Abstract
Neuroblastoma is the most common extra cranial solid tumour of childhood, and survival remains poor for patients with advanced disease. Novel immune therapies are currently in development, but clinical outcomes have not matched preclinical results. Here, we describe key mechanisms in which neuroblastoma inhibits the immune response. We show that murine and human neuroblastoma tumour cells suppress T cell proliferation, through increased arginase activity. Arginase II is the predominant isoform expressed and creates an arginine deplete local and systemic microenvironment. Neuroblastoma arginase activity results in inhibition of myeloid cell activation and suppression of bone marrow CD34+ progenitor proliferation. Finally we demonstrate that the arginase activity of neuroblastoma impairs NY-ESO-1 specific TCR and GD2-specific CAR engineered T cell proliferation and cytotoxicity. High arginase II expression correlates with poor survival for neuroblastoma patients. The results support the hypothesis that neuroblastoma creates an arginase-dependent immunosuppressive microenvironment in both the tumour and blood that leads to impaired immune surveillance and sub-optimal efficacy of immunotherapeutic approaches.
Keywords: Neuroblastoma, arginase, arginine, immunosuppression, immunotherapy
Introduction
Neuroblastoma is the most common extra-cranial malignancy of childhood. Although the prognosis for low risk neuroblastoma has improved, patients with high risk disease have an extremely poor survival despite intensive multi-modal treatment including immunotherapy.(1) Neuroblastoma is associated with an unique interaction with the immune system, clinically evidenced by patients who develop paraneoplastic Opsoclonus-Myoclonus Syndrome and patients whose tumours spontaneously regress. (2,3)
Over the last 10 years as the benefit of conventional therapies has been maximised the focus has moved to enhancing an anti-neuroblastoma immune response. T cells are a major effector arm of the immune system and play a key role in the recognition and targeting of cancer cells. Subsequently engineered chimeric-antigen receptor (CAR) T cells against the predominant neuroblastoma surface antigen GD2, demonstrated anti-neuroblastoma cytotoxicity in vitro and in murine models. (4,5) However, although pre-clinical studies demonstrate that T cells have the potential for anti-neuroblastoma activity, the clinical efficacy of immunotherapies has been controversial. (6,7) Immunotherapeutic approaches are reliant on an active immune system, therefore one likely hypothesis for their failure is that neuroblastoma creates an immunosuppressive microenvironment that inhibits autologous or adoptive immunity. (8,9)
The mechanisms underlying the immunosuppressive microenvironment in neuroblastoma are poorly understood. In this study we identify the key role of neuroblastoma arginase activity in inhibiting both autologous and engineered anti-neuroblastoma immune responses.
Materials and Methods
Neuroblastoma patient samples
Blood and tumour samples were obtained from 26 patients with neuroblastoma treated at the Birmingham Children’s Hospital, Children’s Hospital Oxford, and Great Ormond Street Hospital (Supp Table 1). The samples were taken from patients with newly diagnosed neuroblastoma, at the time of diagnostic biopsy, before the start of treatment.
GD2+ tumour cell isolation
For isolation of GD2+ tumour cells from human and murine tumours, tumours were digested using Type II collagenase, labelled with anti-GD2-PE antibody and bound to anti-PE coated magnetic beads (Miltenyi). Cells were purified according to manufacturer’s instructions (Miltenyi Biotec, Bisley,UK). Purity of GD2+ cells was >98% as confirmed by flow cytometry.
Neuroblastoma murine mode immune characterisation
After weaning,TH-MYCN mice were palpated for intra-abdominal tumours twice weekly. Mice with palpable tumours ranging in size between 5-20mm in diameter were then humanely sacrificed. At sacrifice, unheparinised and heparinized whole blood, as well as tumour tissue and spleen were obtained for further ex vivo analyses. Tumour tissue was processed as above. Spleens were mechanically digested and heparinized whole blood was lysed with red blood cells lysis buffer (Qiagen). Tumour tissue, spleen and blood cell suspensions were stained with anti-mouse Ly6C, Ly6G, F480, CD3 and GD2 antibody (Biolegend) on ice for 30 minutes. The expression of these markers was assessed by flow cytometry.
Arginase enzyme activity
The activity of arginase II present within neuroblastoma cell lines, sorted patient or murine cells, culture supernatants or plasma was determined by measuring the conversion of arginine into urea, as previously described.(!0)
Monocyte polarisation assay
Peripheral blood was collected from healthy donors and monocytes were separated using a Lymphoprep gradient and enriched by negative selection using a Monocyte Isolation Kit II (Miltenyi. Monocytes were cultured in the presence or absence of neuroblastoma, neuroblastoma culture supernatants (50% of final volume) or patient plasma (50% of final volume) overnight, in low-adherent 24 plates (CoStar), and then treated with LPS (10ng/ml, Sigma) for 24hours. The culture supernatants were harvested and analysed for cytokine release. Cells were harvested and stained with anti- CD86 (Biolegend). Propidium iodide added to allow viable cells to be gated. The expression of CD86 on monocytes was assessed by flow cytometry.
Murine myeloid cells were purified from spleens of healthy and tumour bearing mice and from tumour tissue of neuroblastoma mice using flow cytometer sorting. The cells were cultured in R10% or arginine free medium and treated with LPS for 24 hours. The culture supernatants were harvested and analysed for cytokine release as before.
Statistical analysis
A Wilcoxon-rank-sum test was used to determine the statistical significance of the difference in unpaired observations between two groups. All p values are two-tailed and p values <0.05 were considered to represent statistically significant events.
Study approval
In accordance with the Declaration of Helsinki, patient samples were obtained after written, informed consent prior to inclusion in the study. Regional Ethics Committee (REC Number 10/H0501/39) and local hospital trust research approval for the study was granted for United Kingdom hospitals. The Institute of Cancer Research Ethics Committee approved all animal protocols in this study. Procedures were carried out in accordance with UK Home Office Guidelines.
Results
Neuroblastoma suppresses T cell proliferation via arginase II expression and activity
T cells are the major effector arm of the endogenous anti-cancer immune response. However, neuroblastoma is able to develop and metastasise in patients despite the immune system, suggesting impaired immune surveillance. We investigated the effect of neuroblastoma on T cell immunity, using human cell lines as an established model of malignant neuroblastoma. Neuroblastoma cell lines were cultured with T cells from healthy donors. T cell proliferation was significantly suppressed by the presence of neuroblastoma (Fig 1a), even at low neuroblastoma: T cell ratios. Using a transwell assay we identified that the T cell suppression was non-contact dependent (Fig 1b). Cell line supernatant was examined for immunosuppressive molecules, and no detectable levels of IL-10, IL-4, and TGF-β were identified (data not shown), consistent with previous reports of an unknown alternative mechanism of immunosuppression mediated by neuroblastoma cells. (9)
Figure 1. Arginine metabolism regulates the suppressive activity of neuroblastoma.
a) T cell proliferation is suppressed by the presence of different ratios of neuroblastoma cell lines. b) Suppressive activity of neuroblastoma cell lines is maintained when T cells are separated by a transwell. c) Arginase activity from neuroblastoma and AML cell lines measured by the conversion of arginine into urea. d) T cell proliferation is restored by the inhibition of neuroblastoma arginase using the specific inhibitor NOHA, but not by the iNOS inhibitor LNMMA. e) Neuroblastoma cell lines significantly deplete arginine from the microenvironment. All data are representative of three independent experiments (error bars, SD).
We have recently identified that another paediatric malignancy, Acute Myeloid Leukaemia, creates an immunosuppressive microenvironment through altered arginase activity. (10) Although AML is an haematological malignancy and neuroblastoma a solid tumour, both cancers share a common association with pancytopenia, immune alteration and bone marrow infiltration at diagnosis. Therefore we hypothesised that neuroblastoma tumour cells similarly harness arginine depletion to suppress the immune response. Arginine is a semi-essential amino acid, that is metabolised in mammalian cells, principally by the enzymes arginase I and II, and by inducible nitric-oxide synthase (iNOS). (11) As Arginase I, II and iNOS may be responsible for inhibition of T cell proliferation, we tested the functional relevance of each enzyme. (12) By measuring the conversion of arginine into urea we demonstrated that neuroblastoma cells have a measurable arginase activity, albeit lower than AML cell lines (24 mU/106 cells NB vs 67.5 mU/106 cells AML) (Fig 1c). No increased production of reactive nitric oxide species was demonstrated (data not shown). Using the small molecules NOHA and L-NMMA, to inhibit arginase and iNOS respectively, we demonstrated that the immunosuppressive microenvironment is created by arginase activity alone (Fig 1d). NOHA alone was able to rescue T cell proliferation, with no effect from L-NMMA. Moreover analysis of neuroblastoma culture supernatants showed that the concentration of arginine decreases significantly in the local microenvironment (Fig 1e), and leads to downregulation of T cell CD3ζ chain (Supp Fig 1a). Addition of exogenous arginine led to moderate rescue of T cell proliferation (Supp Fig 1b). Both RT-PCR and Western blotting identified that neuroblastoma cell lines express Arginase II as the main isoform (Supp Fig 1c and d), with no measurable Arginase I or iNOS protein expression (Supp Fig 1e and f). The arginase activity correlated with the absolute arginase II intracellular concentration of the various neuroblastoma cell lines analysed (SKN-MC>LAN-1>Kelly) (Supp Fig 1d). Silencing of Arginase II led to restoration of T cell proliferation, confirming the specific role of Arginase II (Supp Fig 1g and h).
Neuroblastoma creates both a local and systemic depletion in arginine, extending its immunosuppressive environment
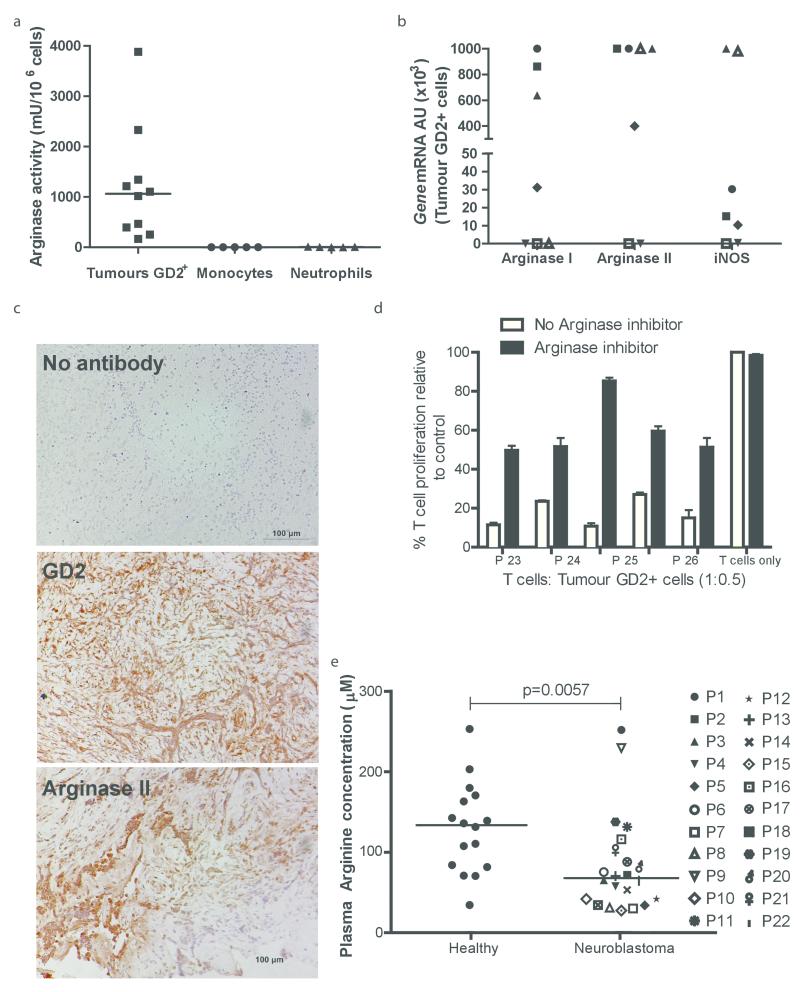
Having established the ability of neuroblastoma cells to suppress T cell proliferation in our model, we investigated the role of arginase in human neuroblastoma samples. High arginase activity (mean 1100mU/106 cells) was confirmed in human GD2+ cells from patients’ tumours at diagnosis, with rapid degradation of arginine into urea (Fig 2a). Monocytes and neutrophils from healthy donors were used as controls. GD2+ cells expressed both arginase isoform genes (Fig 2b). However, immunohistochemical examination of diagnostic tumour biopsy samples, confirmed the specific expression of arginase II within GD2+ tumour cells (Fig 2c and Supp Fig 2), with minimal staining for arginase I and iNOS enzymes. The findings are consistent with the established predominant expression of arginase II in healthy neuronal tissue.(13) We performed T cell proliferation assays using GD2+ tumour cells sorted from the neuroblastoma tumours. GD2+ cells showed a significant ability to suppress T cell proliferation (Fig 2d). Arginase inhibition with NOHA, led to rescue of T cell proliferation consistent with our earlier findings. (10) No correlation was found between patient clinical characteristics and suppressive activity, including patient age, stage, or Myc-N status.
Figure 2. Human neuroblastoma creates a local and systemic microenvironment deficient in arginine.
a) GD2+ neuroblastoma tumour cells have significant arginase activity, compared to nontumour cells. b) Expression of Arginase I, Arginase II, and iNOS from GD2+ cells sorted from the tumours of 7 patients as examined by qPCR. Patients are identified by unique symbols, which are used consistently throughout the manuscript. c) Staining of sections from neuroblastoma tumours at diagnosis with DAPI alone (top), anti-human GD2 (middle), and anti-arginase II (bottom). Representative section from one neuroblastoma patient of five shown. d) GD2+ tumour cells suppress T cell proliferation with is resorted by arginase inhibition. Data are representative of five independent experiments (error bars, SD). e) Plasma arginine is significantly lower in 22 newly diagnosed neuroblastoma patients compared to 22 healthy donors.
Patients with advanced neuroblastoma can present with systemic immune alterations including lymphopenia. (14,15) It has previously been shown that arginase enzymes can be released by tumour cells to enhance the area of immunosuppression. (10) We found no increase in arginase activity of culture supernatants (data not shown), indicating the enzyme was not released. However, patients with neuroblastoma frequently present with large tumour masses. We hypothesised that neuroblastoma may cause a systemic depletion of arginine due to consumption by the tumour mass, despite the failure to secrete free enzyme. Examination of plasma from neuroblastoma patients at diagnosis showed a significant reduction in arginine concentrations in the blood, compared to healthy controls (Fig 2e, Healthy controls 135 μM vs neuroblastoma patients 70μM, p=0.0057). No increased plasma arginase activity was seen compared to the healthy controls, confirming the absence of secreted free arginase enzyme from tumour cells (Supp Fig 2b, p=0.7139). To assess the effect of low arginine on T cell proliferation, donor T cells were cultured in the plasma of neuroblastoma patients or healthy donors. We showed that the low arginine in patient plasma significantly impaired T cell proliferation compared to T cells cultured in plasma from healthy donors (Supp Fig 2c, p=0.045), and induces CD3ζ chain downregulation (Supp Fig 2d). Consistent with previous findings exogenous arginine induces significant rescue of T cell proliferation (p=0.0038, Supp Fig 2e). Thus our data suggests that neuroblastoma creates both a local and systemic immunosuppressive microenvironment through the depletion of arginine by tumour cells.
The neuroblastoma microenvironment leads to altered immune cell frequency and function
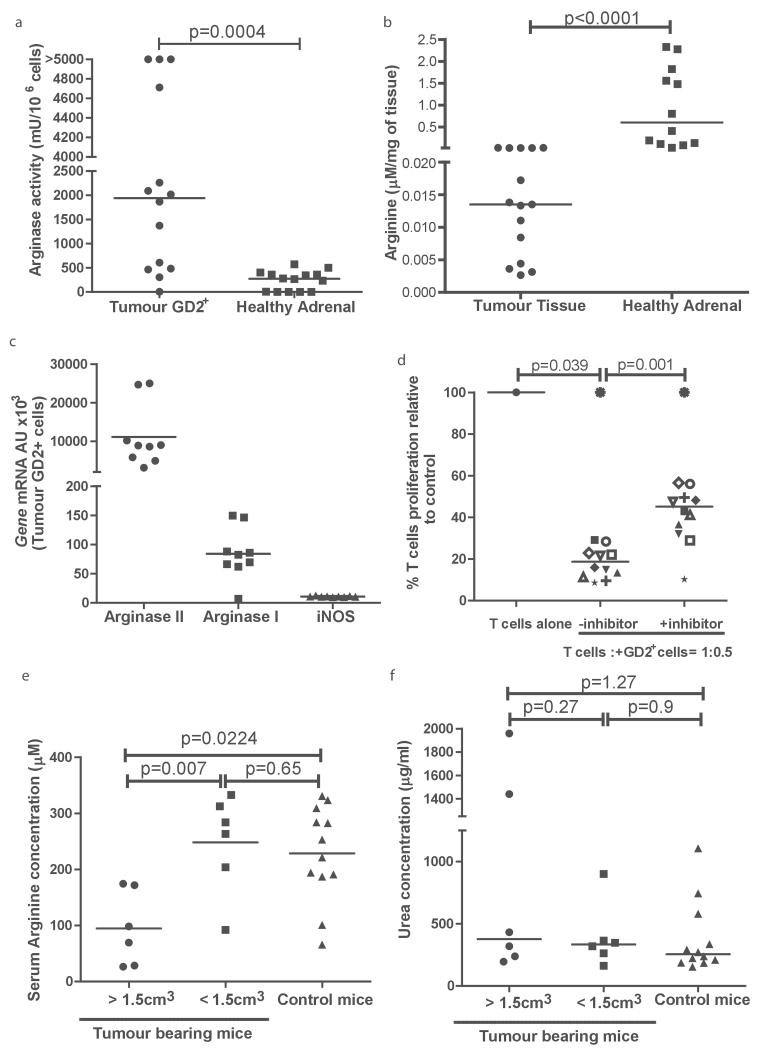
To understand the impact of neuroblastoma on immune populations in vivo, we first characterised the role of neuroblastoma-derived arginase in a neuroblastoma murine model model. TH-MYCN mice are transgenic mice providing the closest biological model of human neuroblastoma development. (16) In patients the most common location for neuroblastoma primary tumours is the adrenal gland. We compared the arginase activity of murine adrenals with murine neuroblastoma tumours, and identified that tumour cells have a significantly higher arginase activity (Fig 3a, p=0.0004). Using a method described by Zhang et al. (17) extracellular fluid was isolated from neuroblastoma tumour tissue and found to have significantly lower arginine concentrations than healthy adrenal tissue (Fig 3b, p<0.0001). Similar to human tissue, GD2+ cells isolated from murine tumours predominantly expressed Arginase II, however low expression of Arginase I is also found (Fig 3c and Supp Fig 3a). GD2+ cells suppress T cell proliferation in an arginase dependent manner (Fig 3d), We observed that mice with a larger tumour burden, had significantly lower serum arginine concentrations, than mice with smaller tumours (p=0.007) or healthy controls (p=0.0224) (large tumours 98 μM vs small tumours 233μM vs. controls 222 μM, Fig 3e). Consistent with our human findings, no increase in serum arginase activity was seen (Fig 3f). Therefore TH-MYCN mice demonstrate the features of the neuroblastoma arginase-dependent microenvironment found in human patients.
Figure 3. The TH-MYC murine neuroblastoma recreates the arginase dependent microenvironment.
a) Sorted GD2+ cells from murine neuroblastoma tumours have significantly higher arginase activity compared to adrenal tissue. b) Arginine concentrations are significantly lower in the extracellular fluid of neuroblatomas compared to healthy adrenals. c) Expression of Arginase I, Arginase II, and iNOS of GD2+ cells sorted from murine neuroblastoma tumours, by qPCR. d) GD2+ tumour cells from murine neuroblastomas suppress T cell proliferation, with is resorted by arginase inhibition. e) Plasma from tumour bearing mice with large (>1.5cm3) and small (<15.cm3) tumours were analysed for arginine concentration. Plasma arginine is significantly lower in tumour bearing mice with large tumours. f) No increase in plasma arginase activity of tumour bearing mice compared to healthy mice.
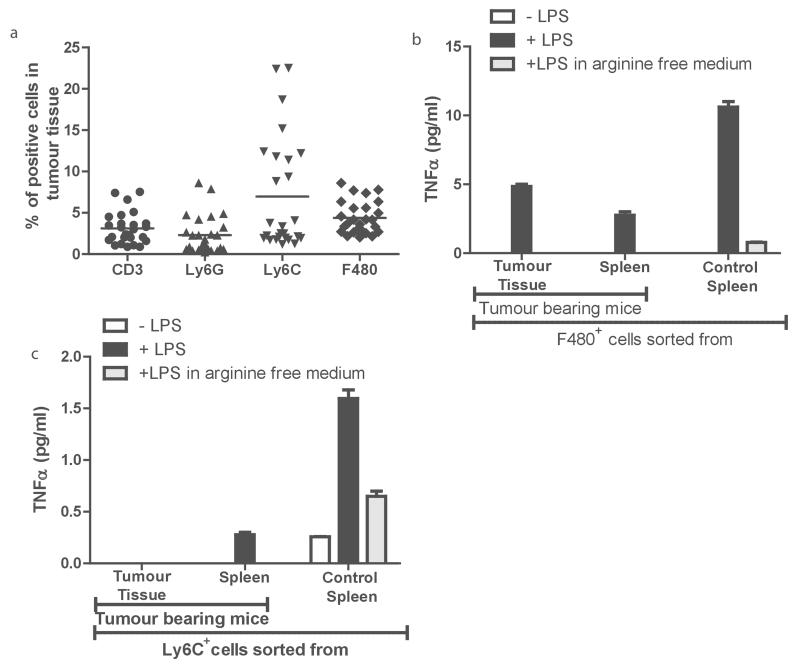
To understand the impact of neuroblastoma derived arginine depletion on the immune system we characterised key immune compartments in the blood, spleen, and tumours of tumour bearing mice. T cell frequency in the spleens of tumour bearing mice were significantly decreased (Supp Fig 3b, bottom, p=0.03 ) and T cells represented only a minor population of cells (3.2 %) within the tumour mass (Fig 4a). Myeloid cells are a second predominant immune population have been shown to promote neuroblastoma chemo- resistance. (18) Within tumours, myeloid populations were composed of F4/80+ macrophages (4.5%), Ly6C+ (6.7%) and Ly6G+ cells (2.7%) (Fig 4a). In our model we identified that tumour bearing mice also had increased numbers of F4/80+ myeloid cells in the blood compared to controls (Supp Fig 3, top; F4/80 p=0.0049). In the spleen all populations of myeloid cells were significantly increased compared to controls (Supp Fig 3; F4/80+ p=0.001; Ly6G+ p=0.0016, Ly6C+ p=0.04).
Figure 4. Neuroblastoma modulates myeloid cell populations in TH-MYC murine models.
a) Neuroblastoma tumours from mice were disassociated and the percentage of CD3+, Ly6G+, Ly6C+, and F4/80+ cells was determined by flow cytometry. b) F4/80+ cells from tumours, spleens of tumour bearing mice, or when cultured in arginine deplete media had significant decreases in LPS-driven TNF-a release. All data are representative of three independent experiments (error bars, SD). c) Ly6C+ cells from tumours, spleens of tumour bearing mice, or when cultured in arginine deplete media had significant decreases in LPS-driven TNF-α release. All data are representative of three independent experiments (error bars, SD).
It is recognised that myeloid cell activation plays a key role in co-ordinating the anti-cancer, pro-inflammatory immune response through cytokine release. (19) To understand if the neuroblastoma microenvironment affects the activation of myeloid cells we isolated the 2 main populations of myeloid cells, Ly6C+ or F4/80+, from the spleen and tumours of tumour-bearing TH-MYCN mice and control mice, and tested their response to the potent immune stimulator LPS. Both myeloid populations had significantly impaired TNF-α release compared to those from healthy controls (Fig 4b and 4c), comparative to the effect of myeloid cells cultured in arginine free conditions. No difference in IL-10, IL-12 or IL-6 was identified suggesting an inactivation of myeloid function, as opposed to polarisation (data not shown).
These murine studies, identify the inhibitory role of the neuroblastoma arginase microenvironment on myeloid cell activation. To understand if the arginine deficient microenvironment in human neuroblastoma can also inactivate human myeloid cell function, CD14+ monocytes from healthy donors were cultured in the presence of neuroblastoma cell supernatant and stimulated overnight with LPS. Analogous to our murine study, neuroblastoma conditioned monocytes had decreased secretion of TNF-α (Fig 5a), and no increase in the T cell activatory cytokine IL-12. (Supp figure 4). Co-culture with arginine deficient media recapitulated these findings, confirming the specificity of the mechanism. Furthermore no increase in the myeloid maturation marker CD86 was observed (Supp Fig 4b). Similarly no upregulation in the M2-polarisation marker CD206 was seen indicating that the monocytes are inactivated but not polarised (data not shown) by the low arginine microenvironment of neuroblastoma.
Figure 5. Neuroblastoma induces immunosuppressive myeloid cells in patients.
a) CD14+ cells cultured with neuroblastoma or arginine deplete media had significant decreases in LPS-driven TNF-α release. All data are representative of three independent experiments (error bars, SD). b) Neuroblastoma conditioned monocytes suppress T cell proliferation. Data are representative of three independent experiments. c) Percentages of CD11b+CD14+ (top) and CD11b+CD15+ (middle) and CD3+ (btoom) in the blood of newly diagnosed neuroblastoma patients compared to healthy donors, as measured by flow cytometry. d) Neuroblastoma patients’ monocytes suppress T cell proliferation.
It is well established that tumour-associated myeloid cells may support tumour pathogenesis by directly inhibiting T cell function. Suppressive myeloid cells have been reported in neuroblastoma, although the mechanism of their induction and their relevance in patients has not been well characterised. (20) We co-cultured monocytes conditioned with arginine deplete neuroblastoma media, with T cells from healthy donors and found they are able to significantly suppress T cell proliferation. (Fig 5b). As neuroblastoma creates a systemic arginine depletion, we also examined the myeloid populations in the blood of newly diagnosed patients (Supp Fig 4c). An increase in circulating CD14+ (p=0.034), but not CD15+ cells, was found compared to healthy controls (Fig 5c upper and middle panels). These CD14+ monocytes from neuroblastoma patients were capable of suppressing allogenic T cell proliferation (Fig 5d). Therefore both the local and systemic arginine depletion created by neuroblastoma not only affects T cell responses directly but also through the induction of immunosuppressive myeloid cells.
Clinical impact of neuroblastoma arginase-microenvironment
Our findings highlighted the key role of arginase activity by neuroblastoma tumours. To understand the clinical significance of our findings we interrogated the ‘R2: microarray analysis and visualization platform’. Analysis of primary patient samples at diagnosis, confirms the enzyme arginase II is expressed by all stages of neuroblastoma tumour, with the exception of Stage 4s, at higher levels than Arginase I (Supp Fig 5a). High Arginase II expression correlates with a significantly worse overall survival (Supp Fig 5b). The result suggests a key role for Arginase II in neuroblastoma pathogenesis. With regard to myeloid populations, patients with low intra-tumoral CD14 expression, suggestive of inactivate myeloid cells, also do significantly worse (Supp Fig 5c).
T cell based immunotherapies commonly require harvesting of T cells from patients for ex vivo manipulation. (21) These studies assume that T cells from patients are fully functional, but our studies suggest these cells would not be in an optimal condition. To test this concept, we isolated T cells from patients at diagnosis and found they have reduced proliferative capacity compared to those from healthy controls, suggesting they have been impaired by the immunosuppressive microenvironment created by the developing neuroblastoma (Fig 6a). This observation complements our finding that T cell frequency is also lower in neuroblastoma patients than healthy controls (p=0.039) (Fig 5c lower panel). The cancer-germline antigen NY-ESO-1 is an antigenic target on neuroblastoma and has been suggested as a target for both endogenous immunity and novel T cell therapies. (22) To evaluate the impact of the arginase microenvironment on engineered T cell immunity, NY-ESO-1-specific TCR engineered T cells were conditioned with neuroblastoma cell lines ex vivo. Neuroblastoma induced an arginase dependent inhibition of proliferation by NY-ESO-1 TCR engineered T cells, which was rescued by the arginase inhibition (Fig 6b). Extending these findings we tested the impact of the neuroblastoma microenvironment on engineered CAR T cells targeting GD2. We demonstrate that neuroblastoma leads to a significant impairment in cytotoxicity and proliferation of CAR T cells (Fig 6c and Supp Fig 4d), with some rescue in cytotoxicity when arginase is inhibited (Fig 6d). These findings provide the first evidence for the role of neuroblastoma arginase activity in impairing anti-neuroblastoma T cell therapies.
Figure 6. Neuroblastoma arginase activity impair T cell immunotherapies and haematopoietic stem cell division.
a) T cells from neuroblastoma patients at diagnosis had decreased proliferative potential compared to those from healthy donors. b) Neuroblastoma conditioning suppresses NY-ESO-1 TCR engineered T cell proliferation. Data representative of 3 independent experiments c) Neuroblastoma conditioning suppresses anti-GD2 CAR T cell cytotoxicity against neuroblastoma. Data representative of 2 independent experiments d). Arginase inhibition during neuroblastoma conditioning rescues anti-GD2 CAR T cell cytotoxicity. Data representative of 2 independent experiments e) Neuroblastoma patient plasma suppresses human CD34+ HSC proliferation. Data are representative of five patient plasma experiments. Independent experiments were performed on two separate occasions. f) CD34+ HSC proliferation is inhibited by the neuroblastoma low-arginine microenvironment. Independent experiments were performed on two separate occasions.
Neuroblastoma patients, particularly those with advanced disease, can present with cytopaenias. However the mechanism by which neuroblastoma can impact on haematopoietic progenitors has never been reported. As neuroblastoma lowers plasma arginine we hypothesised that the low arginine microenvironment could also inhibit the proliferation of bone marrow haematopoietic progenitors. Human CD34+ haematopoietic stem cells (HSCs) have significantly impaired cell division, with no loss of CD34+ expression, in the presence of neuroblastoma patient plasma (red histogram) compared to those cultured in plasma from healthy controls (blue histogram) (Fig 6e). CD34+ cells co-cultured with tumour cells from patients or with arginine deplete media had similar significant decreases in proliferation, compared to controls (Fig 6f). Therefore the arginase-dependent microenvironment of neuroblastoma not only suppresses T cells and myeloid cells, but inhibits the division and proliferation of CD34+HSCs.
Discussion
In this study we identify for the first time, the ability of primary human neuroblastoma cells to directly inhibit T cell proliferation through high arginase activity. We showed that GD2+ neuroblastoma cells from patients, express predominantly the arginase II isoform. Arginase I and II are localised to the cytosol and mitochondria respectively. (23) In healthy states, arginase I is predominantly expressed by hepatocytes, whilst arginase II has wider, tissue-specific expression. (24) Both isoforms catalyse the conversion of arginine into ornithine and urea. Neuroblastoma is a malignancy of the sympathetic nervous system, arising from neural crest progenitors that ordinarily develop into sympathetic ganglia and adrenal medulla. (25,26) Arginase II is the principal isoform expressed in the nervous system, with little or absent arginase I protein, consistent with our findings. (27) Indeed arginine metabolism plays a key role in both healthy neuronal function and in diseases of the nervous system, and may contribute to the relative immunoprivileged niche that that the nervous system exists in.(28)
Using the ‘R2: microarray analysis and visualization platform’ we confirmed that arginase II was expressed by all stages of neuroblastoma suggestive of a fundamental role in neuroblastoma pathogenesis. (29) Stage 4s neuroblastoma is associated with an excellent prognosis, and it is interesting that in this subtype arginase II gene expression is not higher than arginase I. Although the role of arginase I expressing Myeloid-Derived Suppressor Cells (MDSCs) in altering T cell responses in cancer patients has been well established, arginase II in solid tumour immune biology has only received limited attention to date. (30) We recently reported the role of Arginase II in creating an immunosuppressive microenvironment in Acute Myeloid Leukaemia. (10) Similarly, in prostate cancer arginase II expression by both the tumour cells and cancer associated fibroblasts serves to regulate immunosuppressive pathways. (31) The increased expression and immunosuppressive potential of Arginase II alone is likely to be tissue and disease specific. (32)
We examined the impact of neuroblastoma arginase activity, to understand the effect on both local and systemic immunity. We first showed that neuroblastoma tumours lower local arginine concentrations, to levels that inactivate surrounding T and myeloid cells. Increased Arginase II expression correlates with decreased T and myeloid cell tumour infiltration in HNCC, supporting our findings in neuroblastoma. (33) Arginine is exchanged between the blood and Extracellular Fluid (ECF) to maintain homeostasis. (34,35) We investigated plasma levels of arginine in newly diagnosed neuroblastoma patients and found them to be significantly lower than healthy controls. Similar changes were identified in mice with larger tumours. However unlike AML, we show here that neuroblastoma does not release free arginase II enzyme. Therefore uptake and catabolism by the tumour mass must presumably play a significant role in systemic depletion. Patients with high stage neuroblastoma frequently present with significant cachexia. (36) As tumour consumption of arginine lowers systemic arginine concentrations, physiological compensation through protein breakdown and arginine recycling via the intestinal-renal axis, may seek to maintain homeostasis. (37) Corresponding decreases in plasma arginine have been described in patients with renal cell carcinoma and cervical cancer at diagnosis. (38,39)
Arginine is a semi-essential amino acid, required by healthy tissues for a number of cell processes including cell viability, proliferation, and protein synthesis. Thus arginase activity likely provides two key roles in neuroblastoma pathogenesis – firstly maintaining normal cell metabolism but secondly contributing to immune escape. (40,41) It is established that arginine depletion leads to T cell cycle arrest, impaired proliferation and reduced activation. (42,43) This is consistent with our findings of both lower T cell numbers in the peripheral blood and their impaired proliferative capacity. The ‘R2: microarray analysis and visualization platform’ identifies that neuroblastoma tumours with low CD3 expression have a worse overall survival, supporting the pathogenic role of arginine depletion in neuroblastoma tumours.
Monocytes and macrophages play a key role in anti-cancer immunity through co-ordination of other immune effectors by cytokine secretion. Consistent with previous findings we identify that CD14+ myeloid cells are present within the tumour microenvironment. (20,44) However we describe for the first time that neuroblastoma arginase activity, impairs the ability of monocytes to respond to an inflammatory stimulus and decreases their production of the immune activatory cytokine TNF-α from myeloid cells. A previous study noted an impaired TNF-α driven dendritic cell stimulation of T cells in neuroblastoma, but no mechanism was found. (9)
In both our murine model and in patients’ blood we observed a significant increase in the number of immunosuppressive monocytes. It has been shown in xenografts that immunosuppressive myeloid cells are associated with neuroblastoma but the mechanism of cross-talk has not been identified. (45) Here we identify that neuroblastoma arginase activity plays a key role in modulating the CD14 immune phenotype. The clinical finding of cytopaenias in neuroblastoma patients is also well recognised. However, the mechanism of neuroblastoma-induced cytopenia is unclear, particularly in metastatic or end-stage disease where the bone marrow is rarely significantly replaced by tumour cells. We show for the first time that the low arginine microenvironment of neuroblastoma suppresses CD34+ haematopoietic cell division and proliferation.
Immunotherapy requiring an active innate and adaptive immune response, has become a major new approach in the treatment of high-risk neuroblastoma. Despite a promising preclinical rationale, immunotherapy clinical trials in neuroblastoma patients have not resulted in significant improvements in overall survival. (6) Studies show an unexplained fall in adoptive T cell numbers post administration, with no correlation between the dose of engineered cells administered, their numbers in peripheral blood or anti-tumour response. (5) In xenograft studies of neuroblastoma-cell vaccines, T cells from tumour-bearing mice have defective anti-tumour immune responses, both locally at the site of injection but also systemically, although no mechanism has been identified. (46) Furthermore immunotherapies which have activity alone in vitro, often require co-administration with cytokines to maximise therapeutic effect in patients. Early phase clinical trials using autologous patient derived DC vaccines illustrate that moderate benefit is only seen when co-administered with Il-2. (47)
NY-ESO-1 is a cancer-germline antigen, known to be highly immunogenic in melanoma patients, but also expressed by the majority of neuroblastomas. Although it has been demonstrated through ex vivo manipulation that patients can generate a humoral and T cell specific response to this antigen it is unclear why similar responses are not seen in patients. (48) We show that neuroblastoma arginase activity significantly impairs antigen-specific T cell proliferation by NY-ESO-1 TCR engineered T cells.
Following the identification of neuroblastoma surface ganglioside D2 as a potential therapeutic target, Chimeric-Antigen Receptor engineered T cells are an alternative therapeutic approach under development. However, similar to vaccine approaches early phase trial results have not matched preclinical results. We find that the arginase-dependent microenvironment created by neuroblastoma can significantly inhibit the proliferation and cytotoxicity of anti-GD2 CAR T cells. This is the first report identifying a mechanism in which CAR T cell activity can be impaired by tumour immunosuppression. Interestingly a previous report assessing CAR engineered T cell responses against a neuroblastoma cell line in vitro identified a similar decrease in activity but no mechanism for this was identified. (49) Most early trials of novel immune therapies enrol refractory or relapsed patients who may have very significant disease burden, due to treatment failure. It is therefore unsurprising that the extent of the immunosuppressive microenvironment both within the tumour mass and blood has the ability to hamper adoptive T cell responses.
Arginine supplementation has been administered to patients, in non-tumour settings, to enhance immunity and tissue repair. In a subcutaneous xenograft model of neuroblastoma supplemented with combination arginine and IL-2, Lymphokine-Acitaved Killer cells demonstrated increased anti-neuroblastoma immunity and mice had prolonged survival. (50) However concerns that arginine supplementation may feed tumour growth would need to be evaluated further. Therapeutic targeting through arginase inhibition is a more likely strategy. Although small molecules such as NOHA, have been shown to be toxic in vivo, other compounds which act on arginase are already under preclinical evaluation. A recent study identified that the green tea derivative PolyPhenon E can promote antitumor immunity in a murine model of neuroblastoma. Polyphenols have been shown to have activity against arginase. It is possible that the activity previously reported may have been also due to blockade of arginase expressed by neuroblastoma cells.
In conclusion, our findings provide evidence for the key role of arginase activity in the creation of an immunosuppressive microenvironment in neuroblastoma, and have significant clinical implications for T cell immunotherapy approaches. Tumour escape from different arms of the immune response, is likely to be multifactorial, and control of arginase activity by tumour tissue fits rationally with other mechanisms identified in murine models. (48) Targeting of arginase activity in neuroblastoma could provide a new way of enhancing both autologous and therapeutic anti-neuroblastoma immunity.
Supplementary Material
Acknowledgements
The authors thank the patients and parents who contributed samples to the study. Thank you to Jennie Godwin, Jane Cooper and Cay Shakespeare for consent and collection of patient samples. This work was supported by Cancer Research UK and the Birmingham Children’s Hospital Research Fund.
Footnotes
The authors declare no competing financial or conflicts of interest.
References
- 1.Maris J. The biologic basis for neuroblastoma heterogeneity and risk stratification. Curr Opin Pediat. 2005;17:7–13. doi: 10.1097/01.mop.0000150631.60571.89. [DOI] [PubMed] [Google Scholar]
- 2.Rudnick E, Khakoo Y, Antunes N, Seeger R, Brodeur G, Shimada H, et al. Opsoclonusmyoclonus-ataxia syndrome in neuroblastoma: clinical outcome and anti-neuronal antibodies-a report from the Children’s Cancer Group Study. Med Pediatr Oncol. 2001;36:612–22. doi: 10.1002/mpo.1138. [DOI] [PubMed] [Google Scholar]
- 3.Hero B, Simon T, Spitz R, Ernestus K, Gnekow A, Scheel-Walter H, et al. Localized infant neuroblastomas often show spontaneous regression: results of the prospective trials NB95-S and NB97. J Clin Oncol. 2008;26:1504–10. doi: 10.1200/JCO.2007.12.3349. [DOI] [PubMed] [Google Scholar]
- 4.Mujoo K, Kipps T, Yang H, Cheresh D, Wargalla U, Sander D, et al. Functional properties and effect on growth suppression of human neuroblastoma tumours by isotype switch variants of monoclonal antiganglioside GD2 antibody 14.18. Cancer Res. 1989;49:2857–61. [PubMed] [Google Scholar]
- 5.Pule M, Savoldo B, Myers G, Rossig C, Russell H, Doti G, et al. Virus-specific T cells engineered to coexpress tumour-specific receptors: persistence and anti-tumour activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Nierthammer D, et al. Consolidation treatment with chimeric anti-GD2-antibody ch1.18 in children older than 1 year with metastatic neuroblastoma. J Clin Oncol. 2004;22:3549–57. doi: 10.1200/JCO.2004.08.143. [DOI] [PubMed] [Google Scholar]
- 7.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interlukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pistoia V, Morandi F, Bianchi G, Bezzolo A, Prigione I, Raffaghello L. Immunosuppressive microenvironment in neuroblastoma. Front Oncol. 2013;3 doi: 10.3389/fonc.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Doffek K, Sugg SL, Shilyansky J. Neuroblastoma cells inhibit the immunostimulatory fuction of dendritic cells. J Pediatric Surg. 2003;38:901–5. doi: 10.1016/s0022-3468(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 10.Mussai F, De Santo C, Abu-Dayyeh I, Booth S, Quek L, McEwen-Smith RM, et al. Acute Myeloid leukaemia creates an arginase dependent immunosuppressive microenvironment. Blood. 2013;122:749–58. doi: 10.1182/blood-2013-01-480129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grody W, Argyle C, Kern RM, Dizikes GJ, Spector EB, Strickland AD, et al. Differential expression of the two human arginase genes in hyperargininemia. J Clin Invest. 1989;83:602–9. doi: 10.1172/JCI113923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5-inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;27:2691–02. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gotoh T, Araki M, Mori M. Chromosomal localization of the human arginase II gene and tissue distribution of its mRNA. Biochem Biophys Res Commun. 1997;233:487–91. doi: 10.1006/bbrc.1997.6473. [DOI] [PubMed] [Google Scholar]
- 14.Quinn JJ, Altman A. The multiple hematologic manifestations of neuroblastoma. Am J Pediatr Hematol Oncol. 1979;1:201–5. doi: 10.1097/00043426-197923000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Scott JP, Morgan E. Coagulopathy of disseminated neuroblastoma. J Pediat. 1983;103:219–222. doi: 10.1016/s0022-3476(83)80348-5. [DOI] [PubMed] [Google Scholar]
- 16.Terrile M, Bryan K, Vaughan L, Hallsworth A, Webber H, Chesler L, et al. miRNA expression profiling of the murine TH-MYCN neuroblastoma model reveals similarities with human tumors and identifies novel candidate miRNAs. PLoS One. 2011;6:e28356. doi: 10.1371/journal.pone.0028356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Xiang L, Hassan R, Pastan I. Immunotoxin and Taxol synergy results from a decrease in shed mesothelin levels in the extracellular space of tumours. Proc Natl Acad Sci USA. 2007;104:17099–104. doi: 10.1073/pnas.0708101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song L, Asgharzadeh S, Salo J, Engell K, Hong-wei W, Sposto R, et al. Vα24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Inv. 2009;119:1524–36. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu D, Song, Wei J, Courney A, Gao X, Marinova E, et al. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. J Clin Invest. 2012;12:2221–33. doi: 10.1172/JCI59535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asgharzadeh S, Salo JA, Oberthuer A, Fischer M, Bethold F, Hadjidaniel M, et al. Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J Clin Oncol. 2012;30:3525–32. doi: 10.1200/JCO.2011.40.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grupp S, Prak EL, Boyer J, McDonald K, Shusterman S, Thompson E, et al. Adoptive transfer of autologous T cells improves T-cell repertoire diversity and long-term B cell function in pediatric patients with neuroblastoma. Clin Cancer Res. 2012;18:6732–41. doi: 10.1158/1078-0432.CCR-12-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodolfo M, Luksh R, Stockert E, Chen YT, Collini P, Ranzani T, et al. Antigen-specific immunity in neuroblastoma patients: antibody and T-cell recognition of NY-ESO-1 tumor antigen. Cancer Res. 2003;63:6948–55. [PubMed] [Google Scholar]
- 23.Jenkinson C, Grody W, Cederbaum S. Comparative properties of arginases. Comp Biochem Physiol B Biochem Mol Biol. 1996;114:107–32. doi: 10.1016/0305-0491(95)02138-8. [DOI] [PubMed] [Google Scholar]
- 24.Morris SM., Jr Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharm. 2009;15:922–30. doi: 10.1111/j.1476-5381.2009.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber K. The sympathoadrenal cell lineage: specification, diversification, and new perspectives. Dev Biol. 2006;298:335–43. doi: 10.1016/j.ydbio.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 26.De Preter K, Vandesompele J, Heiann P, Yigit N, Beckman S, Schramm A, et al. Human fetal neuroblast and neuroblastoma transcriptome analysis confirms neuroblast origin and highlights neuroblastoma candidate genes. Genome Biol. 2006;7:R84. doi: 10.1186/gb-2006-7-9-r84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braissant O, Gotoh T, Loup M, Mori M, Backmann C. L-arginine uptake, the citrulline-NO cycle and arginase II in the rat brain: an in situ hybridisation study. Brain Res Mol Brain Res. 1999;70:231–41. doi: 10.1016/s0169-328x(99)00151-5. [DOI] [PubMed] [Google Scholar]
- 28.Wiesinger H. Arginine metabolism and the synthesis of nitric oxide in the nervous system. Progress in neurobiology. 2001;64:365–91. doi: 10.1016/s0301-0082(00)00056-3. [DOI] [PubMed] [Google Scholar]
- 29.R2: microarray analysis and visualization platform’. ( http://r2.amc.nl)
- 30.Mussai F, De Santo C, Cerundolo V. Interaction between iNKT cells and MDSCs in cancer patients: Evidence and Therapeutic Opportunities. J Immunother. 2012;35:449–59. doi: 10.1097/CJI.0b013e31825be926. [DOI] [PubMed] [Google Scholar]
- 31.Ino Y, Yamazaki-Itoh R, Oguro S, Shimada K, Kosuge T, Zavada J, et al. Arginase II expressed in cancer-associated fibroblasts indicates tissue hypoxia and predicts poor outcome in patients with pancreatic cancer. PLoS One. 2013;8:e55146. doi: 10.1371/journal.pone.0055146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rotondo R, Mastracci L, Piazza T, Barisione G, Fabbi M, Cassanello M, et al. Arginase 2 is expressed by human lung cancer, but it neither induces immune suppression, nor affects disease progression. Int J Cancer. 2008;123:1108–16. doi: 10.1002/ijc.23437. [DOI] [PubMed] [Google Scholar]
- 33.Bron L, Jandus C, Andrejevic-Blant S, Speiser DE, Monnier P, Romero P, Rivals JP. Prognostic value of arginase-II expression and regulatory T-cell infiltration in head and neck squamous cell carcinoma. Int J Cancer. 2013;132:E85–93. doi: 10.1002/ijc.27728. [DOI] [PubMed] [Google Scholar]
- 34.Borsheim E, Kobayashi H, Traber DL, Wolfe RR. Compartmental distribution of amino acids during hemodialysis-induced hypoaminoacidemeina. Am J Physiology Endocrinol Metab. 2006;290:E643–E652. doi: 10.1152/ajpendo.00267.2005. [DOI] [PubMed] [Google Scholar]
- 35.Lofberg E, Essen P, McNurlan M, Wernerman J, Garlick P, Anderstam B, et al. Effect of hemodialysis on protein synthesis. Clin Nephrol. 2000;54:284–94. [PubMed] [Google Scholar]
- 36.Green GJ, Weitzmann SS, Pencharz PB. Resting energy expenditure in children newly diagnosed with stage IV neuroblastoma. Pediatr Res. 2008;63:332–36. doi: 10.1203/PDR.0b013e318163a2d4. [DOI] [PubMed] [Google Scholar]
- 37.Buijs N, Luttikhold J, Houdijk AP, van Leeuwen PA. The role of a disturbed arginine/NO metabolism in the onset of cancer cachexia: a working hypothesis. Curr Med Chem. 2012;19:5278–86. doi: 10.2174/092986712803833290. [DOI] [PubMed] [Google Scholar]
- 38.Hasim A, Aili A, Maimaiti A, Mamtimin B, Abudula A, Upur H. Plasma-free amino acid profiling of cervical cancer and cervical intraepithelial neoplasia patients and its application for early detection. Mol Biol Rep. 2013;40:5853–59. doi: 10.1007/s11033-013-2691-3. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez P, Ernstoff M, Hernandez C, Atkins M, Zabaleta J, Sierra R, et al. Arginase I-producing myeloid derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–60. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin SE, Wu Fl, Wei MF, Shen LJ. Depletion of arginine by recombinant arginine deiminase induces nNOS-activated neurotoxicity in neuroblastoma cells. Biomed Res Int. 2014;2014:589424. doi: 10.1155/2014/589424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris SM., Jr Arginine Metabolism: Boundaries of Our Knowledge. J Nutr. 2007;137:1602S–1609S. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–73. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zea AH, Rodriguez PC, Culotta KS, Hernandez CP, DeSalvo J, Ochoa JB, et al. L-arginine modulates CD3zeta expression and T cell function in activated human T lymphocytes. Cell Immunol. 2004;232:21–31. doi: 10.1016/j.cellimm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Apps JR, Hasan F, Campus O, Behjati S, Jacques TS, Sebire N, et al. The immune environment of paediatric and solid malignancies: evidence from an immunohistochemical study of clinical cases. Fetal Pediatr Pathol. 2013;32:298–07. doi: 10.3109/15513815.2012.754527. [DOI] [PubMed] [Google Scholar]
- 45.Santilli G, Piotrowska I, Cantilena SA, Chayka O, D’Alicarnasso M, Morgenstern DA, et al. Polyphenon E enhances the antitumor immune response in neuroblastoma by inactivating myeloid suppressor cells. Clin Cancer Res. 2013;19:1116–25. doi: 10.1158/1078-0432.CCR-12-2528. [DOI] [PubMed] [Google Scholar]
- 46.Barr KM, Jing W, Hallett WH, Gershan JA, Johnson BD. Examining T cells at vaccine sites of tumor-bearing hosts provides insights to dysfunctional T-cell immunity. J Immunother. 2013;36:41–51. doi: 10.1097/CJI.0b013e318274590e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowman L, Grossmann M, Rill D, Brown M, Zhong WY, Alexander B, et al. IL-2 adenovector transduced autologous tumor cells induce antitumor immune responses in patients with neuroblastoma. Blood. 1998;92:1941–49. [PubMed] [Google Scholar]
- 48.Rodolfo M, Luksch R, Stockert E, Chen YT, Collini P, Ranzani T, et al. Antigen-specific immunity in neuroblastoma patients: antibody and T-cell recognition of NY-ESO-1 tumour antigen. 2003;63:6948–55. [PubMed] [Google Scholar]
- 49.Sun J, Dotti G, Huye LE, Foster AE, Savoldo B, Gramatges MM, et al. T cells expressing constitutively active Akt resist multiple tumor-associated inhibitory mechanisms. Mol Ther. 2010;18:2006–17. doi: 10.1038/mt.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lieberman M, Nishioka K, Redmond H, Daly J. Enhancement of interlukin-2 immunotherapy with L-arginine. Ann. Surg. 1992;215:157–65. doi: 10.1097/00000658-199202000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.