease in the alveolar oxygen levels increases the activity of pathways that cause pulmonary artery smooth muscle contraction and reduces the activity of those pathways that promote vasodilation. Presently we can only presume that the mechanisms of HPV are the same in the fetus as they are in the adult, because systematic studies regarding HPV in the fetus have not been performed. Work from many laboratories, including our own, show that the process of hypoxia-induced pulmonary vasoconstriction is intrinsic to pulmonary arterial myocytes but that it is modulated by the endothelium. We provide the core elements associated with the effects of acute hypoxia on pulmonary arterial myocytes in (Fig. 2). The depression in oxygen tension initiates an early increase in cytosolic Ca2+, which is termed Phase 1 and lasts approximately 10-15 minutes [210, 211]. The cytosolic Ca2+ then decreases slightly and plateaus, maintaining an elevated level and transitions to the second phase. During this second phase there is an increase in Rho-kinase activity, which helps to sustain the contraction for a longer period. If hypoxia is maintained, as in high altitude fetuses, these and other processes regulate gene transcription that leads to vascular remodeling.
The hypoxia induced increase in cytosolic Ca2+ has been well studied in pulmonary arterial myocytes from adult mice, rats, dogs, and other species [210]. Based on contraction and Ca2+ imaging studies we know that the increase in cytosolic Ca2+ is mediated through a highly coordinated process. Activation of ryanodine receptors on the sarcoplasmic reticulum appears to be critical to the initial response to acute hypoxia and may help coordinate other components. Tantamount to the increase in cytosolic Ca2+ and ensuing contraction is entry of Ca2+ from outside the cell. Many studies have illustrated that acute hypoxia leads to depolarization of the plasma membrane and that contraction is highly reliant on L-type Ca2+ channels (CaL) [212]. One component to the membrane depolarization response is its dependency on inhibition of Kv channels [213, 214]. In addition, there is also a coordinated role for Ca2+ influx through a number of different cation channels including transient receptor potential channels [210, 215-218].
There is intense investigation into the oxygen sensing mechanisms that underlie HPV, and we expect these efforts to help us understand how PVR is maintained in utero. Substantial focus is placed on the ability of mitochondria to sense changes in oxygen tension, and regulate the generation of reactive oxygen species that influence ion channels and other enzymes critical to the HPV process [219, 220]. However, as recently reviewed [208, 210, 221], a large range of proteins are regulated by oxygen tension and generation of reactive oxygen species is just one of several possible mechanisms. The activity of some oxygen-sensitive proteins does not change until the oxygen tension is very low, (less than ~10-15 mmHg). This includes mitochondrial cytochromes and heme-oxygenase-2, which maintain normal activity until very low PO2 levels. A number of proteins, however, are sensitive to changes in oxygen tensions that may be found in arterial blood. This includes NADPH oxidase (NOX2), the activity of which changes gradually over a wide range of oxygen tensions [221]. In addition, hydrogen sulfide production in the lung and pulmonary arterial smooth muscle cells is inversely related to oxygen tension, and there is evidence that this gas is important to HPV responses [221]. Understanding how these and other oxygen sensing pathways change during the course of development and whether or not antenatal hypoxia modifies their function is likely to provide new insights into pulmonary vascular reactivity in utero and the development of antenatal hypoxia induced pulmonary vascular hypertension in the newborn.
As discussed throughout this review, the responses of each species to antenatal hypoxia vary dramatically and range from animals and humans that can fully acclimate to life in a rarified environment, to those who have mild dysfunction, and to those with severe disease. The results of studies that examined vessel reactivity were alluded to in previous sections because many substances that influence vessel remodeling often modulate vessel reactivity too. Therefore contractility responses to antenatal hypoxia cannot be entirely generalized across all species. This is further compounded because the neonatal pulmonary vasculature is immature and vasoactive substances have variable effect on vasomotor tone at different time periods during newborn life [222]. Vessel reactivity to various constrictor as well as dilator substances is often, but not always, attenuated in species that have chronic hypoxia induced pulmonary vascular disease. For example, chronic hypoxia in ovo diminishes chicken pulmonary arterial reactivity to potassium, norepinephrine, ET-1, and thromboxane A2. These animals also had higher mortality, reduced body mass and increased RV and LV wall area and thickness [223]. Yet, we also described studies illustrating that vessels from chronic hypoxic piglets have accentuated contractile responses to ET-1. Such hypoxic exposure also decreases eNOS activity in these piglets [184], which would potentiate vessel contractility.
Perinatal hypoxia-induced PH can program the lung for exaggerated HPV response. SD rats initially exposed to prenatal hypoxia (FiO2 of 0.11, ~5,100 m equivalent altitude) had decreased pulmonary arterial density, though not as severe as that described for FH rats. In this study, prenatal-exposed neonatal SD rats were placed in normoxic conditions for two weeks immediately after birth and then re-exposed to hypoxia two weeks later. Re-exposure of these previously hypoxic-stressed animals accentuated the RV pressure increase compared to rats that had not undergone the initial hypoxic exposure [147]. Our data also show that newborn sheep exposed to antenatal high altitude hypoxia with gestation at 3,801 m have exaggerated HPV responses when exposed acutely to an FiO2 of 0.10 [224]. Similarly, newborn sheep gestating at 3,600 m on the Andean altiplano also have exaggerated HPV responses when exposed to a FiO2 of 0.10 [100, 151]. Newborn infants born at extreme-altitudes (4,540 m Morococha, Peru), where the ambient PO2 is ~90 mmHg, exemplify the pulmonary vascular problems with birth at high altitude and illustrate that acute resolution of elevated pulmonary pressures cannot be uncoupled from remodeling of the cardio-pulmonary circuit. These infants had persistently high pulmonary arterial pressures, remaining at ~60 mmHg for the first 72 hours after birth. This contrasts with infants born at sea level where pulmonary arterial pressure falls from ~75 mmHg to ~20 mmHg over that time period. The pressure was inversely correlated to oxygen saturation, and administration of supplemental oxygen decreased pulmonary arterial pressures to sea-level values [10, 225]. Even still, the RV wall thickness is greater in infants born at high altitude throughout their first year of life compared to sea-level controls [226]. This phenomenon is indicative of persistent PH and may cause lifelong complications. Part of the problem is that when an infant is born at high altitude the change in oxygen tension from the maternal circulation to breathing air is less than in low altitude, thus minimizing the stimulus for vasodilation at birth. This may prolong pulmonary vasoconstriction in the infants pulmonary circulation, slow vessel relaxation and the reduction in pulmonary arterial pressure, contributing to increased prevalence of atrial septal defects [227].
Antenatal hypoxia and vasodilation
Antenatal and perinatal hypoxia can also suppress endothelium-dependent vasodilation. Endothelium-dependent pulmonary arterial relaxation was reduced in newborn piglets exposed to hypobaric hypoxia (~10% PO2, equivalent to ~5,800 m) [207]. What is more, chronic hypoxia (3-10 days at an FiO2 of 0.10 to 0.12, equivalent to ~ 4,500-5,800 m) dramatically altered pulmonary arterial responses to ACh in piglets. In this case, ACh dilated endothelium-intact vessels from control animals, compared to those exposed to chronic hypoxia, which exhibited constriction regardless of the status of the arterial endothelium. Interestingly, superoxide scavengers attenuated the vasoconstrictive response. This led the authors to suggest that chronic hypoxia increased NADPH-oxidase-produced reactive oxygen species, which are thought to be important to ACh dependent vasoconstriction [228]. In a similar series of experiments selective inhibition of cyclooxygenase-2 by NS-398, augmented dilatory responses to arachidonic acid in endothelium-denuded hypoxic pulmonary arteries, as well as diminished ACh-induced constriction and thromboxane production in denuded and non-denuded hypoxic pulmonary arteries from newborn piglets [196].
Ion channels and antenatal hypoxia
Perinatal hypertension and hypoxia cause diverse changes in the function of ion channels expressed on the plasma membrane; channels that are critical regulators of myocyte reactivity and contraction and can contribute to remodeling responses. Previously we provided an overview of the roles of ryanodine receptors, InsP3 receptors on the sarcoplasmic reticulum and non-selective, K+, and Ca2+ channels on the plasma membrane. Presently we know very little regarding the influence of high altitude gestation of ryanodine receptor and InsP3R signaling. Our initial studies suggest antenatal hypoxia reduces the number of cells that have Ca2+ responses before and during 5-HT stimulation [64], but the role of intracellular Ca2+ release and extracellular Ca2+ entry to this blunted response is unclear.
BKCa activation on pulmonary arterial myocytes induces vasodilation and is important to vascular relaxation with birth [68]. However, perinatal hypertension due to ductus arteriosus compression can reduce expression of BKCa channels and their function in the ovine fetal lung, which limits the ability of the arteries to relax [112, 229]. In comparison, 1-2 week old newborn sheep born at 3,600 m have increased BKCa expression [100]. Interestingly, BKCa expression is regulated by HIF-1 and therefore this transcription factor plays a key role in hypoxic-regulation of the O2 sensitivity of the perinatal pulmonary vasculature [117].
Kv channels act to maintain a negative resting membrane potential and are an important component to the vasoconstriction response to acute hypoxia [210]. Loss of expression or blocking channel activation causes membrane depolarization, calcium fluxes, and downstream pathway stimulation that lead to smooth muscle cell contraction as illustrated in (Fig. 2). In newborn piglets, extreme chronic hypoxia (ambient PO2 of 60-72 mmHg, ~6,200-7,500 m equivalent altitude) caused significant changes in pulmonary arterial myocyte membrane potential and Kv channel function [128]. Specifically, the membrane potential of hypoxia-exposed piglet pulmonary arteries was relatively depolarized when compared to their normoxic counterparts. This was accompanied by a selective decrease in Kv1.2 abundance, but not Kv1.5 or Kv2.1 [128]. Furthermore, the generalized importance of Kv channels to pulmonary arterial myocyte proliferation [230], suggests these changes will promote pulmonary vascular remodeling [128].
L-type Ca2+ channel function can also be changed by chronic intrauterine hypoxia. Newborn piglets exposed to severe hypoxia (FiO2 of 0.10, ~ 5,800 m equivalent altitude) had PH and elevated CaL channel-dependent reactivity and vascular tone compared to normoxic piglets [127]. In these hypoxic animals, nifedipine caused a greater decrease in transpulmonary perfusion pressure. This was also associated with an increased nifedipine-sensitive Ca2+ current density in patch voltage-clamped pulmonary artery smooth muscle cells of hypoxic animals compared to controls [127]. This may not occur in all species however. Our studies in pulmonary arteries from fetal sheep exposed to antenatal hypoxia (3,800 m) show that the role of CaL channels to potassium or 5-HT-mediated contraction is maintained, suggesting channel expression is preserved [63, 64].
The preceding paragraphs illustrate that although pulmonary vascular growth and vasodilation are dependent on highly organized systems, very little is known about how antenatal hypoxia influences their function. In particular, efforts have focused on just a few of the many potential players and pathways that are important to pulmonary vascular function and growth. Systematic exploration of the various kinase- and ionic- signaling pathways integral to pulmonary vascular function are therefore warranted.
CURRENT TREATMENT STRATEGIES
The treatment strategies for PH vary significantly based on the etiology of disease. Although there have been significant advances in the pharmacotherapy associated with PAH, most of the data available is for adult patients. Unfortunately, there are very few parallel studies for pediatric patients. This situation has led to the practice of extrapolating treatment strategies to pediatric patients based on data obtained from adults and on expert consensus [231, 232].
The goal of current treatment for pulmonary hypertension in newborns is the same as for patients of any age and is primarily to increase blood oxygenation, with recent reviews outlining these approaches in detail [233, 234]. In brief, these strategies are based on mimicking what should occur naturally during postnatal transition and in so doing reduce PVR. Often hypoxemia can precipitate pulmonary hypertensive crisis by causing vasoconstriction and increasing PVR, such as occurs when infants are born at high altitude. Neonates with mild respiratory distress may therefore only require minimal oxygen supplementation in order to resolve the crisis. The primary rationale is that by providing the oxygen that is required for vasodilation at birth the arteries will dilate and PVR will fall. The risk of oxygen toxicity is minimal when FiO2 is increased by administering supplemental O2 via nasal cannula, but this risk must be considered when prolonged therapy is required to maintain systemic oxygenation. Unfortunately, this type of oxygen therapy may only be beneficial to select infants with very mild disease and is of limited benefit in more serious cases of PH [234, 235].
Moderate to severe hypoxemia and respiratory distress may necessitate mechanical ventilatory support, including high-frequency oscillatory (HFO) ventilation. This method of ventilation carries less risk for barotrauma and atelectrauma compared to more traditional methods of ventilation, but continues to have significant risks, including lower respiratory tract infections, hypoventilation and respiratory acidosis and the need for neuromuscular blockade. Extracorporeal Membrane Oxygenation (ECMO) is generally a last resort, when all other measures of care are exhausted. The process is extremely invasive to the infant, requiring veno-venous access with large bore venous catheters or even veno-arterial access with cannulation of major arteries. The ECMO device, which works as a gas exchange filter, is simple in concept but carries numerous risks mainly associated with the cannulation of major vessels including bleeding, infection, and vascular damage, as well as hemolysis, need for transfusions, and thrombosis.
If oxygen support fails there are a number of therapeutic options that primarily act to induce vasodilation. These include inhaled NO (iNO), phosphodiesterase-5 inhibitors (e.g. sildenafil), prostanoids (eproprostenol and iloprost), endothelin inhibitors (bosentan), and phosphodiesterase-3 inhibitors (milrinone). Although these treatments mainly target the immediate issue of high PVR, some of these (e.g. bosentan) can also suppress vascular remodeling, which is a major component of long-term health problems.
In recent years, nitric oxide inhalation has become an approved treatment for primary pulmonary hypertension of the newborn (PPHN). This therapy improves hemodynamic and oxygenation profiles in multiple pediatric PH populations [236, 237] and lowers pulmonary arterial pressure, improves infant survival, and reduces the need for ECMO [238, 239]. However, approximately 30% of the infants do not respond to iNO. The reason for non-responders is not clear, but may be due to either a dysfunction in guanylate cyclase or PKG signaling. In some cases there may be severe pulmonary hypoplasia to the extent that even when the arteries are dilated there is not an adequate number of vessels to carry the required blood flow. Even for patients who are responsive to iNO, the high cost and technical administration challenges limit its use to mainly large urban medical centers in developed countries [240-244]. Inhibition of phosphodiesterase-5 with sildenafil works through the same signaling systems as iNO, and has fewer practical concerns, but is limited because it can also cause systemic hypotension. Additionally, it may induce non-selective pulmonary vascular relaxation, increasing shunt fraction in the lung, which would worsen oxygenation. Although this can be a significant issue in newborn infants that have low systemic arterial pressures, there is now evidence from placebo-controlled, randomized [245] and open-label, dose-escalation [246] trials in neonates with severe PPHN that phosphodiesterase-5 inhibitors can improve oxygenation index and survival.
Prostanoids such as iloprost are PGI2 mimetics that stimulate PKA dependent signaling. Likewise, phosphodiesterase-3 inhibitors, including milrinone, increase cAMP associated PKA activity. As illustrated in (Fig. 3), there is substantial overlap in the targets important to vasodilation that are phosphorylated by PKA and PKG. Although these similarities between PKA and PKG signaling can be used to improve patient outcome, these overlaps also have shortcomings because therapies that target phosphodiesterase-3 carry similar risks as those that target phosphodiesterase-5. Although there is data indicating that the use of prostanoids results in decreased mortality and improved function in adults with PAH, there are few studies looking into prostanoid therapy in pediatric PH populations. Two clinical studies, showed that prostanoid therapy improved survival in 35 children with idiopathic PAH [247] and improved hemodynamics in 20 children with PH due to cardiac defects [248]. Unfortunately, prostanoid therapy is most efficacious when administered continuously intravenously, something that is accompanied by multiple risks including indwelling central venous catheter associated infections and thrombosis. Alternative routes of administration have been developed and include continuous subcutaneous injection, which can cause significant site pain and infection, as well as inhaled mist treatments, which can cause bronchospasm and other upper airway symptoms. In addition, a significant drawback of prostanoid therapy, especially when using short-half-life intravenous infusions, is a desensitization-like effect. These patients require escalating doses and can have a significant rebound effect if the prostanoids are abruptly discontinued.
Endothelin receptor blockers comprise another class of drugs and act on pathways that are unique to many of the drugs mentioned above. Endothelin-1 binds to two distinct G-protein-coupled receptors (ETA or ETB) that are predominantly expressed on pulmonary vascular smooth muscle, resulting in activation of Rho-kinase and inositol triphosphate pathways that cause vasoconstriction. Bosentan, is an FDA-approved non-specific blocker of both ETA and ETB for the treatment of adult PAH patients [249]. Although there is some data for functional improvement and exercise capacity in children with either idiopathic or associated PAH [250, 251], the drug is not yet widely used in newborns. Nevertheless there are some early indications from a recent randomized, double-blind, placebo-controlled, prospective clinical trial, that bosentan may be an option for newborns with PPHN [252].
There is now a push for combination therapies. This is based on the understanding that multiple pathways and various receptors are part of PH pathophysiology and that monotherapies often result in higher drug doses and increased side-effects. The combination therapies are done with the use of two or three pharmacologic agents, each one from a different class of drugs. Based on studies in adults with PAH that have shown modest improvement in hemodynamics and/or exercise capacity, combination therapies are now being used to treat neonatal and pediatric patients, despite the lack of solid data for this patient population.
FUTURE TREATMENT STRATEGIES
An important consideration in the development and use of new therapies for newborns is that adult treatments cannot always be applied even though some overlap exists. Drugs such as sildenafil, iloprost, and bosentan are of clear relevance in the treatment of PH in adults [233, 234, 253]. Still, there are fewer options for hypertensive newborns, and infants do not always respond favorably to existing treatments. As indicated, as many as 30% of patients with persistent PH of the newborn do not respond to iNO [244]. Moreover, there are few novel therapeutic modalities currently in clinical trials as of June, 2012 [254], necessitating further expansion of our treatment armamentarium.
Future strategies will also need to focus on factors important to the long-term prognosis for infants including suppressing or reversing the arterial wall thickening associated with vascular remodeling. There are practical considerations with therapeutic development because pathways that cause severe remodeling are also critical for normal lung development. For example, inhibiting VEGF in newborn rats impedes normal vascular development and worsens PH and disease [179]. Rho-kinase inhibition can achieve pronounced pulmonary arterial vasodilation but does not affect pulmonary vascular development in newborns with antenatal hypoxia induced hypertension [125]. Such studies illustrate that treatments will need to ensure that lung development continues unabated while remodeling associated with disease is restricted.
There are a number of gene products and associated signaling pathways that have received attention for the treatment of PH in adults that may also hold promise for the treatment of antenatal hypoxia-induced PH in newborns. Particularly interesting is the tyrosine kinase receptor pathway inhibitor imatinib that is in phase III clinical trials for PH in adults [255]. This drug targets the platelet derived growth factor activation pathway and reduces growth and proliferation of smooth muscle. Thus, it has the potential of reversing the course of disease, but because the drug blocks pathways important for normal development, much like VEGF and Rho-kinase, it will need to be examined much further before it can be used for neonatal patients.
Rho kinase inhibition is another pathway that is of great interest, although there are side effects. The pronounced vasodilatory properties of fasudil and other Rho-kinase inhibitors showcase their potential for treatment of PH in newborns and adults [233, 256, 257]. A number of studies show Rho-kinase inhibitors reduce pulmonary arterial pressures in adult patients with idiopathic PH [258] as well as in newborn and adult animal models with PH [85, 125, 259-263].
There are numerous other therapeutic targets for future development including ion channels that are known to regulate the membrane potential of fetal and newborn tissues such as BKCa [68], as well as others that have yet to be explored in fetus including calcium activated chloride channels [72, 264-266]. Channels that regulate intracellular Ca2+ including TRP channels [267], ryanodine receptors and InsP3 metabolism and receptors [76] are important to consider because they regulate contraction as well as cell growth and proliferation. There are also numerous transcriptional regulators that hold future therapeutic promise with HIF-1α receiving the greatest attention [162]. Other transcription factors are important too, including NFkB that is activated by PDGF and other growth factors [268] as well as NFAT that is important for vascular remodeling [126, 269] and is stimulated by calcineurin through calcium signals downstream from TRP channel activation [270]. Gaseous mediators other than NO are also significant. Carbon monoxide signaling is upregulated in llamas at high altitude [99, 100, 271], and hydrogen sulfide (H2S) is an important mediator for HPV responses [221] and protects adult rats from hypoxic induced pulmonary arterial remodeling [272].
Selective Drug Delivery to the Lung
Strategies that target drugs to lung tissues and cells are important for the future of therapeutics, since they will decrease undesirable systemic effects. Nebulization and aerosolization techniques are being used and developed for this purpose. Although the adaptive aerosol delivery techniques currently available remain imperfect [273], they can be used clinically in neonates to provide surfactant, induce bronchodilation, and provide diuretics [274]. These delivery techniques can be used in conjunction with nanoparticles, which are being formulated to deliver a wide range of drugs and therapies from nifedipine to gene therapy targeting VEGF [275-277]. Designer peptides using targeting residues to focus drugs with widespread systemic effects, such as Rho-kinase inhibitors, to select cells is extremely promising. Recently, researchers designed a cyclic peptide, CARSKNKDC that selectively targeted active vascular remodeling in rat lungs due to monocrotaline or the combination of SU5416 and hypoxia [278]. Such selective drug targeting and delivery is ideal, since it combines therapeutic remodeling prevention with preservation of normal lung development.
PERSPECTIVE
In this review we provided evidence that antenatal hypoxia, which represents a significant and worldwide problem, causes prenatal programming of the lung. We presented relevant background regarding lung development and the impact antenatal hypoxia has on that process. We also provided an overview of some of the transcriptional and signaling systems of the lung that are impacted by antenatal hypoxia and are likely important for the fetal origins of hypoxia-related disease after birth. There are relatively few studies regarding the impact of antenatal stress on pulmonary vascular remodeling and reactivity, but the studies that have been performed illustrate there is a continuum of responses. Fetuses and newborns of certain species and specific human populations are well acclimated to the rarified environment. However, antenatal hypoxia causes pulmonary vascular disease in most examined mammalian species and humans, which can range from mild pulmonary vascular pressure increases, to severe vascular remodeling and dangerous elevations in pulmonary artery pressure. The effects of antenatal hypoxia are highly dependent on the timing, length, and magnitude of the stress, but there is also a strong gene-environmental relationship that is not yet completely understood. Determining the origins of pulmonary vascular remodeling and PH and their associated effects is a formidable task, but is necessary in order to develop targeted therapies that can both treat the symptoms and curtail or reverse disease progression.
ACKNOWLEDGEMENTS
The authors would like to thank Rachael Wilson for providing artwork that appears in Figs. 1 and 7.
FUNDING
A portion of this material was performed in the Loma Linda University School of Medicine Advanced Imaging and Microscopy Core that is supported by the National Science Foundation under Major Research Instrumentation, Division of Biological Infrastructure Grant No. 0923559 (SM Wilson) and the Loma Linda University School of Medicine. The work was also supported in part by USPHS Grants HD069746 (SM Wilson), HL095973 (AB Blood), and HD/HL-03807 and HD-31226 (LD Longo).
ABBREVIATIONS
- 5-HT
Serotonin
- AC
Adenylate Cyclase
- ACh
Acetylcholine
- BMP
Bone Morphogenetic Protein
- BK
Bradykinin
- BKCa
Calcium-and voltage-activated K+ Channel
- CaL
L-type Ca2+ channel
- CAM/CAMKII
Calmodulin and Calmodulin kinase type II
- ClCa
Ca2+-dependent chloride conductance
- CO
Carbon monoxide
- CPI-17
17 kDa PKC-potentiated inhibitory protein of PP1
- DAG
Diacylglycerol
- DLCO
Carbon monoxide diffusing capacity
- ECMO
Extracorporeal Membrane Oxygenation
- EDHF
Endothelium derived hyperpolarization factors
- eNOS
Endothelial NO synthase
- Epi
Epinephrine
- ET1
Endothelin-1
- ETA/ETB
Endothelin-1 receptors A and B
- FH
Fawn-Hooded
- Gq/Gs
G-protein alpha-subunit
- GPCRs
G-protein coupled receptors
- HAPE
High altitude pulmonary edema
- HFO
High Frequency Oscillatory Ventilation
- HIF
Hypoxia Inducible Factor
- HO-1 and HO-2
Heme oxygenase -1 and -2
- HPV
Hypoxic pulmonary vasoconstriction
- Hsp90
Heat shock protein 90
- INCAS
International Center for Andean Studies, Chile
- iNO
Inhaled NO
- InsP3
myo-inositol 1,4,5-trisphosphate
- InsP3R
InsP3 receptors
- Kv
Voltage-gated K+-channels
- LV
Left Ventricle
- MLC20
20 kDa myosin light chain
- MLC20-P
Phosphorylated isoform of 20Kd myosin light chain subunit
- MCLK
Myosin light chain kinase
- MLCP
Myosin light chain phosphatase
- NFAT
Nuclear Factor of Activated T Cells
- NO
Nitric oxide
- NOX2
NADPH oxidase
- NSCC
Non-selective cation channels
- PA
Pulmonary artery
- PAF
Platelet activating factor
- PAH
Pulmonary Arterial Hypertension
- PaO2
Arterial partial pressure of oxygen
- PGF2α
Prostaglandin F2α
- PGI2
Prostacyclin I2
- PH
Pulmonary Hypertension
- PKA
Protein kinase A
- PKC
Protein kinase C
- PKG
Protein kinase G
- PLC
Phospholipase C
- PO2
Partial pressure of oxygen
- PP1C
Protein phosphatase 1C
- PVR
Pulmonary Vascular Resistance
- RhoA
Ras homolog gene family member A
- ROCK
Rho-associated, coiled-coil containing protein kinase
- RV
Right Ventricle
- RyR
Ryanodine receptor
- SD
Sprague-Dawley
- SERCA
Sarcoplasmic endoplasmic reticulum Ca2+ ATPase
- TGFβ
Transforming growth factor-β
- TRPC
Canonical transient receptor potential channels
- VEGF
Vascular Endothelial Growth Factor
- WMRS
White Mountain Research Station
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Steinhorn RH. Neonatal pulmonary hypertension. Pediatr Crit Care Med. 2010;11(2 Suppl):S79–84. doi: 10.1097/PCC.0b013e3181c76cdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Osterman MJ. Births: final data for 2008. Natl Vital Stat Rep. 2010;59(1):1, 3–71. [PubMed] [Google Scholar]
- 3.Walsh-Sukys MC, Tyson JE, Wright LL, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics. 2000;105(1 Pt 1):14–20. doi: 10.1542/peds.105.1.14. [DOI] [PubMed] [Google Scholar]
- 4.Niermeyer S, Andrade Mollinedo P, Huicho L. Child health and living at high altitude. Arch Dis Child. 2009;94(10):806–11. doi: 10.1136/adc.2008.141838. [DOI] [PubMed] [Google Scholar]
- 5.Greaves L. Sifting the evidence: Gender and tobacco control. World Health Organization. 2007:1–40. [Google Scholar]
- 6.Weissmann N, Nollen M, Gerigk B, et al. Downregulation of hypoxic vasoconstriction by chronic hypoxia in rabbits: effects of nitric oxide. Am J Physiol Heart Circ Physiol. 2003;284(3):H931–8. doi: 10.1152/ajpheart.00376.2002. [DOI] [PubMed] [Google Scholar]
- 7.Scherrer U, Allemann Y, Jayet PY, Rexhaj E, Sartori C. High altitude, a natural research laboratory for the study of cardiovascular physiology and pathophysiology. Prog Cardiovasc Dis. 2010;52(6):451–5. doi: 10.1016/j.pcad.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Niermeyer S, Yang P. Shanmina, Drolkar, Zhuang J, Moore LG. Arterial oxygen saturation in Tibetan and Han infants born in Lhasa, Tibet. N Engl J Med. 1995;333(19):1248–52. doi: 10.1056/NEJM199511093331903. [DOI] [PubMed] [Google Scholar]
- 9.Grunig E, Dehnert C, Mereles D, et al. Enhanced hypoxic pulmonary vasoconstriction in families of adults or children with idiopathic pulmonary arterial hypertension. Chest. 2005;128(6 Suppl):630S–3S. doi: 10.1378/chest.128.6_suppl.630S-a. [DOI] [PubMed] [Google Scholar]
- 10.Niermeyer S. Cardiopulmonary transition in the high altitude infant. High Alt Med Biol. 2003;4(2):225–39. doi: 10.1089/152702903322022820. [DOI] [PubMed] [Google Scholar]
- 11.Niermeyer S. Going to high altitude with a newborn infant. High Alt Med Biol. 2007;8(2):117–23. doi: 10.1089/ham.2007.1068. [DOI] [PubMed] [Google Scholar]
- 12.Moore LG, Niermeyer S, Zamudio S. Human adaptation to high altitude: regional and life-cycle perspectives. Am J Phys Anthropol. 1998;(Suppl 27):25–64. doi: 10.1002/(sici)1096-8644(1998)107:27+<25::aid-ajpa3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Marconi C, Marzorati M, Cerretelli P. Work capacity of permanent residents of high altitude. High Alt Med Biol. 2006;7(2):105–15. doi: 10.1089/ham.2006.7.105. [DOI] [PubMed] [Google Scholar]
- 14.Wu T, Kayser B. High altitude adaptation in Tibetans. High Alt Med Biol. 2006;7(3):193–208. doi: 10.1089/ham.2006.7.193. [DOI] [PubMed] [Google Scholar]
- 15.Penaloza D, Arias-Stella J. The heart and pulmonary circulation at high altitudes: healthy highlanders and chronic mountain sickness. Circulation. 2007;115(9):1132–46. doi: 10.1161/CIRCULATIONAHA.106.624544. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Raj JU. Regulation of the pulmonary circulation in the fetus and newborn. Physiol Rev. 2010;90(4):1291–335. doi: 10.1152/physrev.00032.2009. [DOI] [PubMed] [Google Scholar]
- 17.Ten Have-Opbroek AA. The development of the lung in mammals: an analysis of concepts and findings. Am J Anat. 1981;162(3):201–19. doi: 10.1002/aja.1001620303. [DOI] [PubMed] [Google Scholar]
- 18.Hislop A, Reid L. Development of the acinus in the human lung. Thorax. 1974;29(1):90–4. doi: 10.1136/thx.29.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campiche MA, Gautier A, Hernandez EI, Reymond A. An Electron Microscope Study of the Fetal Development of Human Lung. Pediatrics. 1963;32:976–94. [PubMed] [Google Scholar]
- 20.Pringle KC. Human fetal lung development and related animal models. Clin Obstet Gynecol. 1986;29(3):502–13. [PubMed] [Google Scholar]
- 21.Hislop AA. Airway and blood vessel interaction during lung development. J Anat. 2002;201(4):325–34. doi: 10.1046/j.1469-7580.2002.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.deMello DE, Reid LM. Embryonic and early fetal development of human lung vasculature and its functional implications. Pediatr Dev Pathol. 2000;3(5):439–49. doi: 10.1007/s100240010090. [DOI] [PubMed] [Google Scholar]
- 23.Thurlbeck WM. Postnatal human lung growth. Thorax. 1982;37(8):564–71. doi: 10.1136/thx.37.8.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald JA. Lung growth and development. M. Dekker; New York: 1997. p. xviii.p. 740. [Google Scholar]
- 25.Harding R, Pinkerton KE, Plopper CG. The lung : development, aging, and environment. 1st ed. Academic Press; San Diego, CA: 2003. [Google Scholar]
- 26.Bancalari E, Polin RA. The newborn lung : neonatology questions and controversies. 2nd ed. Elsevier/Saunders; Amsterdam: 2012. [Google Scholar]
- 27.Hogan BL. Morphogenesis. Cell. 1999;96(2):225–33. doi: 10.1016/s0092-8674(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 28.Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977;14(1):53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- 29.Risau W, Flamme I. Vasculogenesis. Ann Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 30.Burri PH, Tarek MR. A novel mechanism of capillary growth in the rat pulmonary microcirculation. Anat Rec. 1990;228(1):35–45. doi: 10.1002/ar.1092280107. [DOI] [PubMed] [Google Scholar]
- 31.Hislop A, Reid L. Pulmonary arterial development during childhood: branching pattern and structure. Thorax. 1973;28(2):129–35. doi: 10.1136/thx.28.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hislop A, Reid L. Fetal and childhood development of the intrapulmonary veins in man--branching pattern and structure. Thorax. 1973;28(3):313–9. doi: 10.1136/thx.28.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shannon JM. Induction of alveolar type II cell differentiation in fetal tracheal epithelium by grafted distal lung mesenchyme. Dev Biol. 1994;166(2):600–14. doi: 10.1006/dbio.1994.1340. [DOI] [PubMed] [Google Scholar]
- 34.Spooner BS, Wessells NK. Mammalian lung development: interactions in primordium formation and bronchial morphogenesis. J Exp Zool. 1970;175(4):445–54. doi: 10.1002/jez.1401750404. [DOI] [PubMed] [Google Scholar]
- 35.Wessells NK. Mammalian lung development: interactions in formation and morphogenesis of tracheal buds. J Exp Zool. 1970;175(4):455–66. doi: 10.1002/jez.1401750405. [DOI] [PubMed] [Google Scholar]
- 36.Burri PH. Structural aspects of postnatal lung development - alveolar formation and growth. Biol Neonate. 2006;89(4):313–22. doi: 10.1159/000092868. [DOI] [PubMed] [Google Scholar]
- 37.Bucher U, Reid L. Development of the intrasegmental bronchial tree: the pattern of branching and development of cartilage at various stages of intra-uterine life. Thorax. 1961;16:207–18. doi: 10.1136/thx.16.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitaoka H, Burri PH, Weibel ER. Development of the human fetal airway tree: analysis of the numerical density of airway endtips. Anat Rec. 1996;244(2):207–13. doi: 10.1002/(SICI)1097-0185(199602)244:2<207::AID-AR8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 39.Hall SM, Hislop AA, Haworth SG. Origin, differentiation, and maturation of human pulmonary veins. Am J Respir Cell Mol Biol. 2002;26(3):333–40. doi: 10.1165/ajrcmb.26.3.4698. [DOI] [PubMed] [Google Scholar]
- 40.deMello DE, Sawyer D, Galvin N, Reid LM. Early fetal development of lung vasculature. Am J Respir Cell Mol Biol. 1997;16(5):568–81. doi: 10.1165/ajrcmb.16.5.9160839. [DOI] [PubMed] [Google Scholar]
- 41.Hall SM, Hislop AA, Pierce CM, Haworth SG. Prenatal origins of human intrapulmonary arteries: formation and smooth muscle maturation. Am J Respir Cell Mol Biol. 2000;23(2):194–203. doi: 10.1165/ajrcmb.23.2.3975. [DOI] [PubMed] [Google Scholar]
- 42.Hislop AA, Wigglesworth JS, Desai R. Alveolar development in the human fetus and infant. Early Hum Dev. 1986;13(1):1–11. doi: 10.1016/0378-3782(86)90092-7. [DOI] [PubMed] [Google Scholar]
- 43.Caduff JH, Fischer LC, Burri PH. Scanning electron microscope study of the developing microvasculature in the postnatal rat lung. Anat Rec. 1986;216(2):154–64. doi: 10.1002/ar.1092160207. [DOI] [PubMed] [Google Scholar]
- 44.Zeltner TB, Burri PH. The postnatal development and growth of the human lung. II. Morphology. Respir Physiol. 1987;67(3):269–82. doi: 10.1016/0034-5687(87)90058-2. [DOI] [PubMed] [Google Scholar]
- 45.Bancalari E, Polin RA. The newborn lung. 1st ed. Saunders/Elsevier; Philadelphia: 2008. p. xix.p. 487. [Google Scholar]
- 46.Perl AK, Whitsett JA. Molecular mechanisms controlling lung morphogenesis. Clin Genet. 1999;56(1):14–27. doi: 10.1034/j.1399-0004.1999.560103.x. [DOI] [PubMed] [Google Scholar]
- 47.Ten Have-Opbroek AA. Lung development in the mouse embryo. Exp Lung Res. 1991;17(2):111–30. doi: 10.3109/01902149109064406. [DOI] [PubMed] [Google Scholar]
- 48.Warburton D, El-Hashash A, Carraro G, et al. Lung organogenesis. Curr Top Dev Biol. 2010;90:73–158. doi: 10.1016/S0070-2153(10)90003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baker HJ, Lindsey JR, Weisbroth SH. The Laboratory rat. 2nd ed. Academic Press; New York: 2006. [Google Scholar]
- 50.Collins MH, Kleinerman J, Moessinger AC, Collins AH, James LS, Blanc WA. Morphometric analysis of the growth of the normal fetal guinea pig lung. Anat Rec. 1986;216(3):381–91. doi: 10.1002/ar.1092160307. [DOI] [PubMed] [Google Scholar]
- 51.Weichselbaum M, Sparrow MP. A confocal microscopic study of the formation of ganglia in the airways of fetal pig lung. Am J Respir Cell Mol Biol. 1999;21(5):607–20. doi: 10.1165/ajrcmb.21.5.3721. [DOI] [PubMed] [Google Scholar]
- 52.de Zabala LE, Weinman DE. Prenatal development of the bovine lung. Anat Histol Embryol. 1984;13(1):1–14. doi: 10.1111/j.1439-0264.1984.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 53.Maeda S, Suzuki S, Suzuki T, et al. Analysis of intrapulmonary vessels and epithelial-endothelial interactions in the human developing lung. Lab Invest. 2002;82(3):293–301. doi: 10.1038/labinvest.3780423. [DOI] [PubMed] [Google Scholar]
- 54.Schachtner SK, Wang Y, Scott Baldwin H. Qualitative and quantitative analysis of embryonic pulmonary vessel formation. Am J Respir Cell Mol Biol. 2000;22(2):157–65. doi: 10.1165/ajrcmb.22.2.3766. [DOI] [PubMed] [Google Scholar]
- 55.Parera MC, van Dooren M, van Kempen M, et al. Distal angiogenesis: a new concept for lung vascular morphogenesis. Am J Physiol Lung Cell Mol Physiol. 2005;288(1):L141–9. doi: 10.1152/ajplung.00148.2004. [DOI] [PubMed] [Google Scholar]
- 56.Boyden EA. The developing bronchial arteries in a fetus of the twelfth week. Am J Anat. 1970;129(3):357–68. doi: 10.1002/aja.1001290307. [DOI] [PubMed] [Google Scholar]
- 57.Boyden EA. The time lag in the development of bronchial arteries. Anat Rec. 1970;166(4):611–4. doi: 10.1002/ar.1091660407. [DOI] [PubMed] [Google Scholar]
- 58.Hislop A, Reid L. Intra-pulmonary arterial development during fetal life-branching pattern and structure. J Anat. 1972;113(Pt 1):35–48. [PMC free article] [PubMed] [Google Scholar]
- 59.Lewis AB, Heymann MA, Rudolph AM. Gestational changes in pulmonary vascular responses in fetal lambs in utero. Circ Res. 1976;39(4):536–41. doi: 10.1161/01.res.39.4.536. [DOI] [PubMed] [Google Scholar]
- 60.Fike CD, Hansen TN. Hypoxic vasoconstriction increases with postnatal age in lungs from newborn rabbits. Circ Res. 1987;60(2):297–303. doi: 10.1161/01.res.60.2.297. [DOI] [PubMed] [Google Scholar]
- 61.Accurso FJ, Alpert B, Wilkening RB, Petersen RG, Meschia G. Time-dependent response of fetal pulmonary blood flow to an increase in fetal oxygen tension. Respir Physiol. 1986;63(1):43–52. doi: 10.1016/0034-5687(86)90029-0. [DOI] [PubMed] [Google Scholar]
- 62.Storme L, Rairigh RL, Parker TA, Kinsella JP, Abman SH. In vivo evidence for a myogenic response in the fetal pulmonary circulation. Pediatr Res. 1999;45(3):425–31. doi: 10.1203/00006450-199903000-00022. [DOI] [PubMed] [Google Scholar]
- 63.Papamatheakis DG, Vemulakonda S, Patel J, et al. Depolarization-dependent contraction increase after birth and preservation following long-term hypoxia in sheep pulmonary arteries. Pulm Circ. 2012;2:41–53. doi: 10.4103/2045-8932.94832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goyal R, Papamatheakis DG, Loftin M, et al. Long-term maternal hypoxia: the role of extracellular Ca2+ entry during serotonin-mediated contractility in fetal ovine pulmonary arteries. Reprod Sci. 2011;18(10):948–62. doi: 10.1177/1933719111401660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Papamatheakis DG, Vemulakonda S, Blood Q, et al. Preservation of serotonin-mediated contractility in adult sheep pulmonary arteries following long-term high-altitude hypoxia. High Alt Med Biol. 2011;12(3):253–64. doi: 10.1089/ham.2010.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morin FC, 3rd, Egan EA, Ferguson W, Lundgren CE. Development of pulmonary vascular response to oxygen. Am J Physiol. 1988;254(3 Pt 2):H542–6. doi: 10.1152/ajpheart.1988.254.3.H542. [DOI] [PubMed] [Google Scholar]
- 67.Rasanen J, Wood DC, Debbs RH, Cohen J, Weiner S, Huhta JC. Reactivity of the human fetal pulmonary circulation to maternal hyperoxygenation increases during the second half of pregnancy: a randomized study. Circulation. 1998;97(3):257–62. doi: 10.1161/01.cir.97.3.257. [DOI] [PubMed] [Google Scholar]
- 68.Cornfield DN, Reeve HL, Tolarova S, Weir EK, Archer S. Oxygen causes fetal pulmonary vasodilation through activation of a calcium-dependent potassium channel. Proc Natl Acad Sci USA. 1996;93(15):8089–94. doi: 10.1073/pnas.93.15.8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shimoda LA, Sylvester JT, Booth GM, et al. Inhibition of voltage-gated K(+) currents by endothelin-1 in human pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2001;281(5):L1115–L22. doi: 10.1152/ajplung.2001.281.5.L1115. [DOI] [PubMed] [Google Scholar]
- 70.Cornfield DN, Saqueton CB, Porter VA, et al. Voltage-gated K(+)-channel activity in ovine pulmonary vasculature is developmentally regulated. Am J Physiol Lung Cell Mol Physiol. 2000;278(6):L1297–304. doi: 10.1152/ajplung.2000.278.6.L1297. [DOI] [PubMed] [Google Scholar]
- 71.Remillard CV, Lupien MA, Crepeau V, Leblanc N. Role of Ca2+- and swelling-activated Cl- channels in alpha1- adrenoceptor-mediated tone in pressurized rabbit mesenteric arterioles. Cardiovasc Res. 2000;46(3):557–68. doi: 10.1016/s0008-6363(00)00021-3. [DOI] [PubMed] [Google Scholar]
- 72.Forrest AS, Angermann JE, Raghunathan R, Lachendro C, Greenwood IA, Leblanc N. Intricate interaction between store-operated calcium entry and calcium-activated chloride channels in pulmonary artery smooth muscle cells. Adv Exp Med Biol. 2010;661:31–55. doi: 10.1007/978-1-60761-500-2_3. [DOI] [PubMed] [Google Scholar]
- 73.Mitchell C, Syed NI, Gurney AM, Kennedy C. A Ca(2+) - dependent chloride current and Ca(2+) influx via Ca(v) 1.2 ion channels play major roles in P2Y receptor-mediated pulmonary vasoconstriction. Br J Pharmacol. 2012;166(4):1503–12. doi: 10.1111/j.1476-5381.2012.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bao R, Lifshitz LM, Tuft RA, Bellve K, Fogarty KE, ZhuGe R. A close association of RyRs with highly dense clusters of Ca2+-activated Cl- channels underlies the activation of STICs by Ca2+ sparks in mouse airway smooth muscle. J Gen Physiol. 2008;132(1):145–60. doi: 10.1085/jgp.200709933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Janiak R, Wilson SM, Montague S, Hume JR. Heterogeneity of calcium stores and elementary release events in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol. 2001;280(1):C22–33. doi: 10.1152/ajpcell.2001.280.1.C22. [DOI] [PubMed] [Google Scholar]
- 76.Zhang WM, Yip KP, Lin MJ, Shimoda LA, Li WH, Sham JS. ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors. Am J Physiol Lung Cell Mol Physiol. 2003;285(3):L680–L90. doi: 10.1152/ajplung.00067.2003. [DOI] [PubMed] [Google Scholar]
- 77.Ibe BO, Ameer A, Portugal AM, Renteria L, Raj JU. Platelet-activating factor modulates activity of cyclic nucleotides in fetal ovine pulmonary vascular smooth muscle. J Pharmacol Exp Ther. 2007;320(2):728–37. doi: 10.1124/jpet.106.111914. [DOI] [PubMed] [Google Scholar]
- 78.Ibe BO, Portugal AM, Chaturvedi S, Raj JU. Oxygen-dependent PAF receptor binding and intracellular signaling in ovine fetal pulmonary vascular smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2005;288(5):L879–86. doi: 10.1152/ajplung.00341.2004. [DOI] [PubMed] [Google Scholar]
- 79.Goyal R, Creel KD, Chavis E, Smith GD, Longo LD, Wilson SM. Maturation of intracellular calcium homeostasis in sheep pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2008;295(5):L905–14. doi: 10.1152/ajplung.00053.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Remillard CV, Zhang WM, Shimoda LA, Sham JS. Physiological properties and functions of Ca(2+) sparks in rat intrapulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;283(2):L433–L44. doi: 10.1152/ajplung.00468.2001. [DOI] [PubMed] [Google Scholar]
- 81.Fike CD, Kaplowitz MR. Nifedipine inhibits pulmonary hypertension but does not prevent decreased lung eNOS in hypoxic newborn pigs. Am J Physiol. 1999;277(3 Pt 1):L449–56. doi: 10.1152/ajplung.1999.277.3.L449. [DOI] [PubMed] [Google Scholar]
- 82.Resnik ER, Keck M, Sukovich DJ, Herron JM, Cornfield DN. Chronic intrauterine pulmonary hypertension increases capacitative calcium entry in fetal pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292(4):L953–9. doi: 10.1152/ajplung.00327.2006. [DOI] [PubMed] [Google Scholar]
- 83.Alexander S, Harmar A, McGrath I. New updated GRAC Fifth Edition with searchable online version Launch of new portal Guide to Pharmacology in association with NC-IUPHAR Transporter-Themed Issue. Br J Pharmacol. 2011;164(7):1749–50. doi: 10.1111/j.1476-5381.2011.01751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hirano K, Hirano M, Kanaide H. Regulation of myosin phosphorylation and myofilament Ca2+ sensitivity in vascular smooth muscle. J Smooth Muscle Res. 2004;40(6):219–36. doi: 10.1540/jsmr.40.219. [DOI] [PubMed] [Google Scholar]
- 85.Parker TA, Roe G, Grover TR, Abman SH. Rho kinase activation maintains high pulmonary vascular resistance in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol. 2006;291(5):L976–L82. doi: 10.1152/ajplung.00512.2005. [DOI] [PubMed] [Google Scholar]
- 86.Tourneux P, Chester M, Grover T, Abman SH. Fasudil inhibits the myogenic response in the fetal pulmonary circulation. Am J Physiol Heart Circ Physiol. 2008;295(4):H1505–13. doi: 10.1152/ajpheart.00490.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cogolludo A, Moreno L, Lodi F, Tamargo J, Perez-Vizcaino F. Postnatal maturational shift from PKCzeta and voltage-gated K+ channels to RhoA/Rho kinase in pulmonary vasoconstriction. Cardiovasc Res. 2005;66(1):84–93. doi: 10.1016/j.cardiores.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 88.Savineau JP, Marthan R, Crevel H. Contraction of vascular smooth muscle induced by phorbol 12,13 dibutyrate in human and rat pulmonary arteries. Br J Pharmacol. 1991;104(3):639–44. doi: 10.1111/j.1476-5381.1991.tb12482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goyal R, Mittal A, Chu N, Arthur RA, Zhang L, Longo LD. Maturation and long-term hypoxia-induced acclimatization responses in PKC-mediated signaling pathways in ovine cerebral arterial contractility. Am J Physiol Regul Integr Comp Physiol. 2010;299(5):R1377–86. doi: 10.1152/ajpregu.00344.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goyal R, Mittal A, Chu N, Shi L, Zhang L, Longo LD. Maturation and the role of PKC-mediated contractility in ovine cerebral arteries. Am J Physiol Heart Circ Physiol. 2009;297(6):H2242–52. doi: 10.1152/ajpheart.00681.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hall SM, Gorenflo M, Reader J, Lawson D, Haworth SG. Neonatal pulmonary hypertension prevents reorganisation of the pulmonary arterial smooth muscle cytoskeleton after birth. J Anat. 2000;196(Pt 3):391–403. doi: 10.1046/j.1469-7580.2000.19630391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bland RD, McMillan DD, Bressack MA, Dong L. Clearance of liquid from lungs of newborn rabbits. J Appl Physiol. 1980;49(2):171–7. doi: 10.1152/jappl.1980.49.2.171. [DOI] [PubMed] [Google Scholar]
- 93.Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol. 1990;259(6 Pt 2):H1921–7. doi: 10.1152/ajpheart.1990.259.6.H1921. [DOI] [PubMed] [Google Scholar]
- 94.Cassin S, Winikor I, Tod M, et al. Effects of prostacyclin on the fetal pulmonary circulation. Pediatr Pharmacol. 1981;1(3):197–207. [PubMed] [Google Scholar]
- 95.Leffler CW, Hessler JR. Pulmonary and systemic vascular effects of exogenous prostaglandin I2 in fetal lambs. Eur J Pharmacol. 1979;54(1-2):37–42. doi: 10.1016/0014-2999(79)90405-9. [DOI] [PubMed] [Google Scholar]
- 96.Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch. 2010;459(6):863–79. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 97.Skovgaard N, Gouliaev A, Aalling M, Simonsen U. The Role of Endogenous H2S in Cardiovascular Physiology. Curr Pharm Biotechnol. 2011;12(9):1385–93. doi: 10.2174/138920111798280956. [DOI] [PubMed] [Google Scholar]
- 98.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch. 2010;459(6):881–95. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Herrera EA, Reyes RV, Giussani DA, et al. Carbon monoxide: a novel pulmonary artery vasodilator in neonatal llamas of the Andean altiplano. Cardiovasc Res. 2008;77(1):197–201. doi: 10.1093/cvr/cvm013. [DOI] [PubMed] [Google Scholar]
- 100.Herrera EA, Riquelme RA, Ebensperger G, et al. Long-term exposure to high-altitude chronic hypoxia during gestation induces neonatal pulmonary hypertension at sea level. Am J Physiol Regul Integr Comp Physiol. 2010;299(6):R1676–84. doi: 10.1152/ajpregu.00123.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schmetterer L, Strenn K, Kastner J, Eichler HG, Wolzt M. Exhaled NO during graded changes in inhaled oxygen in man. Thorax. 1997;52(8):736–8. doi: 10.1136/thx.52.8.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Farrow KN, Lakshminrusimha S, Czech L, et al. SOD and inhaled nitric oxide normalize phosphodiesterase 5 expression and activity in neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2010;299(1):L109–16. doi: 10.1152/ajplung.00309.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Farrow KN, Wedgwood S, Lee KJ, et al. Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn. Respir Physiol Neurobiol. 2010;174(3):272–81. doi: 10.1016/j.resp.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perez M, Lakshminrusimha S, Wedgwood S, et al. Hydrocortisone normalizes oxygenation and cGMP regulation in lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol. 2012;302(6):L595–603. doi: 10.1152/ajplung.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wedgwood S, Lakshminrusimha S, Farrow KN, et al. Apocynin improves oxygenation and increases eNOS in persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol. 2012;302(6):L616–26. doi: 10.1152/ajplung.00064.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wedgwood S, Lakshminrusimha S, Fukai T, Russell JA, Schumacker PT, Steinhorn RH. Hydrogen peroxide regulates extracellular superoxide dismutase activity and expression in neonatal pulmonary hypertension. Antioxid Redox Signal. 2011;15(6):1497–506. doi: 10.1089/ars.2010.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shaul PW, Farrar MA, Magness RR. Pulmonary endothelial nitric oxide production is developmentally regulated in the fetus and newborn. Am J Physiol. 1993;265(4 Pt 2):H1056–63. doi: 10.1152/ajpheart.1993.265.4.H1056. [DOI] [PubMed] [Google Scholar]
- 108.Porter VA, Reeve HL, Cornfield DN. Fetal rabbit pulmonary artery smooth muscle cell response to ryanodine is developmentally regulated. Am J Physiol Lung Cell Mol Physiol. 2000;279(4):L751–7. doi: 10.1152/ajplung.2000.279.4.L751. [DOI] [PubMed] [Google Scholar]
- 109.Porter VA, Rhodes MT, Reeve HL, Cornfield DN. Oxygen-induced fetal pulmonary vasodilation is mediated by intracellular calcium activation of K(Ca) channels. Am J Physiol Lung Cell Mol Physiol. 2001;281(6):L1379–85. doi: 10.1152/ajplung.2001.281.6.L1379. [DOI] [PubMed] [Google Scholar]
- 110.Rhodes MT, Porter VA, Saqueton CB, Herron JM, Resnik ER, Cornfield DN. Pulmonary vascular response to normoxia and K(Ca) channel activity is developmentally regulated. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1250–7. doi: 10.1152/ajplung.2001.280.6.L1250. [DOI] [PubMed] [Google Scholar]
- 111.Saqueton CB, Miller RB, Porter VA, Milla CE, Cornfield DN. NO causes perinatal pulmonary vasodilation through K+-channel activation and intracellular Ca2+ release. Am J Physiol. 1999;276(6 Pt 1):L925–32. doi: 10.1152/ajplung.1999.276.6.L925. [DOI] [PubMed] [Google Scholar]
- 112.Olschewski A, Hong Z, Linden BC, Porter VA, Weir EK, Cornfield DN. Contribution of the K(Ca) channel to membrane potential and O2 sensitivity is decreased in an ovine PPHN model. Am J Physiol Lung Cell Mol Physiol. 2002;283(5):L1103–9. doi: 10.1152/ajplung.00100.2002. [DOI] [PubMed] [Google Scholar]
- 113.Cheranov SY, Jaggar JH. Sarcoplasmic reticulum calcium load regulates rat arterial smooth muscle calcium sparks and transient K(Ca) currents. J Physiol. 2002;544(Pt 1):71–84. doi: 10.1113/jphysiol.2002.025197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gollasch M, Wellman GC, Knot HJ, et al. Ontogeny of local sarcoplasmic reticulum Ca2+ signals in cerebral arteries: Ca2+ sparks as elementary physiological events. Circ Res. 1998;83(11):1104–14. doi: 10.1161/01.res.83.11.1104. [DOI] [PubMed] [Google Scholar]
- 115.Jaggar JH, Wellman GC, Heppner TJ, et al. Ca2+ channels, ryanodine receptors and Ca(2+)-activated K+ channels: a functional unit for regulating arterial tone. Acta Physiol Scand. 1998;164(4):577–87. doi: 10.1046/j.1365-201X.1998.00462.x. [DOI] [PubMed] [Google Scholar]
- 116.Li A, Adebiyi A, Leffler CW, Jaggar JH. KCa channel insensitivity to Ca2+ sparks underlies fractional uncoupling in newborn cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;291(3):H1118–25. doi: 10.1152/ajpheart.01308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cornfield DN. Developmental regulation of oxygen sensing and ion channels in the pulmonary vasculature. Adv Exp Med Biol. 2010;661:201–20. doi: 10.1007/978-1-60761-500-2_13. [DOI] [PubMed] [Google Scholar]
- 118.Gao Y, Portugal AD, Negash S, Zhou W, Longo LD, Raj JU. Role of Rho kinases in PKG-mediated relaxation of pulmonary arteries of fetal lambs exposed to chronic high altitude hypoxia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L678–L84. doi: 10.1152/ajplung.00178.2006. [DOI] [PubMed] [Google Scholar]
- 119.Wright RJ. Perinatal stress and early life programming of lung structure and function. Biol Psychol. 2010;84(1):46–56. doi: 10.1016/j.biopsycho.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pena JP, Tomimatsu T, Hatran DP, McGill LL, Longo LD. Cerebral blood flow and oxygenation in ovine fetus: responses to superimposed hypoxia at both low and high altitude. J Physiol. 2007;578(Pt 1):359–70. doi: 10.1113/jphysiol.2006.119925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huisman TH. The structure and function of normal and abnormal haemoglobins. Baillieres Clin Haematol. 1993;6(1):1–30. doi: 10.1016/s0950-3536(05)80064-2. [DOI] [PubMed] [Google Scholar]
- 122.Stenmark KR, Fasules J, Hyde DM, et al. Severe pulmonary hypertension and arterial adventitial changes in newborn calves at 4,300 m. J Appl Physiol. 1987;62(2):821–30. doi: 10.1152/jappl.1987.62.2.821. [DOI] [PubMed] [Google Scholar]
- 123.Sheng L, Zhou W, Hislop AA, Ibe BO, Longo LD, Raj JU. Role of epidermal growth factor receptor in ovine fetal pulmonary vascular remodeling following exposure to high altitude long-term hypoxia. High Alt Med Biol. 2009;10(4):365–72. doi: 10.1089/ham.2008.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Llanos AJ, Ebensperger G, Herrera EA, et al. Fetal and postnatal pulmonary circulation in the Alto Andino. Placenta. 2011;32(Suppl 2):S100–3. doi: 10.1016/j.placenta.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 125.Ziino AJ, Ivanovska J, Belcastro R, Kantores C, Xu EZ, Lau M, et al. Effects of rho-kinase inhibition on pulmonary hypertension, lung growth, and structure in neonatal rats chronically exposed to hypoxia. Pediatr Res. 2010;67(2):177–82. doi: 10.1203/PDR.0b013e3181c6e5a7. [DOI] [PubMed] [Google Scholar]
- 126.Bierer R, Nitta CH, Friedman JK, et al. NFATc3 Is Required for Chronic Hypoxia-induced Pulmonary Hypertension in Adult and Neonatal Mice. Am J Physiol Lung Cell Mol Physiol. 2011;301(6):L872–80. doi: 10.1152/ajplung.00405.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hirenallur SD, Haworth ST, Leming JT, et al. Upregulation of vascular calcium channels in neonatal piglets with hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295(5):L915–24. doi: 10.1152/ajplung.90286.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fike CD, Kaplowitz MR, Zhang Y, Madden JA. Voltage-gated K+ channels at an early stage of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol. 2006;291(6):L1169–76. doi: 10.1152/ajplung.00117.2006. [DOI] [PubMed] [Google Scholar]
- 129.Murphy JD, Aronovitz MJ, Reid LM. Effects of chronic in utero hypoxia on the pulmonary vasculature of the newborn guinea pig. Pediatr Res. 1986;20(4):292–5. doi: 10.1203/00006450-198604000-00003. [DOI] [PubMed] [Google Scholar]
- 130.Fagan KA, Fouty BW, Tyler RC, et al. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest. 1999;103(2):291–9. doi: 10.1172/JCI3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Durmowicz AG, Orton EC, Stenmark KR. Progressive loss of vasodilator responsive component of pulmonary hypertension in neonatal calves exposed to 4,570 m. Am J Physiol. 1993;265(6 Pt 2):H2175–83. doi: 10.1152/ajpheart.1993.265.6.H2175. [DOI] [PubMed] [Google Scholar]
- 132.Tucker A, McMurtry IF, Reeves JT, Alexander AF, Will DH, Grover RF. Lung vascular smooth muscle as a determinant of pulmonary hypertension at high altitude. Am J Physiol. 1975;228(3):762–7. doi: 10.1152/ajplegacy.1975.228.3.762. [DOI] [PubMed] [Google Scholar]
- 133.Durmowicz AG, Hofmeister S, Kadyraliev TK, Aldashev AA, Stenmark KR. Functional and structural adaptation of the yak pulmonary circulation to residence at high altitude. J Appl Physiol. 1993;74(5):2276–85. doi: 10.1152/jappl.1993.74.5.2276. [DOI] [PubMed] [Google Scholar]
- 134.Yilmaz C, Dane DM, Hsia CC. Alveolar diffusion-perfusion interactions during high-altitude residence in guinea pigs. J Appl Physiol. 2007;102(6):2179–85. doi: 10.1152/japplphysiol.00059.2007. [DOI] [PubMed] [Google Scholar]
- 135.Hsia CC, Johnson RL, Jr., McDonough P, et al. Residence at 3,800-m altitude for 5 mo in growing dogs enhances lung diffusing capacity for oxygen that persists at least 2.5 years. J Appl Physiol. 2007;102(4):1448–55. doi: 10.1152/japplphysiol.00971.2006. [DOI] [PubMed] [Google Scholar]
- 136.Niermeyer S, Andrade Mollinedo P, Huicho L. Child health and living at high altitude. Arch Dis Child. 2009;94(10):806–11. doi: 10.1136/adc.2008.141838. [DOI] [PubMed] [Google Scholar]
- 137.Allemann Y, Stuber T, de Marchi SF, et al. Pulmonary artery pressure and cardiac function in children and adolescents after rapid ascent to 3450 m. Am J Physiol Heart Circ Physiol. 2012;302(12):H2646–53. doi: 10.1152/ajpheart.00053.2012. [DOI] [PubMed] [Google Scholar]
- 138.Fagan KA, Weil JV. Potential genetic contributions to control of the pulmonary circulation and ventilation at high altitude. High Alt Med Biol. 2001;2(2):165–71. doi: 10.1089/152702901750265279. [DOI] [PubMed] [Google Scholar]
- 139.Haworth SG, Hislop AA. Lung development-the effects of chronic hypoxia. Semin Neonatol. 2003;8(1):1–8. doi: 10.1016/s1084-2756(02)00195-1. [DOI] [PubMed] [Google Scholar]
- 140.ad hoc Statement Committee ATS Mechanisms and limits of induced postnatal lung growth. Am J Respir Crit Care Med. 2004;170(3):319–43. doi: 10.1164/rccm.200209-1062ST. [DOI] [PubMed] [Google Scholar]
- 141.de Meer K, Heymans HS, Zijlstra WG. Physical adaptation of children to life at high altitude. Eur J Pediatr. 1995;154(4):263–72. [PubMed] [Google Scholar]
- 142.Blanco LN, Massaro D, Massaro GD. Alveolar size, number, and surface area: developmentally dependent response to 13% O2. Am J Physiol. 1991;261(6 Pt 1):L370–7. doi: 10.1152/ajplung.1991.261.6.L370. [DOI] [PubMed] [Google Scholar]
- 143.Massaro GD, Olivier J, Dzikowski C, Massaro D. Postnatal development of lung alveoli: suppression by 13% O2 and a critical period. Am J Physiol. 1990;258(6 Pt 1):L321–7. doi: 10.1152/ajplung.1990.258.6.L321. [DOI] [PubMed] [Google Scholar]
- 144.Nicola T, Ambalavanan N, Zhang W, et al. Hypoxia-induced inhibition of lung development is attenuated by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Am J Physiol Lung Cell Mol Physiol. 2011;301(1):L125–34. doi: 10.1152/ajplung.00074.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hsia CC, Carbayo JJ, Yan X, Bellotto DJ. Enhanced alveolar growth and remodeling in Guinea pigs raised at high altitude. Respir Physiol Neurobiol. 2005;147(1):105–15. doi: 10.1016/j.resp.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 146.McDonough P, Dane DM, Hsia CC, Yilmaz C, Johnson RL., Jr. Long-term enhancement of pulmonary gas exchange after high-altitude residence during maturation. J Appl Physiol. 2006;100(2):474–81. doi: 10.1152/japplphysiol.01069.2005. [DOI] [PubMed] [Google Scholar]
- 147.Tang JR, Le Cras TD, Morris KG, Jr., Abman SH. Brief perinatal hypoxia increases severity of pulmonary hypertension after reexposure to hypoxia in infant rats. Am J Physiol Lung Cell Mol Physiol. 2000;278(2):L356–64. doi: 10.1152/ajplung.2000.278.2.L356. [DOI] [PubMed] [Google Scholar]
- 148.Liu SQ. Alterations in structure of elastic laminae of rat pulmonary arteries in hypoxic hypertension. J Appl Physiol. 1996;81(5):2147–55. doi: 10.1152/jappl.1996.81.5.2147. [DOI] [PubMed] [Google Scholar]
- 149.Bixby CE, Ibe BO, Abdallah MF, et al. Role of platelet-activating factor in pulmonary vascular remodeling associated with chronic high altitude hypoxia in ovine fetal lambs. Am J Physiol Lung Cell Mol Physiol. 2007;293(6):L1475–L82. doi: 10.1152/ajplung.00089.2007. [DOI] [PubMed] [Google Scholar]
- 150.Xue Q, Ducsay CA, Longo LD, Zhang L. Effect of long-term high-altitude hypoxia on fetal pulmonary vascular contractility. J Appl Physiol. 2008;104(6):1786–92. doi: 10.1152/japplphysiol.01314.2007. [DOI] [PubMed] [Google Scholar]
- 151.Herrera EA, Pulgar VM, Riquelme RA, et al. High-altitude chronic hypoxia during gestation and after birth modifies cardiovascular responses in newborn sheep. Am J Physiol Regul Integr Comp Physiol. 2007;292(6):R2234–40. doi: 10.1152/ajpregu.00909.2006. [DOI] [PubMed] [Google Scholar]
- 152.Hsia CC, Hyde DM, Ochs M, Weibel ER. How to measure lung structure--what for? On the “Standards for the quantitative assessment of lung structure”. Respir Physiol Neurobiol. 2010;171(2):72–4. doi: 10.1016/j.resp.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 153.Hsia CC, Hyde DM, Ochs M, Weibel ER, Structure AEJTFoQAoL An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181(4):394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ashmore RC, Rodman DM, Sato K, et al. Paradoxical constriction to platelets by arteries from rats with pulmonary hypertension. Am J Physiol. 1991;260(6 Pt 2):H1929–34. doi: 10.1152/ajpheart.1991.260.6.H1929. [DOI] [PubMed] [Google Scholar]
- 155.Kentera D, Susic D, Veljkovic V, Tucakovic G, Koko V. Pulmonary artery pressure in rats with hereditary platelet function defect. Respiration. 1988;54(2):110–4. doi: 10.1159/000195509. [DOI] [PubMed] [Google Scholar]
- 156.Rudofsky UH, Magro AM. Spontaneous hypertension in fawn-hooded rats. Lab Anim Sci. 1982;32(4):389–91. [PubMed] [Google Scholar]
- 157.Le Cras TD, Kim DH, Gebb S, et al. Abnormal lung growth and the development of pulmonary hypertension in the Fawn-Hooded rat. Am J Physiol. 1999;277(4 Pt 1):L709–18. doi: 10.1152/ajplung.1999.277.4.L709. [DOI] [PubMed] [Google Scholar]
- 158.Kuijpers MH, de Jong W. Spontaneous hypertension in the fawn-hooded rat: a cardiovascular disease model. J Hypertens Suppl. 1986;4(3):S41–4. [PubMed] [Google Scholar]
- 159.Rhodes J. Comparative physiology of hypoxic pulmonary hypertension: historical clues from brisket disease. J Appl Physiol. 2005;98(3):1092–100. doi: 10.1152/japplphysiol.01017.2004. [DOI] [PubMed] [Google Scholar]
- 160.Goldberg SJ, Levy RA, Siassi B, Betten J. The effects of maternal hypoxia and hyperoxia upon the neonatal pulmonary vasculature. Pediatrics. 1971;48(4):528–33. [PubMed] [Google Scholar]
- 161.Gebb SA, Jones PL. Hypoxia and lung branching morphogenesis. Adv Exp Med Biol. 2003;543:117–25. doi: 10.1007/978-1-4419-8997-0_8. [DOI] [PubMed] [Google Scholar]
- 162.Shimoda LA, Semenza GL. HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med. 2011;183(2):152–6. doi: 10.1164/rccm.201009-1393PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Del Moral PM, Sala FG, Tefft D, et al. VEGF-A signaling through Flk-1 is a critical facilitator of early embryonic lung epithelial to endothelial crosstalk and branching morphogenesis. Dev Biol. 2006;290(1):177–88. doi: 10.1016/j.ydbio.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 164.Izikki M, Guignabert C, Fadel E, et al. Endothelial-derived FGF2 contributes to the progression of pulmonary hypertension in humans and rodents. J Clin Invest. 2009;119(3):512–23. doi: 10.1172/JCI35070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yin Y, Wang F, Ornitz DM. Mesothelial- and epithelial-derived FGF9 have distinct functions in the regulation of lung development. Development. 2011;138(15):3169–77. doi: 10.1242/dev.065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yin Y, White AC, Huh SH, et al. An FGF-WNT gene regulatory network controls lung mesenchyme development. Dev Biol. 2008;319(2):426–36. doi: 10.1016/j.ydbio.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–80. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- 168.West J, Tada Y, Fagan KA, et al. Suppression of type II bone morphogenic protein receptor in vascular smooth muscle induces pulmonary arterial hypertension in transgenic mice. Chest. 2005;128(6 Suppl):553S. doi: 10.1378/chest.128.6_suppl.553S. [DOI] [PubMed] [Google Scholar]
- 169.Jankov RP, Keith Tanswell A. Growth factors, postnatal lung growth and bronchopulmonary dysplasia. Paediatr Respir Rev. 2004;5(Suppl A):S265–75. doi: 10.1016/s1526-0542(04)90050-4. [DOI] [PubMed] [Google Scholar]
- 170.Warburton D, Bellusci S, De Langhe S, et al. Molecular mechanisms of early lung specification and branching morphogenesis. Pediatr Res. 2005;57(5 Pt 2):26R–37R. doi: 10.1203/01.PDR.0000159570.01327.ED. [DOI] [PubMed] [Google Scholar]
- 171.Vicencio AG, Eickelberg O, Stankewich MC, Kashgarian M, Haddad GG. Regulation of TGF-beta ligand and receptor expression in neonatal rat lungs exposed to chronic hypoxia. J Appl Physiol. 2002;93(3):1123–30. doi: 10.1152/japplphysiol.00031.2002. [DOI] [PubMed] [Google Scholar]
- 172.Groenman F, Rutter M, Caniggia I, Tibboel D, Post M. Hypoxia-inducible factors in the first trimester human lung. J Histochem Cytochem. 2007;55(4):355–63. doi: 10.1369/jhc.6A7129.2006. [DOI] [PubMed] [Google Scholar]
- 173.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–78. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 174.Yu AY, Shimoda LA, Iyer NV, et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest. 1999;103(5):691–6. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Brusselmans K, Compernolle V, Tjwa M, et al. Heterozygous deficiency of hypoxia-inducible factor-2alpha protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest. 2003;111(10):1519–27. doi: 10.1172/JCI15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rajatapiti P, van der Horst IW, de Rooij JD, et al. Expression of hypoxia-inducible factors in normal human lung development. Pediatr Dev Pathol. 2008;11(3):193–9. doi: 10.2350/07-04-0257.1. [DOI] [PubMed] [Google Scholar]
- 177.Bhatt AJ, Amin SB, Chess PR, Watkins RH, Maniscalco WM. Expression of vascular endothelial growth factor and Flk-1 in developing and glucocorticoid-treated mouse lung. Pediatr Res. 2000;47(5):606–13. doi: 10.1203/00006450-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 178.Marti HH, Risau W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc Natl Acad Sci USA. 1998;95(26):15809–14. doi: 10.1073/pnas.95.26.15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Le Cras TD, Markham NE, Tuder RM, Voelkel NF, Abman SH. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol. 2002;283(3):L555–62. doi: 10.1152/ajplung.00408.2001. [DOI] [PubMed] [Google Scholar]
- 180.Murata T, Sato K, Hori M, Ozaki H, Karaki H. Decreased endothelial nitric-oxide synthase (eNOS) activity resulting from abnormal interaction between eNOS and its regulatory proteins in hypoxia-induced pulmonary hypertension. J Biol Chem. 2002;277(46):44085–92. doi: 10.1074/jbc.M205934200. [DOI] [PubMed] [Google Scholar]
- 181.Beall CM, Laskowski D, Strohl KP, et al. Pulmonary nitric oxide in mountain dwellers. Nature. 2001;414(6862):411–2. doi: 10.1038/35106641. [DOI] [PubMed] [Google Scholar]
- 182.John TA, Ibe BO, Usha Raj J. Oxygen alters caveolin-1 and nitric oxide synthase-3 functions in ovine fetal and neonatal lung microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291(5):L1079–93. doi: 10.1152/ajplung.00526.2005. [DOI] [PubMed] [Google Scholar]
- 183.Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106(1):57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 184.Fike CD, Kaplowitz MR, Thomas CJ, Nelin LD. Chronic hypoxia decreases nitric oxide production and endothelial nitric oxide synthase in newborn pig lungs. Am J Physiol. 1998;274(4 Pt 1):L517–26. doi: 10.1152/ajplung.1998.274.4.L517. [DOI] [PubMed] [Google Scholar]
- 185.Hislop AA, Springall DR, Oliveira H, Pollock JS, Polak JM, Haworth SG. Endothelial nitric oxide synthase in hypoxic newborn porcine pulmonary vessels. Arch Dis Child Fetal Neonatal Ed. 1997;77(1):F16–22. doi: 10.1136/fn.77.1.f16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fike CD, Pfister SL, Slaughter JC, et al. Protein complex formation with heat shock protein 90 in chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Heart Circ Physiol. 2010;299(4):H1190–204. doi: 10.1152/ajpheart.01207.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Duplain H, Sartori C, Lepori M, et al. Exhaled nitric oxide in high-altitude pulmonary edema: role in the regulation of pulmonary vascular tone and evidence for a role against inflammation. Am J Respir Crit Care Med. 2000;162(1):221–4. doi: 10.1164/ajrccm.162.1.9908039. [DOI] [PubMed] [Google Scholar]
- 188.Le Cras TD, Kim DH, Markham NE, Abman AS. Early abnormalities of pulmonary vascular development in the Fawn-Hooded rat raised at Denver's altitude. Am J Physiol Lung Cell Mol Physiol. 2000;279(2):L283–91. doi: 10.1152/ajplung.2000.279.2.L283. [DOI] [PubMed] [Google Scholar]
- 189.Fiumana E, Parfenova H, Jaggar JH, Leffler CW. Carbon monoxide mediates vasodilator effects of glutamate in isolated pressurized cerebral arterioles of newborn pigs. Am J Physiol Heart Circ Physiol. 2003;284(4):H1073–9. doi: 10.1152/ajpheart.00881.2002. [DOI] [PubMed] [Google Scholar]
- 190.Leffler CW, Parfenova H, Jaggar JH. Carbon monoxide as an endogenous vascular modulator. Am J Physiol Heart Circ Physiol. 2011;301(1):H1–H11. doi: 10.1152/ajpheart.00230.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xi Q, Tcheranova D, Parfenova H, Horowitz B, Leffler CW, Jaggar JH. Carbon monoxide activates KCa channels in newborn arteriole smooth muscle cells by increasing apparent Ca2+ sensitivity of alpha-subunits. Am J Physiol Heart Circ Physiol. 2004;286(2):H610–8. doi: 10.1152/ajpheart.00782.2003. [DOI] [PubMed] [Google Scholar]
- 192.Leffler CW, Hessler JR, Green RS. Mechanism of stimulation of pulmonary prostacyclin synthesis at birth. Prostaglandins. 1984;28(6):877–87. doi: 10.1016/0090-6980(84)90041-8. [DOI] [PubMed] [Google Scholar]
- 193.Shaul PW, Campbell WB, Farrar MA, Magness RR. Oxygen modulates prostacyclin synthesis in ovine fetal pulmonary arteries by an effect on cyclooxygenase. J Clin Invest. 1992;90(6):2147–55. doi: 10.1172/JCI116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shaul PW, Farrar MA, Magness RR. Oxygen modulation of pulmonary arterial prostacyclin synthesis is developmentally regulated. Am J Physiol. 1993;265(2 Pt 2):H621–8. doi: 10.1152/ajpheart.1993.265.2.H621. [DOI] [PubMed] [Google Scholar]
- 195.Ibe BO, Hillyard RM, Raj JU. Heterogeneity in prostacyclin and thromboxane synthesis in ovine pulmonary vascular tree: effect of age and oxygen tension. Exp Lung Res. 1996;22(3):351–74. doi: 10.3109/01902149609031780. [DOI] [PubMed] [Google Scholar]
- 196.Fike CD, Kaplowitz MR, Zhang Y, Pfister SL. Cyclooxygenase-2 and an early stage of chronic hypoxia-induced pulmonary hypertension in newborn pigs. J Appl Physiol. 2005;98(3):1111–8. doi: 10.1152/japplphysiol.00810.2004. discussion 091. [DOI] [PubMed] [Google Scholar]
- 197.Hoshikawa Y, Voelkel NF, Gesell TL, et al. Prostacyclin receptor-dependent modulation of pulmonary vascular remodeling. Am J Respir Crit Care Med. 2001;164(2):314–8. doi: 10.1164/ajrccm.164.2.2010150. [DOI] [PubMed] [Google Scholar]
- 198.Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159(6):1925–32. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 199.Aguirre JI, Morrell NW, Long L, et al. Vascular remodeling and ET-1 expression in rat strains with different responses to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2000;278(5):L981–7. doi: 10.1152/ajplung.2000.278.5.L981. [DOI] [PubMed] [Google Scholar]
- 200.Noguchi Y, Hislop AA, Haworth SG. Influence of hypoxia on endothelin-1 binding sites in neonatal porcine pulmonary vasculature. Am J Physiol. 1997;272(2 Pt 2):H669–78. doi: 10.1152/ajpheart.1997.272.2.H669. [DOI] [PubMed] [Google Scholar]
- 201.Schindler MB, Hislop AA, Haworth SG. Porcine pulmonary artery and bronchial responses to endothelin-1 and norepinephrine on recovery from hypoxic pulmonary hypertension. Pediatr Res. 2006;60(1):71–6. doi: 10.1203/01.pdr.0000219577.01928.78. [DOI] [PubMed] [Google Scholar]
- 202.McMurtry IF, Abe K, Ota H, Fagan KA, Oka M. Rho kinase-mediated vasoconstriction in pulmonary hypertension. Adv Exp Med Biol. 2010;661:299–308. doi: 10.1007/978-1-60761-500-2_19. [DOI] [PubMed] [Google Scholar]
- 203.McNamara PJ, Murthy P, Kantores C, et al. Acute vasodilator effects of Rho-kinase inhibitors in neonatal rats with pulmonary hypertension unresponsive to nitric oxide. Am J Physiol Lung Cell Mol Physiol. 2008;294(2):L205–13. doi: 10.1152/ajplung.00234.2007. [DOI] [PubMed] [Google Scholar]
- 204.Chiappara G, Gagliardo R, Siena A, et al. Airway remodelling in the pathogenesis of asthma. Curr Opin Allergy Clin Immunol. 2001;1(1):85–93. doi: 10.1097/01.all.0000010990.97765.a1. [DOI] [PubMed] [Google Scholar]
- 205.Droma Y, Hanaoka M, Hotta J, et al. Pathological features of the lung in fatal high altitude pulmonary edema occurring at moderate altitude in Japan. High Alt Med Biol. 2001;2(4):515–23. doi: 10.1089/152702901753397081. [DOI] [PubMed] [Google Scholar]
- 206.Wojciak-Stothard B, Tsang LY, Haworth SG. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288(4):L749–60. doi: 10.1152/ajplung.00361.2004. [DOI] [PubMed] [Google Scholar]
- 207.Tulloh RM, Hislop AA, Boels PJ, Deutsch J, Haworth SG. Chronic hypoxia inhibits postnatal maturation of porcine intrapulmonary artery relaxation. Am J Physiol. 1997;272(5 Pt 2):H2436–45. doi: 10.1152/ajpheart.1997.272.5.H2436. [DOI] [PubMed] [Google Scholar]
- 208.Ward JP, McMurtry IF. Mechanisms of hypoxic pulmonary vasoconstriction and their roles in pulmonary hypertension: new findings for an old problem. Curr Opin Pharmacol. 2009;9(3):287–96. doi: 10.1016/j.coph.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yuan JXJ. Hypoxic pulmonary vasoconstriction : cellular and molecular mechanisms. Kluwer Academic Pub.; Boston: 2004. p. xvii.p. 590. [Google Scholar]
- 210.Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev. 2012;92(1):367–520. doi: 10.1152/physrev.00041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Robertson TP, Hague D, Aaronson PI, Ward JP. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol. 2000;525(Pt 3):669–80. doi: 10.1111/j.1469-7793.2000.t01-1-00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jabr RI, Toland H, Gelband CH, Wang XX, Hume JR. Prominent role of intracellular Ca2+ release in hypoxic vasoconstriction of canine pulmonary artery. Br J Pharmacol. 1997;122(1):21–30. doi: 10.1038/sj.bjp.0701326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Post JM, Hume JR, Archer SL, Weir EK. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol. 1992;262(4 Pt 1):C882–90. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- 214.Sham JS, Crenshaw BR, Jr., Deng LH, Shimoda LA, Sylvester JT. Effects of hypoxia in porcine pulmonary arterial myocytes: roles of K(V) channel and endothelin-1. Am J Physiol Lung Cell Mol Physiol. 2000;279(2):L262–72. doi: 10.1152/ajplung.2000.279.2.L262. [DOI] [PubMed] [Google Scholar]
- 215.Peng G, Lu W, Li X, et al. Expression of store-operated Ca2+ entry and transient receptor potential canonical and vanilloid-related proteins in rat distal pulmonary venous smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2010;299(5):L621–30. doi: 10.1152/ajplung.00176.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Urban N, Hill K, Wang L, Kuebler WM, Schaefer M. Novel pharmacological TRPC inhibitors block hypoxia-induced vasoconstriction. Cell Calcium. 2012;51(2):194–206. doi: 10.1016/j.ceca.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 217.Weigand L, Foxson J, Wang J, Shimoda LA, Sylvester JT. Inhibition of hypoxic pulmonary vasoconstriction by antagonists of store-operated Ca2+ and nonselective cation channels. Am J Physiol Lung Cell Mol Physiol. 2005;289(1):L5–L13. doi: 10.1152/ajplung.00044.2005. [DOI] [PubMed] [Google Scholar]
- 218.Ng LC, Wilson SM, Hume JR. Mobilization of sarcoplasmic reticulum stores by hypoxia leads to consequent activation of capacitative Ca2+ entry in isolated canine pulmonary arterial smooth muscle cells. J Physiol. 2005;563(Pt 2):409–19. doi: 10.1113/jphysiol.2004.078311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294(2):H570–8. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 220.Michelakis ED, Thebaud B, Weir EK, Archer SL. Hypoxic pulmonary vasoconstriction: redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells. J Mol Cell Cardiol. 2004;37(6):1119–36. doi: 10.1016/j.yjmcc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 221.Olson KR, Dombkowski RA, Russell MJ, et al. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J Exp Biol. 2006;209(Pt 20):4011–23. doi: 10.1242/jeb.02480. [DOI] [PubMed] [Google Scholar]
- 222.Ghanayem NS, Gordon JB. Modulation of pulmonary vasomotor tone in the fetus and neonate. Respir Res. 2001;2(3):139–44. doi: 10.1186/rr50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Villamor E, Kessels CG, Ruijtenbeek K, et al. Chronic in ovo hypoxia decreases pulmonary arterial contractile reactivity and induces biventricular cardiac enlargement in the chicken embryo. Am J Physiol Regul Integr Comp Physiol. 2004;287(3):R642–51. doi: 10.1152/ajpregu.00611.2003. [DOI] [PubMed] [Google Scholar]
- 224.Blood AB, Terry MH, Merritt T, et al. Effect of chronic perinatal hypoxia on the role of rho-kinase in pulmonary artery contraction in newborn lambs. Am J Physiol Regul Integr Comp Physiol. 2012 doi: 10.1152/ajpregu.00126.2012. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gamboa R, Marticorena E. [Pulmonary arterial pressure in newborn infants in high altitude]. Arch Inst Biol Andina. 1971;4(2):55–66. [PubMed] [Google Scholar]
- 226.Aparicio Otero O, Romero Gutierrez F, Harris P, Anand I. Echocardiography shows persistent thickness of the wall of the right ventricle in infants at high altitude. Cardioscience. 1991;2(1):63–9. [PubMed] [Google Scholar]
- 227.Miao CY, Zuberbuhler JS, Zuberbuhler JR. Prevalence of congenital cardiac anomalies at high altitude. J Am Coll Cardiol. 1988;12(1):224–8. doi: 10.1016/0735-1097(88)90378-6. [DOI] [PubMed] [Google Scholar]
- 228.Fike CD, Slaughter JC, Kaplowitz MR, Zhang Y, Aschner JL. Reactive oxygen species from NADPH oxidase contribute to altered pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295(5):L881–8. doi: 10.1152/ajplung.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Cornfield DN, Resnik ER, Herron JM, Abman SH. Chronic intrauterine pulmonary hypertension decreases calcium-sensitive potassium channel mRNA expression. Am J Physiol Lung Cell Mol Physiol. 2000;279(5):L857–62. doi: 10.1152/ajplung.2000.279.5.L857. [DOI] [PubMed] [Google Scholar]
- 230.Platoshyn O, Golovina VA, Bailey CL, et al. Sustained membrane depolarization and pulmonary artery smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2000;279(5):C1540–9. doi: 10.1152/ajpcell.2000.279.5.C1540. [DOI] [PubMed] [Google Scholar]
- 231.Cerro MJ, Abman S, Diaz G, et al. A consensus approach to the classification of pediatric pulmonary hypertensive vascular disease: Report from the PVRI Pediatric Taskforce, Panama 2011. Pulm Circ. 2011;1(2):286–98. doi: 10.4103/2045-8932.83456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573–619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 233.Abman SH. Recent advances in the pathogenesis and treatment of persistent pulmonary hypertension of the newborn. Neonatology. 2007;91(4):283–90. doi: 10.1159/000101343. [DOI] [PubMed] [Google Scholar]
- 234.Oishi P, Datar SA, Fineman JR. Advances in the management of pediatric pulmonary hypertension. Respir Care. 2011;56(9):1314–39. doi: 10.4187/respcare.01297. discussion 39-40. [DOI] [PubMed] [Google Scholar]
- 235.Ohashi N, Matsushima M, Maeda M, Yamaki S. Advantages of oxygen inhalation therapy for postoperative pulmonary hypertension. Pediatr Cardiol. 2005;26(1):90–2. doi: 10.1007/s00246-003-0663-4. [DOI] [PubMed] [Google Scholar]
- 236.Bizzarro M, Gross I. Inhaled nitric oxide for the postoperative management of pulmonary hypertension in infants and children with congenital heart disease. Cochrane Database Syst Rev. 2005;(4):CD005055. doi: 10.1002/14651858.CD005055.pub2. [DOI] [PubMed] [Google Scholar]
- 237.Goldman AP, Delius RE, Deanfield JE, et al. Pharmacological control of pulmonary blood flow with inhaled nitric oxide after the fenestrated Fontan operation. Circulation. 1996;94(9 Suppl):II44–8. [PubMed] [Google Scholar]
- 238.Clark RH, Kueser TJ, Walker MW, et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000;342(7):469–74. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- 239.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med. 2003;349(22):2099–107. doi: 10.1056/NEJMoa031154. [DOI] [PubMed] [Google Scholar]
- 240.Pierce CM, Peters MJ, Cohen G, Goldman AP, Petros AJ. Cost of nitric oxide is exorbitant. BMJ. 2002;325(7359):336. doi: 10.1136/bmj.325.7359.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Subhedar NV, Jauhari P, Natarajan R. Cost of inhaled nitric oxide therapy in neonates. Lancet. 2002;359(9319):1781–2. doi: 10.1016/S0140-6736(02)08632-4. [DOI] [PubMed] [Google Scholar]
- 242.Martin RJ. Nitric oxide for preemies--not so fast. N Engl J Med. 2003;349(22):2157–9. doi: 10.1056/NEJMe038165. [DOI] [PubMed] [Google Scholar]
- 243.Kinsella JP, Griebel J, Schmidt JM, Abman SH. Use of inhaled nitric oxide during interhospital transport of newborns with hypoxemic respiratory failure. Pediatrics. 2002;109(1):158–61. doi: 10.1542/peds.109.1.158. [DOI] [PubMed] [Google Scholar]
- 244.Goldman AP, Delius RE, Deanfield JE, de Leval MR, Sigston PE, Macrae DJ. Nitric oxide might reduce the need for extracorporeal support in children with critical postoperative pulmonary hypertension. Ann Thorac Surg. 1996;62(3):750–5. doi: 10.1016/s0003-4975(96)00375-x. [DOI] [PubMed] [Google Scholar]
- 245.Baquero H, Soliz A, Neira F, Venegas ME, Sola A. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics. 2006;117(4):1077–83. doi: 10.1542/peds.2005-0523. [DOI] [PubMed] [Google Scholar]
- 246.Steinhorn RH, Kinsella JP, Pierce C, et al. Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. J Pediatr. 2009;155(6):841–7. e1. doi: 10.1016/j.jpeds.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 247.Yung D, Widlitz AC, Rosenzweig EB, Kerstein D, Maislin G, Barst RJ. Outcomes in children with idiopathic pulmonary arterial hypertension. Circulation. 2004;110(6):660–5. doi: 10.1161/01.CIR.0000138104.83366.E9. [DOI] [PubMed] [Google Scholar]
- 248.Rosenzweig EB, Kerstein D, Barst RJ. Long-term prostacyclin for pulmonary hypertension with associated congenital heart defects. Circulation. 1999;99(14):1858–65. doi: 10.1161/01.cir.99.14.1858. [DOI] [PubMed] [Google Scholar]
- 249.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346(12):896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 250.Maiya S, Hislop AA, Flynn Y, Haworth SG. Response to bosentan in children with pulmonary hypertension. Heart. 2006;92(5):664–70. doi: 10.1136/hrt.2005.072314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rosenzweig EB, Ivy DD, Widlitz A, et al. Effects of long-term bosentan in children with pulmonary arterial hypertension. J Am Coll Cardiol. 2005;46(4):697–704. doi: 10.1016/j.jacc.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 252.Mohamed WA, Ismail M. A randomized, double-blind, placebo-controlled, prospective study of bosentan for the treatment of persistent pulmonary hypertension of the newborn. J Perinatol. 2012;32(8):608–13. doi: 10.1038/jp.2011.157. [DOI] [PubMed] [Google Scholar]
- 253.Mourani PM, Sontag MK, Ivy DD, Abman SH. Effects of Long-Term Sildenafil Treatment for Pulmonary Hypertension in Infants with Chronic Lung Disease. J Pediatr. 2009;154(3):379–84. doi: 10.1016/j.jpeds.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.ClinicalTrials.gov “Search String: Pulmonary Hypertension Newborn”. 2012. [2012 June 18].
- 255.Chhina MK, Nargues W, Grant GM, Nathan SD. Evaluation of imatinib mesylate in the treatment of pulmonary arterial hypertension. Future Cardiol. 2010;6(1):19–35. doi: 10.2217/fca.09.54. [DOI] [PubMed] [Google Scholar]
- 256.Abman SH. Pulmonary hypertension in older children: new approaches and therapies. Paediatr Respir Rev. 2006;7(Suppl 1):S177–9. doi: 10.1016/j.prrv.2006.04.210. [DOI] [PubMed] [Google Scholar]
- 257.Castro M, Ramirez MI, Gern JE, et al. Strategic plan for pediatric respiratory diseases research: an NHLBI working group report. Proc Am Thorac Soc. 2009;6(1):1–10. doi: 10.1513/pats.200810-116CB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ishikura K, Yamada N, Ito M, et al. Beneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ J. 2006;70(2):174–8. doi: 10.1253/circj.70.174. [DOI] [PubMed] [Google Scholar]
- 259.Doggrell SA. Rho-kinase inhibitors show promise in pulmonary hypertension. Expert Opin Investig Drugs. 2005;14(9):1157–9. doi: 10.1517/13543784.14.9.1157. [DOI] [PubMed] [Google Scholar]
- 260.Nagaoka T, Morio Y, Casanova N, et al. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol. 2004;287(4):L665–L72. doi: 10.1152/ajplung.00050.2003. [DOI] [PubMed] [Google Scholar]
- 261.Mouchaers KT, Schalij I, de Boer MA, et al. Effective reduction of MCT-PAH by Fasudil. Comparison with Bosentan and Sildenafil. Eur Respir J. 2010;36(4):800–7. doi: 10.1183/09031936.00130209. [DOI] [PubMed] [Google Scholar]
- 262.McMurtry IF, Bauer NR, Fagan KA, Nagaoka T, Gebb SA, Oka M. Hypoxia and Rho/Rho-kinase signaling. Lung development versus hypoxic pulmonary hypertension. Adv Exp Med Biol. 2003;543:127–37. [PubMed] [Google Scholar]
- 263.Nagaoka T, Gebb SA, Karoor V, et al. Involvement of RhoA/Rho kinase signaling in pulmonary hypertension of the fawn-hooded rat. J Appl Physiol. 2006;100(3):996–1002. doi: 10.1152/japplphysiol.01028.2005. [DOI] [PubMed] [Google Scholar]
- 264.Alapati VR, McKenzie C, Blair A, Kenny D, MacDonald A, Shaw AM. Mechanisms of U46619- and 5-HT-induced contraction of bovine pulmonary arteries: role of chloride ions. Br J Pharmacol. 2007;151(8):1224–34. doi: 10.1038/sj.bjp.0707338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Oriowo MA. Chloride channels and alpha1-adrenoceptor-mediated pulmonary artery smooth muscle contraction: effect of pulmonary hypertension. Eur J Pharmacol. 2004;506(2):157–63. doi: 10.1016/j.ejphar.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 266.Yamamura A, Yamamura H, Zeifman A, Yuan JX. Activity of Ca - activated Cl channels contributes to regulating receptor- and store-operated Ca entry in human pulmonary artery smooth muscle cells. Pulm Circ. 2011;1(2):269–79. doi: 10.4103/2045-8932.83447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Zhang S, Patel HH, Murray F, et al. Pulmonary artery smooth muscle cells from normal subjects and IPAH patients show divergent cAMP-mediated effects on TRPC expression and capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1202–10. doi: 10.1152/ajplung.00214.2006. [DOI] [PubMed] [Google Scholar]
- 268.Ogawa A, Firth AL, Yao W, Rubin LJ, Yuan JX. Prednisolone inhibits PDGF-induced nuclear translocation of NF-kappaB in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2008;295(4):L648–57. doi: 10.1152/ajplung.90245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.de Frutos S, Spangler R, Alo D, Bosc LV. NFATc3 mediates chronic hypoxia-induced pulmonary arterial remodeling with alpha-actin up-regulation. J Biol Chem. 2007;282(20):15081–9. doi: 10.1074/jbc.M702679200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kuwahara K, Wang Y, McAnally J, et al. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116(12):3114–26. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Nachar RA, Pastene CM, Herrera EA, et al. Low-dose inhaled carbon monoxide reduces pulmonary vascular resistance during acute hypoxemia in adult sheep. High Alt Med Biol. 2001;2(3):377–85. doi: 10.1089/15270290152608552. [DOI] [PubMed] [Google Scholar]
- 272.Hongfang J, Cong B, Zhao B, et al. Effects of hydrogen sulfide on hypoxic pulmonary vascular structural remodeling. Life Sci. 2006;78(12):1299–309. doi: 10.1016/j.lfs.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 273.Mazela J, Polin R. Aerosol delivery to ventilated newborn infants: historical challenges and new directions. Eur J Pediatr. 2011;170(4):433–44. doi: 10.1007/s00431-010-1292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Brion LP, Primhak RA, Yong W. Aerosolized diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst Rev. 2006;3:CD001694. doi: 10.1002/14651858.CD001694.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Azarmi S, Roa WH, Lobenberg R. Targeted delivery of nanoparticles for the treatment of lung diseases. Adv Drug Deliv Rev. 2008;60(8):863–75. doi: 10.1016/j.addr.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 276.Mansour HM, Rhee YS, Wu X. Nanomedicine in pulmonary delivery. Int J Nanomed. 2009;4:299–319. doi: 10.2147/ijn.s4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Sung JC, Pulliam BL, Edwards DA. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007;25(12):563–70. doi: 10.1016/j.tibtech.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 278.Urakami T, Jarvinen TA, Toba M, et al. Peptide-directed highly selective targeting of pulmonary arterial hypertension. Am J Pathol. 2011;178(6):2489–95. doi: 10.1016/j.ajpath.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]