Abstract
β-Glucans are important carbohydrate antigens on the surface of fungal cells useful for antifungal vaccine development. This paper has described a highly convergent and efficient strategy for the synthesis of structurally defined branched β-glucan oligosaccharides that can be used for detailed studies of β-glucans and for the design of β-glucan-based vaccines. The strategy was highlighted by assembling the title compounds via preactivation-based glycosylation with thioglycosides as glycosyl donors. It was used to successfully prepare β-glucan oligosaccharides that had a β-1,3-linked nonaglucan backbone with β-1,6-glucotetraose, β-1,3-glucodiose and β-1,3-glucotetraose branches at the 6-O-position of the nonaglucan central sugar unit. The structure and size of the glycosyl donors and acceptors used in the syntheses did not significantly affect the glycosylation efficiency, suggesting that the strategy can be generally useful for the synthesis of more complex structures.
Keywords: fungus, carbohydrate, β-glucan, preactivation-based glycosylation
Graphical Abstract
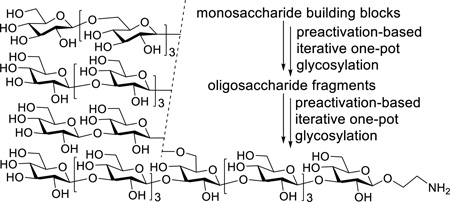
A highly convergent and efficient strategy relying on preactivation-based iterative glycosylation with thioglycosides as glycosyl donors was developed for the synthesis of 6-O-branched β-glucan oligosaccharides that can be used for detailed structure-activity relationship studies of β-glucans and for the design of β-glucan-based vaccines.
Introduction
With the drastic increase in fungal infections and antifungal drug resistance in the past two decades,[1] antifungal vaccines are in urgent demand.[2] For antifungal vaccine development, the unique polysaccharides,[3] especially β-1,3-linked glucans (known as β-glucans),[4] on the surface of fungal cells are attractive antigens.[5] Studies have shown that β-glucans are not only exposed but also consistently expressed and highly conserved on the cell surface of all pathogenic fungi.[6] It has also been shown that β-glucans could induce strong immune responses[4] and that a vaccine composed of natural β-glucans could engender effective protection against Candida albican and Aspergillus fumigatus infections in mouse.[7] In addition, the CRM197 protein conjugates of β-glucan oligosaccharides have been revealed to elicit immune responses comparable to that induced by the conjugates of natural β-glucans,[8] demonstrating that the oligosaccharide analogs of natural β-glucans are useful for antifungal vaccine development.
The structure of β-glucans has been well established.[3, 6] Their main carbohydrate chain is composed of approximately 1500 β-1,3-linked glucose units, with ca. 40–50 additional short β-1,6- or β-1,3-glucans attached to the main chain glucose 6-O-positions as branches.[9] However, the functions of the branches in β-glucans and their influence on the immunological properties of β-glucans have not been clarified. To study these issues and to develop β-glucan-based vaccines, it is essential to have access to homogeneous and structurally defined β-glucan oligosaccharides. Although several linear β-glucan oligosaccharides and an oligosaccharide with monosaccharide branches were previously prepared by hydrolysis of natural β-glucans or by chemical synthesis,[8] current strategies have drawbacks that have limited their synthetic efficiency and applicability, especially in the synthesis of complex structures. Evidently, there is a need for an efficient and generally applicable method for β-glucan oligosaccharide synthesis. Recently, we described the efficient synthesis of linear β-glucan oligosaccharides[10] via preactivation-based glycosylation.[11] In the current paper, we report a highly convergent, efficient, and potentially generally applicable strategy for the synthesis of branched β-glucan oligosaccharides.
Results and Discussion
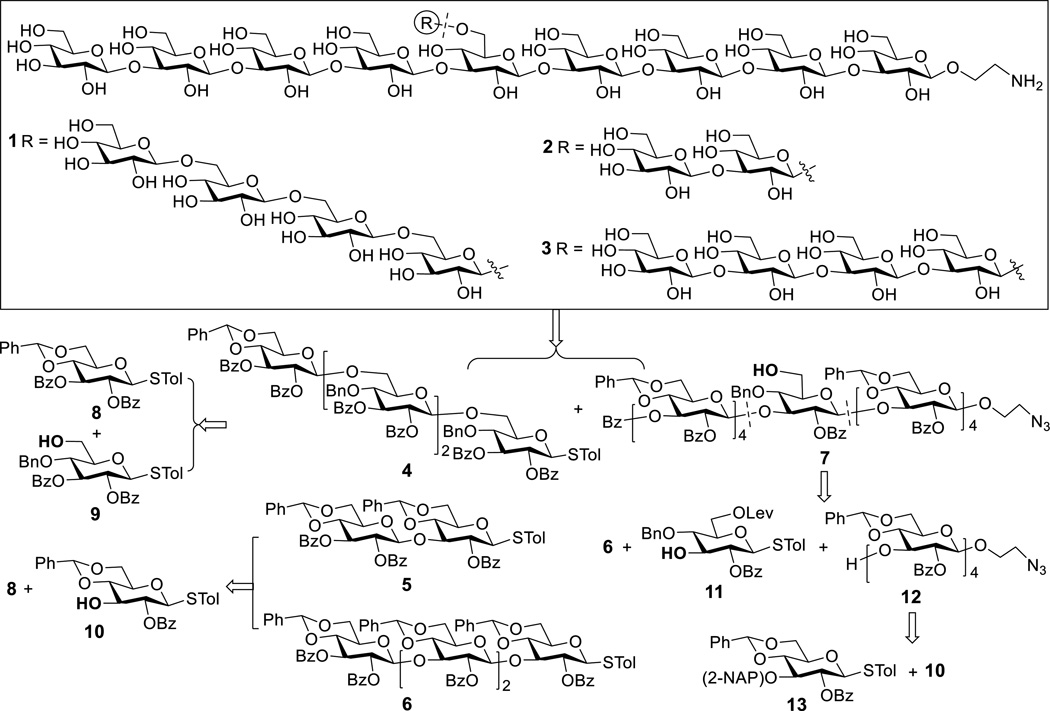
Our synthetic targets were 1–3 (Scheme 1), which had a β-1,3-linked nonaglucan backbone with branches, including β-1,6-glucotetraose (1), β-1,3-glucodiose (2) and β-1,3-glucotetraose (3), attached to the 6-O-position of the central sugar unit of the nona-β-glucan. They were supposed to span different structural properties and immunological determinant epitopes of natural β-glucans.[12] Moreover, we planned to attach a free aminoethyl group to the reducing end of these oligosaccharides to facilitate their conjugation with various biomolecules and tags, such as carrier proteins, to be useful for biological studies and conjugate vaccine development.
Scheme 1.
Structures of the designed β-glucan oligosaccharides 1–3 having β-1,6-tetraglucose (1), β-1,3-diglucose (2) and β-1,3-tetraglucose (3) branches attached to the 6-O-position of the central glucose unit of a nonasaccharide and their retrosynthetic plan
For the synthesis of target molecules 1–3, we were interested in a strategy that was built on preactivation-based iterative one-pot glycosylation employing thioglycosides as glycosyl donors, as the synthetic strategy has been proved to be simple and efficient due to elimination of multiple intermediate conversion and separation steps.[11] Accordingly, our synthetic plan (Scheme 1) was to separately prepare branches 4–6 as glycosyl donors and backbone nonasaccharide 7 as glycosyl acceptor and then stitch them together to get fully protected oligosaccharides, which were finally deprotected. Such a synthetic strategy can be widely applicable by using different donor branches and/or backbone acceptors that can also have varied sites of branch. In turn, oligosaccharides 4–6 could be prepared from monosaccharides 8, 9, and 10 via iterative one-pot glycosylation. Key intermediate 7 could be also constructed through one-pot glycosylation using 6, 11 and 12. The synthesis of 12 would be similar to that of 6, with 13 and 10 as building blocks. In 13, the 3-O-position would be temporarily protected with the 2-naphthylmethyl (NAP) group, which can be removed with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ)[13] but more stable to acids than the comparable p-methoxybenzyl group. As a result, this position would be selectively exposed for the sugar chain elongation later on. In addition, the 2-O-positions of all glycosyl donors were protected with the benzoyl (Bz) group to ensure β-selective glycosylations by taking advantage of neighboring group participation.
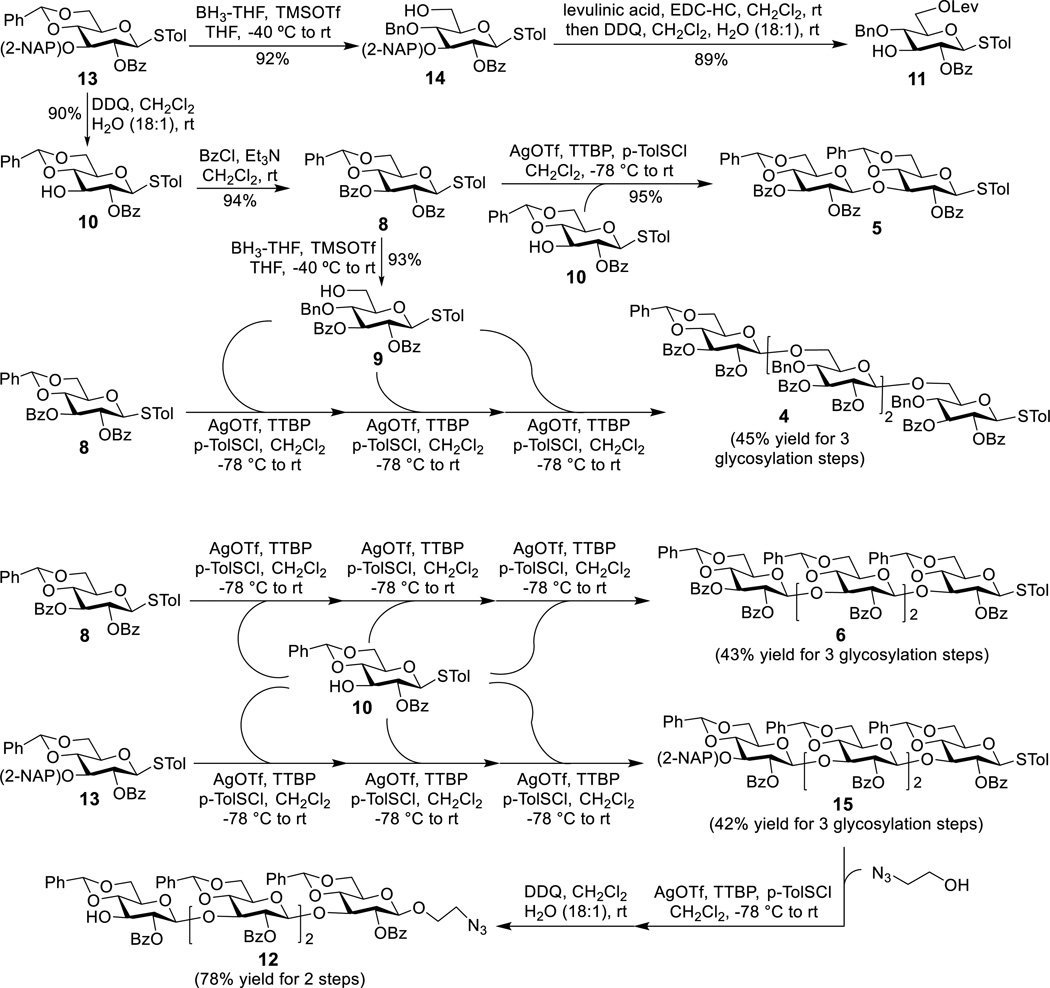
Our synthesis commenced with the preparation of 13 according to a reported procedure.[14] Regioselective removal of the NAP group at the 3-O-position in 13 with DDQ, followed by 3-O-benzoylation and then regioselective reductive ring opening of the benzylidene acetal in 8 using BH3·THF and trimethylsilyl trifluoromethanesulfonate (TMSOTf), afforded 9 (Scheme 2). On the other hand, reductive ring opening of the benzylidene acetal in 13, followed by protection of the exposed 6-O-position with a levulinoyl (Lev) group through reaction with levulinic acid and N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC·HCl) and then deprotection of the 3-O-position with DDQ, produced 11. Consequently, all of the required monosaccharide building blocks were readily synthesized from 13 with excellent overall yields.
Scheme 2.
Synthesis of the mono-, di- and tetrasaccharide building blocks
For the synthesis of disaccharide building block 5, the preactivation glycosylation protocol was applied.[11a] First, glycosyl donor 8 was treated with the promoter p-toluenesulfenyl triflate (p-TolSOTf, 1.0 equiv.), which was formed in situ from the reaction of p-toluenesulfenyl chloride (p-TolSCl) with silver triflate (AgOTf), at −78 °C for 10 min, and then glycosyl acceptor 10 (0.9 equiv.) was added for glycosylation. The reaction was β-specific to accomplish 5 in a 95% yield. Starting from 8, tetrasaccharide building blocks 4 and 6 were prepared through preactivation-based iterative one-pot glycosylation using 9 and 10 as glycosyl donors, respectively (Scheme 2).[11] Preactivation of the thioglycosyl donors with p-TolSOTf was carried out at −78 °C for 10 min in a mixture of dichloromethane and acetonitrile. After the donor was completely consumed (in ca. 5 min at −78 °C, shown by TLC), 0.9 equivalent of an acceptor was added together with 2,4,6-tri-tert-butylpyrimidine (TTBP), which was used to neutralize trifluoromethanesulfonic acid formed from the glycosylation reaction. The reaction was warmed to room temperature for ca. 20 min to guarantee complete consumption of the accepter as indicated by TLC. Then, the mixture was cooled to −78 °C to perform another round of preactivation and glycosylation by the same protocol. After the third round of glycosylation and then workup, 4 and 6 were obtained in 45% and 43% isolated yields, respectively. Similarly, tetrasaccharide 15 was prepared from 13 and 10 via iterative one-pot glycosylation in an overall yield of 42%. These results suggested that each glycosylation step gave an average of more than 75% yield and that the overall yields did not show a significant difference for β-1,6- and β-1,3-linked tetrasaccharides. Eventually, 15 was transformed into building block 12 upon glycosylation with 2-azidoethanol in the presence of p-TolSCl/AgOTf and removal of the 2-NAP protecting group with DDQ. All of the glycosylation reactions were β-specific, confirmed by the 1H NMR spectra of 4, 5, 6 and 12 with the coupling constants in the range of 6.2–10.1 Hz for all anomeric protons.
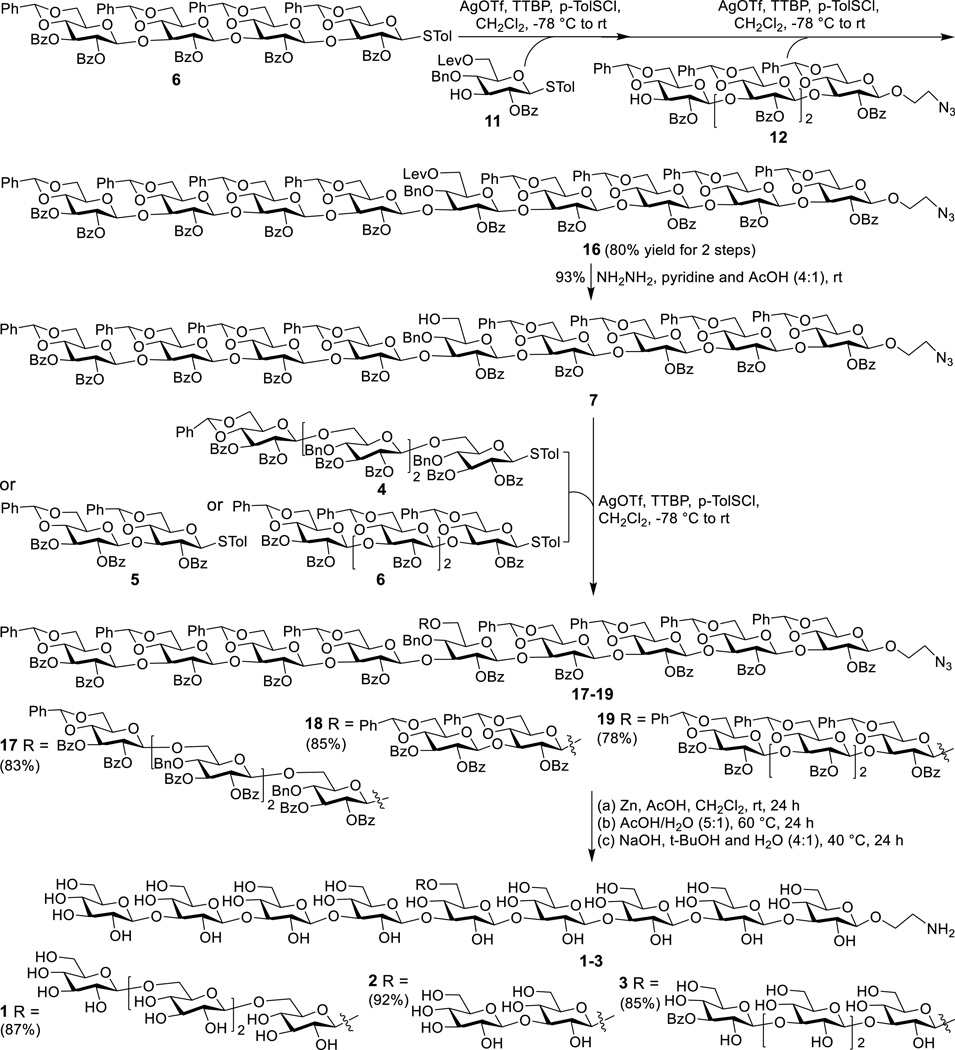
The preactivation-based one-pot glycosylation protocol was also used to prepare protected nonasaccharide 16 from 6, 11 and 12 (Scheme 3). Delightfully, these reactions gave an excellent overall yield (80%), despite that they involved rather complex glycosyl donors and acceptors. Thereafter, the Lev group at the 6-O-position of the central sugar residue in 16 was selectively removed with hydrazine to accomplish 7. Glycosylation of 7 with 4, 5 and 6 in the presence of p-TolSCl/AgOTf to install the branches was smooth and gave full protected target molecules 17, 18 and 19, respectively, in very good yields. Global deprotection of 17–19 was performed by a stepwise, one-pot protocol to deal with the solubility problem of various partially deprotected reaction intermediates. Thus, 17–19 were first treated with Zn and acetic acid in dichloromethane to reduce the azide group. After filtration to remove solids and concentration to remove solvents, the crude product was dissolved in acetic acid and water (5:1) and was heated at 60 °C to remove all of the benzylidene groups. Finally, the benzoyl groups were removed with sodium hydroxide in tert-butanol and water (4:1) to afford the desired products 1, 2 and 3 that were purified with a Sephadex-G25 size exclusion column and fully characterized with 1D and 2D NMR, as well as HR MS. Although the intermediates involved in the global deprotection process were not purified, the reactions were carefully monitored with MS to make sure that each step was complete. This was critical for the successful and clean global deprotection and for product purification.
Scheme 3.
Synthesis of the target oligosaccharides 1–3
Conclusion
Research towards β-glucan-based antifungal vaccines has made promising progresses in the recent decades, but a significant hindrance in this area is the access to complex, structurally well-defined β-glucan oligosaccharides used for detailed structure-activity relationship studies and for glycoconjugate vaccine preparation. We described herein a new, highly convergent and efficient strategy for the synthesis of branched β-glucan oligosaccharides. The strategy was highlighted by the application of preactivation-based iterative one-pot glycosylation to the efficient construction of the intermediate oligosaccharide fragments and target molecules. This can remarkably reduce the number of synthetic and purification steps as compared to the reported methods.[8b, 15] Three complex β-glucan oligosaccharides with β-1,3- and β-1,6-oligoglucose branches were efficiently assembled by the new strategy. It was further observed that the structure and the size of glycosyl donors and acceptors employed for preactivation-based glycosylation had little influence on the reaction efficiency, suggesting that this synthetic strategy may be broadly useful for constructing more complex oligosaccharides with good yields. For example, significantly larger branch and backbone oligosaccharides can be readily assembled by the procedures shown in Scheme 2 and utilized to construct large oligosaccharides by the procedures shown in Scheme 3. Furthermore, the synthetic targets were designed to bear a free amino group at their reducing end, which will facilitate their conjugation with other molecules, such as carrier proteins, via bifunctional linkers. The resulting glycoconjugates can be used to investigate the functions of branches in β-glucans, gain insights into their structure-activity relationships, and so on.
Experimental Section
General Methods
Chemicals and materials were obtained from commercial sources and were used as received without additional purification unless otherwise noted. 4Å MS was flame-dried under high vacuum and used immediately after cooling under a N2 atmosphere. Analytical TLC was carried out on silica gel 60Å F254 plates with detection by a UV detector and/or by charring with 15% (v/v) H2SO4 in EtOH. NMR spectra were recorded on a 400, 500 or 600 MHz machine with chemical shifts reported in ppm (δ) downfield from tetramethylsilane (TMS) that was used as an internal reference. Mass spectrometry (MS) was performed using either a Bruker Daltonics Ultraflex MALDI TOF MS or Waters LCT Premier XE high resolution ESI TOF MS instrument.
p-Tolyl 2-O-Benzoyl-4:6-O-benzylidene-1-thio-β-D-glucopyranoside (10).[13] To a stirred solution of 13 (7.88 g, 12.73 mmol) in CH2Cl2 (400 mL) and water (22 mL) was added DDQ (5.78 g, 25.47 mmol) at room temperature (rt). After the reaction was stirred at rt for 8 h, saturated aq. NaHCO3 solution was added, and the mixture was extracted with CH2Cl2. The extracts were washed with saturated aq. NaHCO3 solution and dried over Na2SO4. After evaporation of the solvent in vacuum, the product was purified by silica gel column chromatography (toluene/ethyl acetate 15:1 to 10:1) to give 10 (5.48 g, 90% yield) as a white solid. 1H NMR (600 MHz, CDCl3) δ: 8.14–8.02 (m, 2H), 7.60 (t, J = 7.4 Hz, 1H), 7.52–7.42 (m, 4H), 7.36 (m, 5H), 7.28–7.06 (m, 3H), 5.55 (s, 1H), 5.17–5.05 (t, J = 9.12 Hz, 1H), 4.83 (d, J = 10.0 Hz, 1H, H-1), 4.41 (dd, J = 10.6, 4.9 Hz, 1H), 4.04 (m, 1H), 3.81 (t, J = 10.2 Hz, 1H), 3.66–3.50 (m, 2H), 2.76 (d, J = 3.2 Hz, 1H), 2.34 (d, J = 8.8 Hz, 3H). 13C-NMR (150 MHz, CDCl3) δ: 165.88, 138.72, 136.79, 133.76, 133.43, 130.03, 129.73, 129.50, 129.33, 128.46, 128.34, 127.83, 126.26, 101.91, 86.76 (C-1), 80.61, 77.21, 77.00, 76.79, 73.73, 73.24, 70.39, 68.50, 21.18. MS (ESI TOF): calcd. for C27H26NaO6S [M+Na]+ m/z, 501.1; found, 501.1.
p-Tolyl 2,3-di-O-Benzoyl-4:6-O-benzylidene-1-thio-β-D-glucopyranoside (8).[16] To a solution of 10 (10.00 g, 20.09 mmol), TEA (17.9 mL, 127.27 mmol) and catalytic amount of DMAP in anhydrous CH2Cl2 (160 mL) was added benzoyl chloride (3.7 mL, 31.37 mmol) at 0 °C. After being stirred for 12 h, the reaction mixture was washed with saturated aq. NaHCO3 solution and brine, dried over Na2SO4, and concentrated under vacuum. The residue was purified by silica gel column chromatography (ethyl acetate/toluene 1:20) to afford 8 (11.44 g, 94%) as a white solid. 1H NMR (600 MHz, CDCl3) δ: 7.98 (d, J = 7.3 Hz, 2H, Ph), 7.93 (d, J = 7.3 Hz, 2H, Ph), 7.52 (t, J = 7.4 Hz, 1H, Ph), 7.47 (t, J = 7.4 Hz, 1H, Ph), 7.43–7.21 (m, 11H, Ph), 7.12 (d, J = 7.9 Hz, 2H, Ph), 5.78 (t, J = 9.5 Hz, 1H, H-3), 5.53 (s, 1H, CHPh), 5.45 (t, J = 9.6 Hz, 1H, H-2), 4.96 (d, J = 10.0 Hz, 1H, H-1), 4.45 (dd, J = 10.6, 4.9 Hz, 1H, H-6), 3.94–3.82 (m, 2H, H4, H5), 3.74 (dd, J = 9.6, 4.9 Hz, 1H, H6), 2.35 (s, 3H, CH3). 13C NMR (150 MHz, CDCl3) δ: 165.56, 165.15, 138.74, 136.71, 133.73, 133.27, 133.05, 129.86, 129.77, 129.76, 129.37, 129.25, 129.01, 128.37, 128.26, 128.16, 127.90, 126.10, 101.44, 87.25 (C-1), 78.57, 77.22, 77.01, 76.80, 73.34, 71.07, 70.91, 68.53, 21.19. HRMS (ESI TOF): calcd. for C34H31O7S [M+H]+ m/z, 583.1790; found, 583.1799.
p-Tolyl 2,3-di-O-Benzoyl-4-O-benzyl-1-thio-β-D-glucopyranoside (9). After a mixture of 8 (3.50 g, 6.00 mmol) and 4Å MS (8 g) in anhydrous THF (120 mL) was stirred at rt for 1 h and then cooled to −40 °C, BH3·THF (29.7 mL, 30.00 mmol; 1 M solution in THF) was added. The mixture was stirred for 15 min, followed by the addition of TMSOTf (1.41 mL, 7.80 mmol) and stirring at −40 °C for another hour. The reaction mixture was slowly warmed to rt and stirred for 24 h. Then, saturated aq. NaHCO3 solution was added at 0 °C, and the mixture was diluted with CH2Cl2 and filtrated to remove insoluble materials. The organic layer was washed with saturated aq. NaHCO3 solution and brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography (ethyl acetate/toluene 1:25) to produce 9 (3.26 g, 93%) as a white solid. 1H NMR (600 MHz, CDCl3) δ: 7.95 (dd, J = 8.2, 1.0 Hz, 2H, Ph), 7.90 (dd, J = 8.2, 1.0 Hz, 2H, Ph), 7.53–7.46 (m, 2H, Ph), 7.40–7.32 (m, 6H, Ph), 7.20–7.07 (m, 7H, Ph), 5.72 (t, J = 9.4 Hz, 1H, H-3), 5.33 (t, J = 9.8 Hz, 1H, H-2), 4.89 (d, J = 10.0 Hz, 1H, H-1), 4.57 (s, 2H, CH2Ph), 4.00 – 3.94 (m, 1H, H-6), 3.89 (t, J = 9.5 Hz, 1H, H-4), 3.79 (m, 1H, H-6), 3.65–3.56 (m, 1H, H-5), 2.33 (s, 3H), 2.08– 1.95 (br, 1H, OH). 13C NMR (150 MHz, CDCl3) δ: 165.65, 165.29, 138.61, 137.08, 133.41, 133.22, 133.17, 129.85, 129.78, 129.71, 129.33, 129.26, 128.35, 128.16, 127.96, 86.29 (C-1), 79.53, 76.23, 75.33, 74.84, 70.88, 61.73, 21.17. HRMS (ESI TOF): calcd. for C34H33O7S [M+H]+ m/z, 585.1947; found, 585.1941.
p-Tolyl 2-O-Benzoyl-4-O-benzyl-3-O-(naphthalen-2-ylmethyl)-1-thio-β-D-glucopyranoside (14).[13] Compound 14 (1.84 g, 92%) was prepared from 13 (2.00 g, 3.23 mmol) by the same procedure described for 9. 1H-NMR (500 MHz, CDCl3) δ: 7.99 (d, J = 7.32 Hz, 2H, Ph), 7.70–7.67 (m, 1H, Ph), 7.63-7.60 (m, 1H, Ph), 7.57–7.51 (m, 3H, Ph), 7.42-7.29 (m, 11H, Ph), 7.27–7.24 (m, 1H, Ph), 7.10 (d, J = 7.63 Hz, 2H, Ph), 5.28 (dd, J = 9.77, 8.55 Hz, 1H, H-2), 4.93 (d, J = 11.60 Hz, 1H, ½ CH2Ar), 4.90 (d, J = 11.29 Hz, 1H, ½CH2Ar), 4.82 (d, J = 11.29 Hz, 1H, ½CH2Ar), 4.76 (d, J = 10.07 Hz, 1H, H-1), 4.71 (d, J = 10.68 Hz, 1H, ½CH2Ar), 3.96-3.90 (m, 2H, H-3 and H-6), 3.78-3.71 (m, 2H, H-4 and H-6), 3.53–3.49 (m, 1H, H-5), 2.33 (s, 3H, CH3), 1.93 (br s, 1H, OH). 13C-NMR (125 MHz, CDCl3) δ: 165.20, 138.43, 137.73, 135.10, 133.32, 133.21, 133.07, 132.90, 129.78, 129.73, 128.56, 128.49, 128.36, 128.16, 128.10, 128.04, 127.85, 127.64, 126.90, 126.07, 125.94, 125.81, 86.36 (C-1), 83.91, 79.54, 77.62, 75.39, 75.23, 72.42, 62.04, 21.17. HRMS (ESI TOF): calcd. for C38H36NaO6S [M+Na]+ m/z, 643.2130; found, 643.2139.
p-Tolyl 2-O-Benzoyl-4-O-benzyl-6-O-levulinoyl-1-thio-β-D-glucopyranoside (11). A mixture of 14 (3.72 g, 6.00 mmol), levulinic acid (0.84 g, 7.23 mmol) and EDC•HCl (1.38g, 7.20 mmol) in CH2Cl2 (50 mL) was stirred at rt for 4 h. The reaction mixture was washed with water and brine, dried over Na2SO4 and concentrated under vacuum. The residue was dissolved in a solution of CH2Cl2 (100 mL) and water (1.5 mL) at rt and then DDQ (2.72 g, 12.00 mmol) was added. After being stirred at rt for 6 h, the mixture was washed with saturated aq. NaHCO3 solution and brine, dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (ethyl acetate/toluene 1:10) to produce 11 (3.10 g, 89%) as a foamy solid. 1H NMR (500 MHz, CDCl3) δ: 8.16–8.06 (m, 2H, Ph), 7.66–7.56 (m, 1H, Ph), 7.49 (t, J = 7.8 Hz, 2H, Ph), 7.39–7.21 (m, 7H, Ph), 7.10 (d, J = 7.9 Hz, 2H, Ph), 4.98 (t, J = 9.5 Hz, 1H, H-3), 4.87 (d, J = 11.2 Hz, 1H, H-1), 4.72 (dd, J = 15.6, 10.6 Hz, 2H, CH2Ph), 4.46 (dd, J = 11.8, 2.0 Hz, 1H, H-6), 4.28 (dd, J = 11.8, 5.2 Hz, 1H, H-6), 3.96 (td, J = 8.9, 2.6 Hz, 1H, H-3), 3.61 (m, 1H, H-5), 3.56–3.48 (m, 1H, H-4), 2.88 (d, J = 3.4 Hz, 1H, OH), 2.77 (t, J = 6.6 Hz, 2H, CH2CO2), 2.69–2.56 (m, 2H, CH2CO), 2.34 (s, 3H, CH3), 2.21 (s, 3H, CH3). 13C NMR (125 MHz, CDCl3) δ: 206.50, 172.43, 166.43, 138.44, 137.74, 133.53, 133.48, 130.05, 129.63, 129.45, 128.59, 128.51, 128.26, 128.08, 85.70, 77.69, 77.59, 76.86, 74.97, 73.34, 63.28, 37.91, 29.92, 27.88, 21.19. HRMS (ESI TOF): calcd. for C32H35O8S[M+H]+ m/z, 579.2053; found, 579.2051.
p-Tolyl [2,3-di-O-Benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-2-O-benzoyl-4:6-O-benzylidene-1-thio-β-D-glucopyranoside (5). After a mixture of glycosyl donor 8 (300.0 mg, 0.52 mmol) and 4Å MS (1.50 g) in anhydrous CH2Cl2 (10 mL) was stirred at rt for 1 h and then cooled to −78 °C, AgOTf (397.0 mg, 1.55 mmol in 3 mL dry acetonitrile) was added, followed by p-TolSCl (74 µL, 0.52 mmol) addition using a micro-syringe 10 min later. The mixture was stirred at −78 °C for another 15 min, when TLC showed that 8 was completely consumed. A solution of acceptor 10 (221.8 mg, 0.46 mmol) and TTBP (127.9 mg, 0.52 mmol) in CH2Cl2 (3 mL) was added. The mixture was stirred at −78 °C for 20 min and warmed to rt, followed by filtration to remove 4Å MS. The filtrate was washed with saturated aq. NaHCO3 solution and brine, dried over Na2SO4, and concentrated under vacuum. The residue was purified by silica gel column chromatography (ethyl acetate/toluene 1:30) to produce 5 (411.4 mg, 95%). 1H NMR (500 MHz, CDCl3) δ: 7.89 (d, J = 7.6 Hz, 2H), 7.81 (d, J = 7.6 Hz, 2H), 7.58 (t, J = 6.5 Hz, 4H), 7.48–7.19 (m, 19H), 7.08 (d, J = 7.9 Hz, 2H), 5.69–5.56 (m, 2H), 5.44 (t, J = 7.9 Hz, 1H), 5.34 (dd, J = 17.9, 8.7 Hz, 2H), 5.05 (d, J = 7.2 Hz, 1H, H″), 4.82 (d, J = 10.0 Hz, 1H′), 4.44 (dd, J = 10.5, 4.7 Hz, 1H), 4.33–4.22 (m, 2H), 3.95 (t, J = 9.5 Hz, 1H), 3.90–3.82 (m, 2H), 3.78 (t, J = 10.2 Hz, 1H), 3.68–3.53 (m, 2H), 2.34 (s, 3H). 13C NMR (125 MHz, CDCl3) δ: 165.54, 164.90, 164.64, 138.50, 137.12, 136.84, 133.37, 133.07, 132.75, 129.83, 129.72, 129.41, 129.33, 129.08, 128.99, 128.51, 128.40, 128.37, 128.25, 128.15, 128.11, 126.15, 126.11, 101.52, 101.30, 100.72, 87.63, 79.56, 79.32, 78.30, 72.90, 72.35, 72.07, 70.75, 68.68, 68.60, 66.27, 21.19. HRMS (ESI TOF): calcd. for C54H49NO13S [M+H]+ m/z, 937.2894; found, 937.2864.
p-Tolyl [2,3-di-O-Benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→6)-[2,3-di-O-benzoyl-4-O-benzyl-β-D-glucopyranosyl]-(1→6)-[2,3-di-O-benzoyl-4-O-benzyl-β-D-glucopyranosyl]-(1→6)-2,3-di-O-benzoyl-4-O-benzyl-1-thio-β-D-glucopyranoside (4). After a mixture of donor 8 (349.8 mg, 0.60 mmol) and activated 4Å MS in CH2Cl2 (8 mL) was stirred at rt for 1 h and then cooled to −78 °C, AgOTf (462.5 mg, 1.80 mmol in 1.5 mL dry acetonitrile) was added, followed by p-TolSCl (86 µL, 0.60 mmol) addition using a micro-syringe 10 min later. The mixture was stirred for another 15 min when TLC showed that 8 was completely consumed. Then, a solution of acceptor 9 (316.2 mg, 0.54 mmol) and TTBP (122.1 mg, 0.54 mmol) in CH2Cl2 (2 mL) was added. The mixture was allowed to warm to rt slowly over 1 h and stirred at rt for another 20 min. The mixture was then cooled to −78 °C to perform another round of glycosylation with 9 (288.1 mg, 0.49 mmol) as the glycosyl acceptor by the same protocol, which was followed by the third round of glycosylation also with 9 (262.3 mg, 0.45 mmol) as glycosyl acceptor. Finally, the reaction mixture was warmed to rt, stirred for 20 min, and then quenched with saturated aq. NaHCO3 solution. The mixture was filtered to remove insoluble materials, and the organic layer was washed with saturated aq. NaHCO3 solution and brine, dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (ethyl acetate/toluene1:12) to give 4 (395.7 mg, 45%) as a white solid. 1H NMR (600 MHz, CDCl3) δ: 8.03 (d, J = 7.9 Hz, 2H), 8.01–7.89 (m, 11H), 7.85 (d, J = 8.0 Hz, 2H), 7.60–7.04 (m, 40H), 7.01 (t, J = 7.4 Hz, 1H), 6.99–6.91 (m, 4H), 6.88 (t, J = 7.5 Hz, 2H), 6.73 (d, J = 7.7 Hz, 2H), 5.85 (t, J = 9.6 Hz, 1H), 5.78 (t, J = 9.5 Hz, 1H), 5.71–5.60 (m, 3H), 5.47 (dd, J = 10.7, 7.0 Hz, 3H), 5.30 (t, J = 9.7 Hz, 1H), 4.98–4.87 (m, 3H, anomeric), 4.58 (d, J = 7.9 Hz, 1H, anomeric), 4.35 (dt, J = 10.5, 5.2 Hz, 4H), 4.19 (m, 5H), 4.06 (d, J = 11.3 Hz, 1H), 3.91 (m, 5H), 3.74 (m, 4H), 3.63 (d, J = 9.5 Hz, 1H), 3.54 (dd, J = 11.5, 6.3 Hz, 1H), 2.39 (s, 3H). 13C NMR (150 MHz, CDCl3) δ: 165.79, 165.68, 165.64, 165.60, 165.29, 165.23, 165.10, 164.81, 138.08, 137.14, 137.06, 136.92, 134.31, 133.30, 133.16, 133.09, 133.01, 132.97, 132.88, 132.77, 130.02, 129.89, 129.82, 129.78, 129.73, 129.67, 129.62, 129.56, 129.49, 129.46, 129.39, 129.33, 128.95, 128.93, 128.58, 128.50, 128.40, 128.29, 128.26, 128.22, 128.19, 128.14, 127.98, 127.92, 127.88, 127.80, 127.54, 126.08, 102.93, 101.70, 101.10, 101.06, 85.45, 79.17, 78.38, 77.83, 76.69, 76.62, 75.61, 75.15, 74.96, 74.89, 74.86, 74.77, 73.66, 72.94, 72.48, 72.15, 71.99, 70.75, 69.75, 69.39, 68.88, 68.36, 66.82, 21.30. HRMS (ESI TOF): calcd. for C115H106NO28S [M+NH4]+ m/z, 1980.6622; found, 1980.6481.
p-Tolyl [2,3-di-O-Benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-2-O-benzoyl-4:6-O-benzylidene-1-thio-β-D-glucopyranoside (6). Compound 6 (415.3 mg, 43%) was prepared from 8 (475.0 mg, 0.82 mmol) and 10 (1st glycosylation: 341.5 mg, 0.72 mmol; 2nd glycosylation: 310.8 mg, 0.65 mmol; 3rd glycosylation: 282.8 mg, 0.59 mmol) after 3 rounds of glycosylation reactions by the protocol described for 4 and was purified by silica gel column chromatography (ethyl acetate/toluene 1:12). 1H NMR (600 MHz, CDCl3) δ: 7.90 (t, J = 7.6 Hz, 4H), 7.81 (d, J = 7.4 Hz, 2H), 7.70 (d, J = 7.5 Hz, 2H), 7.67 (d, J = 7.4 Hz, 2H), 7.60 (t, J = 7.4 Hz, 1H), 7.54 (d, J = 7.4 Hz, 2H), 7.52–7.11 (m, 34H), 7.07 (d, J = 8.0 Hz, 2H), 5.67 (t, J = 9.2 Hz, 1H), 5.48 (dd, J = 10.4, 5.8 Hz, 2H), 5.39 (s, 1H), 5.27 (t, J = 6.5 Hz, 1H), 5.16 (s, 1H), 5.12 (d, J = 7.5 Hz, 1H, anomeric), 5.07 (d, J = 6.4 Hz, 1H, anomeric), 4.89–4.81 (m, 2H, anomeric), 4.76 (t, J = 9.3 Hz, 1H), 4.66 (d, J = 10.1 Hz, 1H, anomeric), 4.50 (s, 1H), 4.34 (dd, J = 10.5, 4.9 Hz, 1H), 4.27 (dd, J = 10.5, 4.9 Hz, 1H), 4.22–4.15 (m, 2H), 4.14–4.07 (m, 2H), 3.94 (ddd, J = 18.8, 15.5, 9.3 Hz, 3H), 3.76 (dt, J = 14.0, 9.6 Hz, 2H), 3.71–3.57 (m, 4H), 3.53 – 3.37 (m, 3H), 3.12 (t, J = 9.5 Hz, 1H), 2.32 (s, 3H). 13C NMR (150 MHz, CDCl3) δ: 165.48, 165.08, 164.86, 164.57, 164.54, 138.23, 137.30, 137.09, 136.88, 133.60, 133.17, 132.98, 132.73, 130.16, 129.82, 129.76, 129.70, 129.66, 129.45, 129.39, 129.11, 129.08, 129.04, 129.01, 128.94, 128.77, 128.47, 128.39, 128.34, 128.27, 128.23, 128.18, 128.12, 127.92, 126.47, 126.18, 126.14, 126.05, 125.31, 101.99, 101.31, 101.00, 100.62, 99.74, 97.62, 97.11, 87.89, 78.81, 78.60, 78.45, 77.74, 77.19, 75.15, 74.71, 73.19, 72.87, 72.78, 72.63, 72.29, 70.68, 68.69, 68.49, 66.21, 65.86, 65.26, 21.13. HRMS (ESI TOF): calcd. for C94H88NO25S [M+NH4] m/z, 1662.5366; found, 1662.5244.
p-Tolyl [2-O-Benzoyl-4:6-O-benzylidene-3-O-(naphthalen-2-ylmethyl)-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-2-O-benzoyl-4:6-O-benzylidene-1-thio-β-D-glucopyranoside (15). Compound 15 (312.5 mg, 42%) was prepared from 13 (475.0 mg, 0.82 mmol) and 10 (1st glycosylation: 258.4 mg, 0.54 mmol; 2nd glycosylation: 235.2 mg, 0.49 mmol; 3rd glycosylation: 214.4 mg, 0.45 mmol) after 3 rounds of glycosylation reactions by the same protocol described for 4, which was purified by silica gel column chromatography (ethyl acetate/toluene 1:12). 1H NMR (600 MHz, CDCl3) δ: 7.86 (d, J = 7.3 Hz, 2H), 7.79 (d, J = 7.3 Hz, 2H), 7.75 (d, J = 7.4 Hz, 2H), 7.69 (d, J = 8.0 Hz, 1H), 7.62 (d, J = 7.3 Hz, 2H), 7.59–7.14 (m, 40H), 7.06 (d, J = 8.0 Hz, 2H), 5.53 (s, 1H), 5.44 (s, 1H), 5.32 (t, J = 7.8 Hz, 1H), 5.17 (t, J = 5.8 Hz, 1H), 5.00–4.94 (m, 3H), 4.90 (d, J = 12.4 Hz, 1H), 4.85–4.75 (m, 4H), 4.66 (d, J = 10.1 Hz, 1H, anomeric), 4.61 (s, 1H), 4.35 (dd, J = 10.5, 4.9 Hz, 1H), 4.20 (dd, J = 10.4, 5.0 Hz, 1H), 4.16–4.04 (m, 4H), 3.95 (t, J = 8.8 Hz, 1H), 3.91–3.86 (m, 2H), 3.83 (t, J = 8.7 Hz, 1H), 3.72 (td, J = 10.2, 7.4 Hz, 2H), 3.58 (t, J = 9.1 Hz, 3H), 3.49–3.37 (m, 4H), 3.24 (t, J = 9.4 Hz, 1H), 2.32 (s, 3H). 13C NMR (150 MHz, CDCl3) δ: 164.99, 164.82, 164.54(2C), 138.25, 137.33, 137.29, 137.24, 137.07, 135.38, 133.50, 133.26, 133.17, 133.10, 133.03, 132.83, 132.74, 129.81, 129.78, 129.75, 129.70, 129.63, 129.56, 129.44, 129.41, 129.37, 129.04, 129.01, 128.96, 128.87, 128.63, 128.49, 128.39, 128.36, 128.24, 128.07, 127.97, 127.90, 127.88, 127.56, 126.67, 126.42, 126.24, 126.09, 126.00, 125.77, 125.62, 125.30, 101.93, 101.14, 100.87, 100.84, 99.30, 97.99, 96.93, 87.93, 81.22, 78.57, 78.49, 78.17, 77.10, 75.69, 74.29, 73.72, 73.45, 73.20, 73.01, 72.66, 70.67, 68.72, 68.51, 66.01, 65.67, 65.38, 21.15. HRMS (ESI TOF): calcd. for C98H92NO24S [M+NH4]+ m/z, 1698.5730; found, 1698.5598.
2-Azidoethyl [2-O-Benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranoside (12). Glycosylation of azidoethanol (15.8 mg, 0.18 mmol) with 15 (305.5 mg, 0.18 mmol) by the same protocol described for 5 afforded a crude trisaccharyl glycoside intermediate that was directly dissolved in CH2Cl2 (10 mL) and water (0.5 mL) and treated with DDQ (82.5 mg, 0.36 mmol). After the reaction mixture was stirred at rt for 6 h, it was washed with saturated aq. NaHCO3 solution and brine, dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (ethyl acetate/toluene 1:8) to produce 12 (213.6 mg, 78%). 1H NMR (500 MHz, CDCl3) δ: 7.95 (d, J = 7.8 Hz, 2H), 7.90 (d, J = 7.9 Hz, 2H), 7.82 (d, J = 7.8 Hz, 2H), 7.70 (d, J = 7.9 Hz, 2H), 7.62–7.16 (m, 32H), 5.57 (s, 1H), 5.44 (s, 1H), 5.21 (dd, J = 9.3, 6.6 Hz, 2H, anomeric), 5.08 (d, J = 7.4 Hz, 1H, anomeric), 5.03 (d, J = 5.2 Hz, 1H, anomeric), 4.97 (t, J = 8.1 Hz, 1H), 4.91–4.85 (m, 2H), 4.59 (d, J = 7.6 Hz, 1H, anomeric), 4.37 (dd, J = 10.4, 4.8 Hz, 1H), 4.23 (dd, J = 10.4, 4.9 Hz, 1H), 4.14 (m, 3H), 3.98 (m, 3H), 3.89 (dd, J = 10.4, 4.8 Hz, 1H), 3.80–3.38 (m, 11H), 3.33 – 3.22 (m, 2H), 2.71 (bs, 1H). 13C NMR (125 MHz, CDCl3) δ: 165.82, 164.68, 164.64, 164.60, 137.34, 137.26, 137.13, 137.06, 133.62, 133.41, 133.16, 133.11, 129.91, 129.77, 129.72, 129.68, 129.46, 129.41, 129.31, 129.25, 129.11, 129.07, 128.94, 128.66, 128.59, 128.40, 128.37, 128.33, 128.29, 128.26, 128.07, 126.42, 126.33, 126.11, 125.34, 101.88, 101.76, 101.28, 101.16, 100.79, 98.85, 98.42, 97.06, 80.81, 78.74, 78.30, 77.52, 76.94, 75.08, 74.88, 74.43, 73.78, 73.50, 72.65, 72.49, 68.71, 68.65, 67.89, 66.56, 66.04, 65.58, 50.68. HRMS (ESI TOF): calcd. for C82H81N4O25 [M+NH4]+ m/z, 1521.5190; found, 1521.5090.
2-Azidoethyl [2,3-di-O-Benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4-O-benzyl-6-O-levulinoyl-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranoside (16). After a mixture of 6 (329.1 mg, 0.20 mmol) and activated 4Å MS in CH2Cl2 (4 mL) was stirred at rt for 1 h and then cooled to −78 °C, AgOTf (154.2 mg, 0.60 mmol in 1.5 mL dry acetonitrile) was added, followed by addition of p-TolSCl (29 µL, 0.20 mmol) via a micro-syringe 10 min later. The mixture was stirred for another 15 min, when TLC indicated that donor 6 was completely consumed. A solution of 11 (104.2 mg, 0.18 mmol) and TTBP (44.7 mg, 0.18 mmol) in CH2Cl2 (1.5 mL) was added, and the mixture was warmed to rt slowly over 1 h. After stirring at rt for another 20 min, the mixture was cooled to −78 °C to perform glycosylation with 12 (246.4 mg, 0.16 mmol) by the same protocol using AgOTf (138.7 mg, 0.54 mmol in acetonitrile 1 mL), p-TolSCl (26 µL, 0.18 mmol), and TTBP (40.7 mg, 0.16 mmol). The reaction was finally quenched with saturated aq. NaHCO3 solution, and filtered to remove insoluble materials. The organic layer was washed with saturated aq. NaHCO3 solution and brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (ethyl acetate/toluene 1:10) to give 16 (455.3 mg, 80%). 1H NMR (600 MHz, CDCl3) δ: 7.89 (d, J = 8.2 Hz, 2H), 7.86 (d, J = 8.1 Hz, 2H), 7.74 (m, 10H), 7.62 (m, 8H), 7.55–7.13 (m, 73H), 5.65 (t, J = 9.2 Hz, 1H), 5.53 (s, 1H), 5.46–5.42 (m, 1H), 5.38 (s, 1H), 5.30 (s, 1H), 5.13 (dd, J = 9.7, 6.6 Hz, 2H), 4.98–4.73 (m, 17H), 4.73–4.68 (m, 2H), 4.55 (d, J = 7.6 Hz, 1H), 4.33 (dd, J = 10.3, 4.8 Hz, 1H), 4.25 (td, J = 11.0, 4.6 Hz, 2H), 4.20–4.01 (m, 11H), 4.00–3.84 (m, 8H), 3.78–3.67 (m, 3H), 3.63–3.34 (m, 20H), 3.26 (ddd, J = 13.9, 13.2, 7.1 Hz, 5H), 2.59 (dd, J = 11.3, 6.3 Hz, 2H), 2.44 (dd, J = 10.9, 6.3 Hz, 2H), 2.10 (s, 3H). 13C NMR (150 MHz, CDCl3) δ: 206.42, 172.46, 170.62, 165.47, 165.11, 164.69, 164.55, 164.48, 138.18, 137.86, 137.46, 137.30, 137.24, 137.18, 136.91, 133.67, 133.58, 133.48, 133.35, 133.18, 133.00, 129.88, 129.77, 129.72, 129.64, 129.54, 129.48, 129.42, 129.33, 129.17, 129.07, 128.96, 128.65, 128.53, 128.47, 128.42, 128.30, 128.26, 128.11, 127.73, 126.48, 126.41, 126.34, 126.15, 126.08, 125.34, 101.98, 101.50, 101.31, 101.25, 101.21, 101.16, 101.08, 100.80, 99.19, 99.11, 98.40, 98.01, 97.46, 97.11, 96.79, 96.70, 79.31, 78.78, 78.75, 78.67, 78.36, 77.94, 77.91, 77.58, 77.27, 75.26, 75.02, 74.96, 74.70, 74.60, 74.45, 74.15, 73.79, 73.64, 73.56, 73.23, 73.15, 73.02, 72.57, 72.49, 72.31, 72.05, 68.72, 68.65, 67.94, 66.49, 66.39, 66.27, 65.62, 65.53, 63.75, 50.66, 37.87, 29.85, 29.53, 27.90. HRMS (ESI TOF): calcd. for C194H181N3O58 [M+2H]2+ m/z, 1740.0653; found, 1740.0428.
2-Azidoethyl [2,3-di-O-Benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4-O-benzyl-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranoside (7). A mixture of 16 (420.0 mg, 120.7 µmol) and 10 mL of 0.5 M hydrazine solution in pyridine-acetic acid (4:1) buffer was stirred under an Ar atmosphere at rt for 1h. Then 2,4-pentanedione (1 ml) was added, and the stirring continued for another 20 min. The mixture was diluted with CH2Cl2, washed sequentially with saturated aq. NaHCO3, CuSO4 and NH4Cl solutions, dried over Na2SO4, and concentrated under vacuum. The residue was purified by silica gel column chromatography (ethyl acetate/toluene 1:8) to give 7 (380.6 mg, 93%) as a white foamy solid. 1H NMR (600 MHz, CDCl3) δ: 7.90 (d, J = 7.6 Hz, 2H), 7.86 (d, J = 7.6 Hz, 2H), 7.77 (d, J = 7.7 Hz, 2H), 7.73–7.57 (m, 16H), 7.55–7.42 (m, 11H), 7.42–7.19 (m, 62H), 5.66 (t, J = 9.2 Hz, 1H), 5.52 (s, 1H), 5.48–5.43 (m, 1H), 5.39 (s, 1H), 5.27 (s, 1H), 5.13 (dd, J = 9.5, 4.2 Hz, 2H), 4.93–4.79 (m, 14H), 4.78–4.70 (m, 5H), 4.55 (d, J = 7.6 Hz, 1H), 4.34 (dd, J = 10.4, 4.7 Hz, 1H), 4.25 (dt, J = 10.7, 5.3 Hz, 2H), 4.18–4.08 (m, 7H), 4.06–4.01 (m, 2H), 3.99–3.85 (m, 8H), 3.76–3.68 (m, 2H), 3.66–3.58 (m, 3H), 3.57–3.36 (m, 20H), 3.34–3.21 (m, 5H). 13C NMR (151 MHz, CDCl3) δ: 165.43, 165.07, 164.66, 164.62, 164.60, 164.57, 164.54, 164.52, 164.47, 164.40, 138.30, 137.29, 137.20, 137.17, 137.13, 136.87, 133.51, 133.44, 133.36, 133.32, 133.10, 132.96, 129.81, 129.75, 129.72, 129.62, 129.47, 129.39, 129.29, 129.24, 129.17, 129.08, 129.02, 128.95, 128.62, 128.57, 128.54, 128.47, 128.43, 128.38, 128.35, 128.29, 128.26, 128.21, 128.16, 128.11, 128.10, 128.07, 127.86, 127.69, 126.43, 126.34, 126.32, 126.29, 126.28, 126.26, 126.12, 126.04, 101.94, 101.44, 101.30, 101.24, 101.14, 101.11, 101.09, 100.78, 99.13, 99.06, 98.27, 98.23, 97.61, 97.44, 97.35, 96.87, 78.89, 78.69, 78.67, 78.34, 78.04, 77.95, 77.58, 77.46, 77.22, 75.50, 75.24, 75.02, 74.86, 74.83, 74.66, 74.34, 74.22, 73.78, 73.56, 73.53, 73.30, 73.26, 73.12, 72.94, 72.53, 72.45, 72.27, 68.69, 68.61, 67.91, 66.49, 66.29, 66.23, 65.62, 65.58, 65.54, 65.49, 62.13, 50.66. HRMS (ESI TOF): calcd. for C189H181N5O56 [M+2NH4]2+ m/z, 1708.0734; found, 1708.0634.
2-Azidoethyl [2,3-di-O-Benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4-O-benzyl-6-O-{[2,3-di-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→6)-[2,3-di-O-benzoyl-4-O-benzyl-β-D-glucopyranosyl]-(1→6)-[2,3-di-O-benzoyl-4-O-benzyl-β-D-glucopyranosyl]-(1→6)-2,3-di-O-benzoyl-4-O-benzyl-β-D-glucopyranosyl}-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranoside (17). Compound 17 (155.0 mg, 83%) was prepared from 4 (77.4 mg, 39.4 µmol) and 7 (120.0 mg, 35.5 µmol) by the same protocol described for 5 and was purified by silica gel column chromatography (ethyl acetate/toluene 1:12). 1H NMR (600 MHz, CDCl3) δ: 8.37 – 6.69 (m, 155H), 5.82 (d, J = 9.6 Hz, 1H), 5.75 (m, 2H), 5.69–5.64 (m, 2H), 5.61–5.56 (m, 2H), 5.49–5.46 (m, 1H), 5.45–5.38 (m, 3H), 5.31 (s, 1H), 5.21 (d, J = 7.6 Hz, 1H), 5.14 (m, 1H), 5.09 (m, 1H), 5.04–4.67 (m, 20H), 4.65–4.57 (m, 4H), 4.51 (m, 1H), 4.41 (d, J = 10.8 Hz, 2H), 4.36 (d, J = 6.2 Hz, 2H), 4.31–4.24 (m, 4H), 4.23–4.04 (m, 15H), 4.03–3.74 (m, 19H), 3.72–3.33 (m, 22H), 3.32–3.22 (m, 5H), 3.17 (d, J = 8.5 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ: 165.75, 165.72, 165.69, 165.40, 165.30, 165.07, 164.88, 164.81, 164.72, 164.62, 164.57, 164.50, 164.42, 163.91, 138.83, 137.74, 137.39, 137.35, 137.29, 137.21, 137.18, 137.12, 136.91, 134.45, 133.57, 133.22, 133.11, 133.07, 132.99, 132.93, 132.82, 132.64, 132.58, 130.08, 129.92, 129.74, 129.70, 129.52, 129.45, 129.40, 129.32, 129.22, 129.18, 129.10, 129.00, 128.92, 128.84, 128.77, 128.69, 128.48, 128.44, 128.36, 128.26, 128.21, 128.16, 128.12, 128.06, 127.99, 127.86, 127.80, 127.65, 127.49, 127.39, 126.87, 126.38, 126.35, 126.25, 126.17, 126.12, 126.07, 103.55, 102.49, 101.81, 101.63, 101.42, 101.30, 101.25, 101.15, 100.89, 100.80, 100.72, 100.66, 100.60, 98.73, 98.57, 97.91, 97.81, 97.57, 97.31, 96.68, 78.91, 78.83, 78.77, 78.22, 78.15, 78.03, 77.95, 77.53, 76.38, 75.89, 75.64, 75.53, 75.36, 75.11, 75.02, 74.87, 74.81, 74.63, 74.25, 73.98, 73.61, 73.30, 73.21, 73.10, 72.94, 72.64, 72.37, 72.33, 72.01, 68.65, 68.01, 67.84, 66.83, 66.51, 66.25, 65.63, 65.37, 60.03, 50.65. MS (MALDI TOF): calcd. for C297H267KN3O84 [M+K]+ m/z, 5257.6; found, 5257.9.
2-Azidoethyl [2,3-di-O-Benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4-O-benzyl-6-O-{[2,3-di-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl}-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranoside (18). Compound 18 (126.6 mg, 85%) was prepared from 5 (36.9 mg, 39.4 µmol) and 7 (120.0 mg, 35.5 µmol) by the protocol described for 5 and was purified by silica gel column chromatography (ethyl acetate/toluene 1:15). 1H NMR (600 MHz, CDCl3) δ 7.87 (m, 6H), 7.76 (m, 3H), 7.73–6.98 (m, 111H), 5.67–5.63 (m, 1H), 5.61–5.58 (m, 1H), 5.55–5.49 (m, 2H), 5.47–5.40 (m, 2H), 5.37 (d, J = 7.5 Hz, 2H), 5.21 (dd, J = 11.5, 5.1 Hz, 2H), 5.15–5.09 (m, 2H), 4.99–4.93 (m, 2H), 4.91 (d, J = 5.3 Hz, 2H), 4.87–4.69 (m, 13H), 4.64 (d, J = 6.0 Hz, 2H), 4.55 (dd, J = 14.0, 8.2 Hz, 2H), 4.45 (d, J = 7.9 Hz, 1H), 4.33 (s, 2H), 4.24 (s, 2H), 4.14 (d, J = 7.1 Hz, 3H), 4.11–4.04 (m, 5H), 4.00–3.81 (m, 11H), 3.74 (dt, J = 18.7, 9.7 Hz, 5H), 3.67 – 3.16 (m, 32H). 13C NMR (150 MHz, CDCl3) δ: 165.50, 165.43, 165.07, 164.86, 164.63, 164.59, 164.53, 164.20, 138.50, 137.57, 137.33, 137.28, 137.16, 136.87, 136.84, 133.42, 133.35, 133.29, 133.21, 133.14, 133.06, 132.98, 132.94, 132.68, 129.79, 129.70, 129.67, 129.61, 129.52, 129.48, 129.38, 129.36, 129.32, 129.21, 129.07, 129.01, 128.98, 128.93, 128.58, 128.48, 128.44, 128.39, 128.36, 128.25, 128.20, 128.14, 128.11, 128.06, 127.85, 127.68, 127.59, 127.43, 126.53, 126.43, 126.39, 126.29, 126.27, 126.11, 126.04, 101.90, 101.62, 101.43, 101.29, 101.22, 101.10, 100.91, 100.77, 100.66, 99.09, 98.54, 98.46, 98.20, 97.40, 96.93, 96.87, 96.64, 79.27, 78.78, 78.68, 78.41, 78.32, 78.12, 77.85, 77.56, 75.32, 75.26, 74.75, 74.66, 74.55, 73.87, 73.66, 73.55, 73.51, 73.43, 73.36, 72.99, 72.93, 72.81, 72.74, 72.57, 72.50, 72.44, 72.33, 72.28, 68.64, 68.60, 68.21, 67.87, 66.47, 66.31, 66.22, 66.16, 65.62, 65.55, 65.40, 60.03, 50.64. MS (MALDI TOF): calcd. for C236H213KN3O69 [M+K]+ m/z, 4231.3; found, 4231.5.
2-Azidoethyl [2,3-di-O-Benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4-O-benzyl-6-O-{[2,3-di-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-2-O-benzoyl-4,6-O-benzylidene-β-D-glucopyranosyl}-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-[2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranosyl]-(1→3)-2-O-benzoyl-4:6-O-benzylidene-β-D-glucopyranoside (19). Compound 19 (135.8 mg, 78%) was prepared from 6 (64.8 mg, 39.4 µmol) and 7 (120.0 mg, 35.5 µmol) by the same protocol described for 5 and was purified by silica gel column chromatography (ethyl acetate/toluene 1:12). 1H NMR (600 MHz, CDCl3) δ: 8.05–6.90 (m, 140H), 5.64 (m, 2H), 5.51 (s, 1H), 5.44 (m, 3H), 5.36 (d, J = 14.3 Hz, 3H), 5.27 (m, 2H), 5.18 (m, 1H), 5.15–5.08 (m, 3H), 5.00 (d, J = 5.5 Hz, 2H), 4.94–4.64 (m, 22H), 4.54 (d, J = 7.6 Hz, 1H), 4.35–4.30 (m, 2H), 4.22 (dd, J = 10.1, 4.6 Hz, 4H), 4.16–4.06 (m, 8H), 4.03 (d, J = 8.3 Hz, 1H), 3.99–3.80 (m, 13H), 3.70 (ddd, J = 33.2, 21.1, 11.0 Hz, 6H), 3.62–3.21 (m, 33H), 3.15 (d, J = 8.8 Hz, 1H), 3.06–3.01 (m, 1H). 13C NMR (150 MHz, CDCl3) δ: 165.44, 165.05, 165.00, 164.95, 164.78, 164.65, 164.61, 164.55, 164.51, 164.48, 164.30, 164.19, 138.57, 137.60, 137.30, 137.19, 137.16, 136.88, 133.47, 133.39, 133.23, 133.15, 133.10, 132.96, 129.83, 129.75, 129.71, 129.67, 129.47, 129.43, 129.39, 129.29, 129.20, 129.06, 129.01, 128.92, 128.62, 128.56, 128.54, 128.50, 128.41, 128.37, 128.26, 128.21, 128.11, 128.07, 128.01, 127.65, 127.54, 126.64, 126.50, 126.42, 126.29, 126.12, 126.04, 101.95, 101.73, 101.55, 101.42, 101.30, 101.23, 101.15, 100.98, 100.91, 100.76, 100.65, 99.64, 99.27, 99.07, 98.93, 98.83, 98.42, 98.23, 97.47, 97.02, 96.79, 96.66, 78.74, 78.68, 78.53, 78.49, 78.30, 77.96, 77.88, 77.66, 77.60, 77.55, 77.40, 75.67, 75.36, 75.13, 74.91, 74.66, 74.50, 74.16, 74.02, 73.88, 73.73, 73.60, 73.49, 73.41, 73.18, 73.11, 73.06, 72.76, 72.66, 72.51, 72.44, 72.28, 68.65, 68.43, 68.24, 67.91, 66.47, 66.23, 65.65, 65.58, 65.53, 65.45, 60.39, 60.03, 50.65. MS (MALDI TOF): calcd. for C276H249KN3O81 [M+K]+ m/z, 4939.5; found, 4939.4.
2-Aminoethyl β-D-Glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→3)-{6-O-[β-D-glucopyranosyl-(1→6)-β-D-glucopyranosyl-(1→6)-β-D-glucopyranosyl-(1→6)-β-D-glucopyranosyl]-β-D-glucopyranosyl}-(1→3)-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→3)-β-D-glucopyranoside (1). To a solution of 17 (20.0 mg, 3.8 µmol) in CH2Cl2 (4 mL) was added acetic acid (4 drops) and zinc powder (20 mg). After vigorously stirring at rt for 24 h, the mixture was filtered through a Celite pad and concentrated under vacuum. The residue was dissolved in AcOH and H2O (5:1, 15 mL) and heated at 60 °C for 24 h. The solvents were removed in vacuum and co-evaporated with toluene 5 times. The resulting residue was dissolved in t-BuOH and H2O (4:1, 15 mL), and NaOH (15 mg in 1.5 mL H2O) was added in portions. After the mixture was heated at 40 °C for 24 h, the solvents were removed by lyophilization. The residue was dissolved in water and neutralized with 0.25 N HCl, and then lyophilized to give the crude product that was purified on a Sephadex G-25 gel filtration column with water as the eluent. Lyophilization gave 1 (7.1 mg, 87%) as a white fluffy solid. 1H NMR (600 MHz, D2O) δ: 4.58 (m, 8H), 4.39–4.33 (m, 5H), 4.08–4.02 (m, 4H), 3.97–3.94 (m, 1H), 3.77 (m, 10H), 3.69 (m, 5H), 3.65–3.50 (m, 18H), 3.50–3.26 (m, 34H), 3.26–3.11 (m, 9H), 3.10 (t, J = 4.8 Hz, 1H). HRMS (ESI TOF): calcd. for C80H138NNaO66 [M+H+Na]2+ m/z, 1095.8686; found, 1095.8658.
2-Aminoethyl β-D-Glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→3)-{6-O-[β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl]-β-D-glucopyranosyl}-(1→3)-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→3)-β-D-glucopyranoside (2). Compound 2 (5.8 mg, 92%) was prepared from 18 (15.0 mg, 3.6 µmol) by the same protocol described for 1. 1H NMR (600 MHz, D2O) δ: 4.58 (m, 9H), 4.40 (d, J = 8.2 Hz, 2H), 4.06 (d, J = 10.7 Hz, 1H), 4.00–3.95 (m, 1H), 3.83–3.71 (m, 12H), 3.58 (m, 20H), 3.41 (m, 30H), 3.26–3.23 (m, 2H), 3.22–3.18 (m, 2H), 3.12 (d, J = 4.7 Hz, 2H). HRMS (ESI TOF): calcd. for C68H118NO56 [M+H]+ m/z, 1844.6416; found, 1844.6464.
2-Aminoethyl β-D-Glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→3)-{6-O-[β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl]-β-D-glucopyranosyl}-(1→3)-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→3)-β-D-glucopyranoside (3). Compound 3 (6.0 mg, 85%) was prepared from 19 (15.0 mg, 3.1 µmol) by the same protocol described for 1. 1H NMR (600 MHz, D2O) δ: 4.60 (m, 11H), 4.39 (d, J = 7.8 Hz, 2H), 4.06 (d, J = 12.1 Hz, 1H), 3.98–3.93 (m, 1H), 3.77 (m, 14H), 3.58 (m, 25H), 3.49–3.28 (m, 35H), 3.25 (t, J = 8.0 Hz, 2H), 3.20 (t, J = 8.6 Hz, 2H), 3.09 (s, 2H). HRMS (ESI TOF): calcd. for C80H138NNaO66 [M+H+Na]2+ m/z, 1095.8686; found, 1095.8641.
Supplementary Material
Acknowledgement
This work was supported in part by an NIH/NCI grant (R01 CA95142). The authors thank Dr. B. Ksebati, Department of Chemistry at Wayne State University, for some 2D NMR measurements, and the 600 MHz NMR instrument used in this research was supported by an NSF grant (CHE-0840413).
References
- 1.(a) Limper AH, Knox KS, Sarosi GA, Ampel NM, Bennett JE, Catanzaro A, Davies SF, Dismukes WE, Hage CA, Marr KA, Mody CH, Perfect JR, Stevens DA. Am. J. Respir. Crit. Care Med. 2011;183:96–128. doi: 10.1164/rccm.2008-740ST. [DOI] [PubMed] [Google Scholar]; (b) Pfaller MA, Diekema DJ. Clin. Microbiol. Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lass-Florl C. Mycoses. 2009;52:197–205. doi: 10.1111/j.1439-0507.2009.01691.x. [DOI] [PubMed] [Google Scholar]
- 2.(a) Cassone A, Casadevall A. Curr. Opin. Microbiol. 2012;15:427–433. doi: 10.1016/j.mib.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Edwards JE., Jr J. Med. Microbiol. 2012;61:895–903. doi: 10.1099/jmm.0.041665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masuoka J. Clin. Microbiol. Rev. 2004;17:281–310. doi: 10.1128/CMR.17.2.281-310.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Cassone A. Nat. Rev. Microbiol. 2013;11:884–891. doi: 10.1038/nrmicro3156. [DOI] [PubMed] [Google Scholar]; (b) Johnson MA, Bundle DR. Chem. Soc. Rev. 2013;42:4327–4344. doi: 10.1039/c2cs35382b. [DOI] [PubMed] [Google Scholar]
- 5.Klis FM, de Koster CG, Brul S. Future Microbiol. 2011;6:941–951. doi: 10.2217/fmb.11.72. [DOI] [PubMed] [Google Scholar]
- 6.Bowman SM, Free SJ. BioEssays. 2006;28:799–808. doi: 10.1002/bies.20441. [DOI] [PubMed] [Google Scholar]
- 7.Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, Norelli F, Bellucci C, Polonelli L, Costantino P, Rappuoli R, Cassone A. J. Exp. Med. 2005;202:597–606. doi: 10.1084/jem.20050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Bromuro C, Romano M, Chiani P, Berti F, Tontini M, Proietti D, Mori E, Torosantucci A, Costantino P, Rappuoli R, Cassone A. Vaccine. 2010;28:2615–2623. doi: 10.1016/j.vaccine.2010.01.012. [DOI] [PubMed] [Google Scholar]; (b) Adamoa R, Tontinia M, Brogionia G, Romanoa MR, Costantinia G, Danielia E, Proiettia D, Bertia F, Costantinoa P. J. Carbohydr. Chem. 2011;30:249–280. [Google Scholar]; (c) Hu Q-Y, Allan M, Adamo R, Quinn D, Zhai H, Wu G, Clark K, Zhou J, Ortiz S, Wang B, Danieli E, Crotti S, Tontini M, Brogioni G, Berti F. Chem. Sci. 2013;4:3827–3832. [Google Scholar]; (d) Adamo R, Hu Q-Y, Torosantucci A, Crotti S, Brogioni G, Allan M, Chiani P, Bromuro C, Quinn D, Tontini M, Berti F. Chem. Sci. 2014;5:4302–4311. [Google Scholar]
- 9.(a) Fontaine T, Simenel C, Dubreucq G, Adam O, Delepierre M, Lemoine J, Vorgias CE, Diaquin M, Latge JP. J. Biol. Chem. 2000;275:27594–27607. doi: 10.1074/jbc.M909975199. [DOI] [PubMed] [Google Scholar]; (b) Manners DJ, Masson AJ, Patterson JC. Biochem. J. 1973;135:19–30. doi: 10.1042/bj1350019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao G, Zhou Z, Burgular S, Liao J, Yuan C, Wu Q, Guo Z. Bioconjug. Chem. 2015;26 doi: 10.1021/bc500575a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Huang X, Huang L, Wang H, Ye XS. Angew. Chem. Int. Ed. 2004;43:5221–5224. doi: 10.1002/anie.200460176. [DOI] [PubMed] [Google Scholar]; (b) Wang Z, Zhou L, El-Boubbou K, Ye XS, Huang X. J. Org. Chem. 2007;72:6409–6420. doi: 10.1021/jo070585g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anish C, Schumann B, Pereira CL, Seeberger PH. Chem. Biol. 2014;21:38–50. doi: 10.1016/j.chembiol.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 13.de Jong AR, Hagen B, van der Ark V, Overkleeft HS, Codee JDC, van der Marel GA. J. Org. Chem. 2012;77:108–125. doi: 10.1021/jo201586r. [DOI] [PubMed] [Google Scholar]
- 14.(a) Liptak A, Jodal I, Harangi J, Nanasi P. Acta Chim. Hung. 1983;113:415–422. [Google Scholar]; (b) Ellervik U, Grundberg H, Magnusson G. J. Org. Chem. 1998;63:9323–9338. [Google Scholar]
- 15.(a) Zeng Y, Ning J, Kong F. Carbohydr. Res. 2003;338:307–311. doi: 10.1016/s0008-6215(02)00455-x. [DOI] [PubMed] [Google Scholar]; (b) Mo KF, Li H, Mague JT, Ensley HE. Carbohydr. Res. 2009;344:439–447. doi: 10.1016/j.carres.2008.12.014. [DOI] [PubMed] [Google Scholar]; (c) Tanaka H, Kawai T, Adachi Y, Ohno N, Takahashi T. Chem. Commun. 2010;46:8249–8251. doi: 10.1039/c0cc03153d. [DOI] [PubMed] [Google Scholar]
- 16.Dasgupta S, Mukhopadhyay B. Eur. J. Org. Chem. 2008:5770–5777. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.