Abstract
Purpose
Although long-term outcomes after initial placement of artificial urinary sphincters are established, limited data exist comparing sphincter survival in patients with compromised urethras (prior radiation, artificial urinary sphincter placement or urethroplasty). We evaluated artificial urinary sphincter failure in patients with compromised and noncompromised urethras.
Materials and Methods
We performed a retrospective analysis of 86 sphincters placed at a single institution between December 1997 and September 2012. We assessed patient demographic, comorbid disease and surgical characteristics. All nonfunctioning, eroded or infected devices were considered failures.
Results
Of the 86 patients reviewed 67 (78%) had compromised urethras and had higher failure rates than the noncompromised group (34% vs 21%, p=0.02). Compared to the noncompromised group, cases of prior radiation therapy (HR 4.78; 95% CI 1.27, 18.04), urethroplasty (HR 8.61; 95% CI 1.27, 58.51) and previous artificial urinary sphincter placement (HR 8.14; 95% CI 1.71, 38.82) had a significantly increased risk of failure. The risk of artificial urinary sphincter failure increased with more prior procedures. An increased risk of failure was observed after 3.5 cm cuff placement (HR 8.62; 95% CI 2.82, 26.36) but not transcorporal placement (HR 1.21; 95% CI 0.49, 2.99).
Conclusions
Artificial urinary sphincter placement in patients with compromised urethras from prior artificial urinary sphincter placement, radiation or urethroplasty had a statistically significant higher risk of failure than placement in patients with noncompromised urethras. Urethral mobilization and transection performed during posterior urethroplasty surgeries likely compromise urethral blood supply, predisposing patients to failure. Patients with severely compromised urethras from multiple prior procedures may have improved outcomes with transcorporal cuff placement rather than a 3.5 cm cuff.
Keywords: urinary sphincter, artificial; urinary incontinence; radiation
Soon after its introduction in 1972, the artificial urinary sphincter became a mainstay of treatment of male stress urinary incontinence. After several advances in mechanical design, the AMS 800™ was released in 1983 and remains the primary AUS used today. Although various continence promoting devices, most notably bone anchored,1 transobturator2 and adjustable male slings,3 have been used as a treatment modality for mild to moderate SUI, the AUS is considered the gold standard for the treatment of severe SUI.4
Acceptable long-term patient satisfaction and device durability have been demonstrated in multiple cohorts chiefly comprised of uncomplicated patients, with 63% to 77% of original sphincters still in place with long-term followup.5–8 Outcome data from these cohorts may not be applicable to patients with a history of pelvic radiation, AUS explant or urethroplasty.
More post-prostatectomy cases are now receiving adjuvant radiation due to the trend toward multimodal treatment of aggressive prostate cancer.9 Radiation causes small vessel obliteration and endarteritis, resulting in localized ischemic tissue changes such as fibrosis and necrosis.10 Although the bulbar urethra is outside the radiated field, urethral blood supply may be compromised during its pelvic course, which could predispose these patients to urethral erosion after AUS placement.11–13 Although several studies showed little difference in sphincter survival between the radiated and nonradiated groups,14–16 others have reported a significantly higher failure rate, primarily from atrophy and infection/erosion, in radiated cases.11,12,17–19
The number of patients undergoing revision and reimplantation procedures is increasing.11 Simple revision operations to replace older malfunctioning devices or downsize the cuff appear to have durability similar to that of the initial placement.20,21 The recent availability of the 3.5 cm cuff has allowed physicians to achieve functional success in patients who have spongiosal atrophy with acceptable 1-year erosion rates (9%).10 Patients undergoing secondary AUS reimplant after removal of an eroded/infected AUS are more likely to experience re-erosion.11,20 Since cuff placement around the poorly perfused scar tissue at the prior erosion site will likely re-erode, further mobilization of the urethra and placement of the new cuff in an alternate location are recommended.22 In addition to the negative impact this has on collateral blood flow, longitudinal blood flow through the scarred, previously eroded portion of the urethra is likely impaired in these patients.11 Also, a smaller cuff often has to be placed around the less robust distal bulbar urethra since the initial cuff is generally placed around the thicker, proximal bulbar urethra.11,20
In this analysis we compared compromised (prior AUS, radiation or urethroplasty) and non-compromised AUS cases to determine risk factors for AUS failure. We hypothesized that conditions which negatively impact spongiosal blood supply, including urethroplasty, would lead to increased AUS failure.
MATERIALS AND METHODS
After institutional review board approval was granted we evaluated all male patients who underwent bulbar urethra AUS placement at the University of California, San Francisco from December 1997 through September 2012. A retrospective chart review was conducted to identify patient demographics and surgical variables including age at implantation, BMI (kg/m2), medical comorbidities by patient self-report including CAD, diabetes and smoking, prior urethroplasty, AUS placement or radiation (EBR and/or brachytherapy), cuff size (3.5, 4, 4.5, 5 cm) and placement technique (single vs double cuff, transcorporal). All patients were contacted by telephone by a single surgeon (JBM) and were asked if they still had a functioning artificial urinary sphincter in place. Failure was defined as sphincter explant. To account for tissue atrophy, explant for nonfunctioning devices was also considered a failure. Postoperative variables including continued sphincter function, time to failure (explant) and etiology of failure were gathered. We included patients with at least 6 months of followup and all patients who experienced failure before 6 months. Followup time was defined as the last clinic visit or telephone contact, whichever was later. Patients with clinical signs of erosion or infection underwent confirmatory office cystoscopy and subsequent AUS explantation.
All patients in the study underwent placement of the AMS 800 with a 61 to 70 cm reservoir for the treatment of SUI. A single surgeon (JWM) placed the majority of sphincters (97%, 83 of 86), and the remainder were placed by another faculty member and former fellow (BNB) using the same surgical technique through separate perineal and suprapubic incisions. Patients with non-compromised urethras were compared across categories of demographic and clinical characteristics with those who had compromised urethras, using Fisher’s exact test for categorical variables. The t-test was used for continuous variables to compare means across groups. We enumerated the reasons for failures by those with non-compromised vs compromised urethras. In all patients Cox proportional hazards models were used to analyze associations between demographic indicators, clinical characteristics and history, and time to failure. All Cox proportional hazards models were adjusted for age at surgery. Hazard ratios and 95% CIs were calculated. In the analysis of time to failure, patients were evaluated from time of surgery to date of last followup. We used a Kaplan-Meier plot to illustrate failure-free survival. All analyses were performed using SAS® version 9.3 and results with a 2-sided p <0.05 were considered statistically significant.
RESULTS
The study population included a total of 86 sphincters placed in 69 patients. Of these, 19 (22%) were placed in patients with noncompromised urethras and 67 (78%) were placed in patients with compromised urethras. There was no significant difference in demographics between these groups (table 1). Median followup was 39.2 months (range 1 to 126). Four patients lacking followup within the last 3 years were not included in the study.
Table 1.
Patient demographics
| Noncompromised Urethra |
Compromised Urethra |
p Value | |
|---|---|---|---|
| No. pts | 18 | 56 | |
| Mean pt age (SD) | 63.9 (11.3) | 67.5 (11.7) | 0.25 |
| History of CAD (%) | 20.0 | 7.4 | 0.17 |
| History of diabetes (%) | 13.3 | 5.7 | 0.26 |
| Current smoker (%) | 40.0 | 49.1 | 0.58 |
| Mean kg/m2 BMI (SD) | 28.7 (5.0) | 28.2 (4.9) | 0.75 |
| Mos followup | 51.6 | 35.8 |
Demographic characteristics were assessed only at initial sphincter placement at University of California, San Francisco for patients included in the data set multiple times.
There were 38 patients with a history of at least 1 prior perineal surgery, including 25 prior AUS placements and 23 prior urethroplasties. All patients treated with urethroplasty underwent excision primary anastomosis posterior urethroplasty. A total of 42 patients previously received radiation (EBR 25, brachytherapy 7, both 10). Since some patients had a history of more than 1 type of compromising procedure (ie urethroplasty, prior AUS placement and/or radiation) there was overlap between subgroups. A total of 16 patients had 2 prior compromising interventions and 4 had a history of all 3. Overall, patients with a history of 1 type of compromising procedure had a lower risk of failure (34%, 16 of 47) than those with 2 (38%, 6 of 16) or 3 (75%, 3 of 4) types of procedures.
The cause of failure differed between patients with and without compromised urethras (table 2). Only 1 case of failure (25%, 1 of 4) in the non-compromised group was due to infection or erosion. Conversely, infection and erosion were responsible for 70% (16 of 23) of the failures in the compromised group. Failure from infection or erosion was also more common in urethroplasty cases (35%, 8 of 23) than those with a history of radiation (26%, 11 of 42) or prior AUS placement (20%, 5 of 25). Cases of compromised urethras had a higher rate of failure than noncompromised cases (34% vs 21% failure rate, HR 4.59; 95% CI 1.32, 15.96; p=0.02, table 3).
Table 2.
Reasons for failure
| No. Noncompromised Urethra | No. All Compromised | No. Prior Urethroplasty | No. Prior Sphincter | No. Radiation | |
|---|---|---|---|---|---|
| Erosion | 0 | 8 | 3 | 2 | 5 |
| Infection | 1 | 8 | 5 | 3 | 6 |
| Erosion with stricture/diverticulum formation | 1 | 1 | 0 | 1 | 1 |
| Atrophy | 2 | 2 | 1 | 2 | 1 |
| Malfunction | 0 | 3 | 1 | 1 | 1 |
| Removed per pt request | 0 | 1 | 1 | 1 | 0 |
Patients may be counted in multiple columns.
Table 3.
Hazard of AUS failure across history and other factors
| No. Events | HR (95% CI) | p Value | |
|---|---|---|---|
| History of CAD | 4 | 1.83 (0.61, 5.42) | 0.28 |
| History of diabetes | 0 | — | — |
| History of smoking | 10 | 1.33 (0.56, 3.20) | 0.52 |
| BMI: | |||
| Less than 25 | 7 | 1.00 | |
| 25 - Less than 30 | 14 | 1.45 (0.46, 4.51) | 0.52 |
| 30 or Greater | 3 | 0.89 (0.20, 4.05) | 0.88 |
| Noncompromised urethra | 4 | 1.00 | |
| Compromised urethra:* | 23 | 4.59 (1.32, 15.96) | 0.02 |
| Prior urethroplasty* | 11 | 8.61 (1.27, 58.51) | 0.03 |
| Prior sphincter* | 10 | 8.14 (1.71, 38.82) | 0.009 |
| Prior radiation:* | 14 | 4.78 (1.27, 18.04) | 0.02 |
| External beam* | 11 | 4.05 (1.04, 15.83) | 0.04 |
| Brachytherapy* | 6 | 5.96 (1.05, 33.74) | 0.04 |
| Prior sling* | 2 | 6.39 (0.34, 120.23) | 0.22 |
| Cuff size (cm): | |||
| 3.5 | 6 | 8.62 (2.82, 26.36) | 0.0002 |
| 4.0 | 18 | 1.00 | |
| 4.5 | 3 | 0.25 (0.06, 1.11) | 0.07 |
| 5.0 | 0 | — | — |
| Tandem cuff | 3 | 4.11 (1.09, 15.51) | 0.04 |
| Transcorporal cuff | 8 | 1.21 (0.49, 2.99) | 0.68 |
Adjusted for age at surgery.
Compared to noncompromised.
On subgroup analysis, patients with a history of urethroplasty (HR 8.61; 95% CI 1.27, 58.51; p=0.03), AUS (HR 8.14; 95% CI 1.71, 38.82; p=0.009) or radiation (HR 4.78; 95% CI 1.27, 18.04) had a significantly higher risk of failure than those with a noncompromised urethra. Sample sizes were too small to compare hazard ratios between these individual subgroups. The urethroplasty subgroup had slightly longer followup (mean 37 months) than the AUS and radiation subgroups (25 and 34 months, respectively).
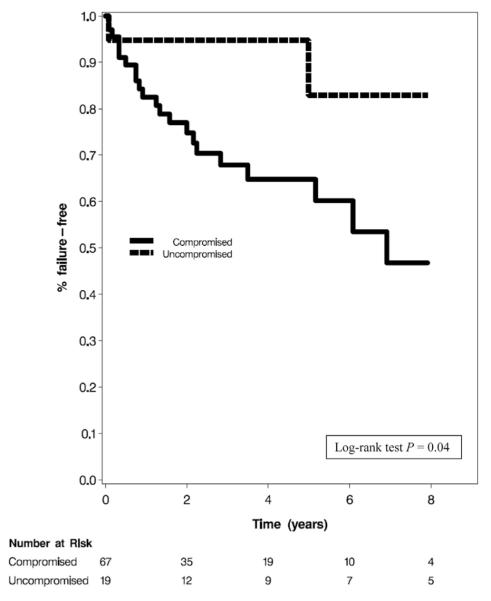
The cuff was placed transcorporally in 22 patients, 9 had 3.5 cm cuffs placed and 3 had tandem cuffs inserted. Patients with tandem and 3.5 cm cuff placement were more likely to experience failure (HR 4.11; 95% CI 1.09, 15.51; p=0.04 and HR 8.62; 95% CI 2.82, 26.36; p=0.0002, respectively). Of the 9 patients who had 3.5 cm cuffs placed, 67% (6 of 9) experienced failure at a mean followup of 7 months. All failures were secondary to infection or erosion. Transcorporally placed cuffs did not have a significantly higher rate of failure (HR 1.21; 95% CI 0.49, 2.99; p=0.68). Of this group 36% (8 of 22) had failure at a mean followup of 52 months. Infection or erosion was the reason for failure in 63% (5 of 8) of these patients. The proportions of patients free of AUS failure at 5 years was 64.79% for compromised cases and 82.89% for noncompromised cases (log rank test p=0.04, see figure).
Figure.
Kaplan-Meier curves for AUS failure
DISCUSSION
Compared to noncompromised cases, those with previous radiation therapy, urethroplasty or AUS implantation had an increased risk of AUS failure, which was defined as a nonfunctioning or explanted device. To our knowledge this study is the first to specifically evaluate AUS survival in patients with a history of urethroplasty.
This study represents a large number of patients with compromised urethras due to tertiary care referrals. Other studies have reported outcomes for reoperative patient cohorts.11,15,21–23 Frank et al reported on 23 patients who underwent secondary AUS placement after prior device explantation due to erosion or infection.23 They reported only an 8.7% erosion risk at a mean followup of 32.6 months. In 2005 Raj et al compared the outcomes of 435 primary and 119 secondary AUS placements and, surprisingly, found patients undergoing AUS revision/secondary reimplantation had a better 5-year survival than those with primary implants (88% vs 79%).21 The majority (82%) of these were simple revisions and after 6 and a half years the survival curve for the secondary implant/revision cohort did drop off and the Kaplan-Meier curves crossed.21 Later, in a followup study at the same institution examining preoperative risk factors for 46 patients requiring 54 explantations, systemic symptoms such as hypertension (p=0.006) and CAD (p=0.003) as well as prior radiation therapy (p=0.006) and AUS explant (p=0.0001) were found to increase the risk of subsequent AUS erosion.11 Patients with a prior explant due to erosion had a 34.8% chance of another erosion.
Initially, after reviewing 218 cases (including 60 radiated and 31 with revision/reimplantation) that underwent AUS implantation, Lai et al reported similar complication rates in radiated, nonradiated, neurogenic and revision/reimplantation cases.15 Five years later, when they separated out primary revision (37) and secondary reimplant (21) cases, and compared outcomes to virgin (169) cases, they noted a fourfold higher risk of erosion in the secondary reimplant group (p=0.02, 14% vs 3.6%, RR 4.02; 95% CI 1.09, 14.91).20 The findings of this study and the 2006 study by Raj et al11 emphasize the increased risk of failure after a secondary reimplant. In our study patients with prior AUS had a 40% (10 of 25) chance of failure, half (5 of 10) due to infection/erosion. Similar to the earlier study by Lai and Boone,20 our prior AUS group included patients who underwent a simple revision for atrophy or malfunction (11) and those requiring an explant with delayed reimplant for infection or urethral injury (14). Our findings differed since we found a statistically higher failure rate in the prior AUS group without distinguishing between delayed reimplant and revision cases.
We also found radiated cases had a significantly increased risk of failure (HR 4.59; 95% CI 1.32, 15.96; p=0.02), with infection or erosion responsible for 79% (11 of 14) of failures in this group. The majority of patients received EBR (25) while the rest had brachytherapy (7) or EBR and brachytherapy (10). Although failure rates among these subgroups were fairly similar at 32% (8 of 25), 43% (3 of 7) and 30% (3 of 10), respectively, future evaluation of the relationship between radiation type/dose and AUS failure rates may be useful.
We hypothesized that during urethroplasty, extensive mobilization and transection of the urethra as well as ligation of any remaining bulbar arterial supply severely compromise blood flow to the urethra and likely increase the risk of a future AUS failure. Although we were unable to measure bulbospongiosus blood supply, we found urethroplasty cases, like other complicated cases, were more likely to experience failure than virgin cases (p=0.03). Unfortunately our ability to compare outcomes among the individual subgroups within the compromised population was limited by sample size and group overlap. Our small sample sizes within group subsets are reflected in the large confidence intervals associated with the HR. Still, patients with a history of urethroplasty did have a slightly higher failure rate (48%, 11 of 23) than those with prior AUS (43%, 10 of 25) or radiation (33%, 14 of 43). After excluding all overlapping cases there was an even greater disparity in failure rates between the prior urethroplasty (44%, 4 of 9) and the radiation (29%, 8 of 28) or AUS placement groups (20%, 2 of 10). Although perineal radiation and prior AUS failure likely impair urethral blood flow, the combination of insults inherent to urethroplasty may result in an equivalent or possibly higher risk of failure in this group.13 A larger study will be needed before any firm conclusions can be made regarding the comparative degree of impact prior radiation, AUS failure and urethroplasty have on AUS survival.
What constitutes the safest and most effective approach for AUS placement in patients with frail urethras or reoperative cases has been an ongoing debate. Surgical options include proximal or distal cuff relocation, tandem cuff placement, external urethral bulking with various agents, transcorporal cuff placement, tandem transcorporal placement24 and smaller cuff placement.25 Several recent studies have advocated transcorporal cuff placement, reporting good sphincter survival and minimal erectile problems. However, others have found the technique to have variable survival, often resulting in postoperative urinary retention.26-30 A recent review of 3.5 cm cuffs placed in 45 patients, of which 47% had a history of radiation and 20% had prior urethral surgery, reported a 9% erosion rate and 2% revision rate at 1-year followup.10
We found increased rates of failure with tandem and 3.5 cm cuffs. All 3 patients who had tandem cuffs placed had treatment failure. Of these, 2 failures were from infection/erosion. Both of these patients had a history of urethroplasty. The third patient had failure due to urethral atrophy after 5 years. Although we noted a high failure rate (67%, 6 of 9) in the 3.5 cm cuff group, the complexity of the 6 patients in whom treatment failed should be noted since 5 had a prior urethroplasty, 4 had a prior AUS explant and 3 had received pelvic radiation.
The patients who had transcorporal cuff placement were also a complex group. All but 1 (95%, 21 of 22) had a compromised urethra and half (11 of 22) had a prior urethroplasty. Overall, only 36% (8 of 22) of the transcorporal group experienced failure compared to 67% (6 of 9) of the 3.5 cm group. These data suggest that patients with a severely compromised urethra secondary to multiple insults, especially if 1 of the insults is urethroplasty, fare better with transcorporal than with 3.5 cm cuff placement.
One of the limitations of our data set is the retrospective nature of the analysis. As a result, outcomes were limited to sphincter survival and lacked any measure of functionality, which ideally would include objective measures such as validated questionnaires and pad weight. Although we performed subgroup analysis, we evaluated patients who underwent different surgical techniques with transcorporal, tandem and 3.5 cm cuff placement. We also reported on a small number of 3.5 cm cuff and tandem cuff cases. Comparison among subgroups of the complicated patients could not be performed due to small sample size.
CONCLUSIONS
AUS placement in patients with a compromised urethra from prior AUS placement, radiation or urethroplasty was associated with a statistically significant risk of failure. The extensive urethral mobilization and transection performed during most urethroplasty surgeries likely compromise urethral blood supply and predispose these patients to failure. Transcorporal placement rather than 3.5 cm cuff placement may fare better in patients with a thin, severely compromised urethra.
Abbreviations and Acronyms
- AUS
artificial urinary sphincter
- BMI
body mass index
- CAD
coronary artery disease
- EBR
external beam radiation
- SUI
stress urinary incontinence
REFERENCES
- 1.Claudon P, Spie R, Bats M, et al. Male stress urinary incontinence: medium-term results of treatment by sub-urethral bone anchored sling InVance. Prog Urol. 2011;21:625. doi: 10.1016/j.purol.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Rehder P, Haab F, Cornu JN, et al. Treatment of postprostatectomy male urinary incontinence with the transobturator retroluminal repositioning sling suspension: 3-year follow-up. Eur Urol. 2012;62:140. doi: 10.1016/j.eururo.2012.02.038. [DOI] [PubMed] [Google Scholar]
- 3.Hoda MR, Primus G, Fischereder K, et al. Early results of a European multicentre experience with a new self-anchoring adjustable transobturator system for treatment of stress urinary incontinence in men. BJU Int. 2013;111:296. doi: 10.1111/j.1464-410X.2012.11482.x. [DOI] [PubMed] [Google Scholar]
- 4.Tuygun C, Imamoglu A, Gucuk A, et al. Comparison of outcomes for adjustable bulbourethral male sling and artificial urinary sphincter after previous artificial urinary sphincter erosion. Urology. 2009;73:1363. doi: 10.1016/j.urology.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 5.Elliott DS, Barrett DM. Mayo Clinic long-term analysis of the functional durability of the AMS 800 artificial urinary sphincter: a review of 323 cases. J Urol. 1998;159:1206. [PubMed] [Google Scholar]
- 6.Arai Y, Takei M, Nonomura K, et al. Current use of the artificial urinary sphincter and its long-term durability: a nationwide survey in Japan. Int J Urol. 2009;16:101. doi: 10.1111/j.1442-2042.2008.02176.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim SP, Sarmast Z, Daignault S, et al. Long-term durability and functional outcomes among patients with artificial urinary sphincters: a 10-year retrospective review from the University of Michigan. J Urol. 2008;179:1912. doi: 10.1016/j.juro.2008.01.048. [DOI] [PubMed] [Google Scholar]
- 8.Venn SN, Greenwell TJ, Mundy AR. The long-term outcome of artificial urinary sphincters. J Urol. 2000;164:702. doi: 10.1097/00005392-200009010-00020. [DOI] [PubMed] [Google Scholar]
- 9.Bastian PJ, Boorjian SA, Bossi A, et al. High-risk prostate cancer: from definition to contemporary management. Eur Urol. 2012;61:1096. doi: 10.1016/j.eururo.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 10.Hudak SJ, Morey AF. Impact of 3.5 cm artificial urinary sphincter cuff on primary and revision surgery for male stress urinary incontinence. J Urol. 2011;186:1962. doi: 10.1016/j.juro.2011.06.062. [DOI] [PubMed] [Google Scholar]
- 11.Raj GV, Peterson AC, Webster GD. Outcomes following erosions of the artificial urinary sphincter. J Urol. 2006;175:2186. doi: 10.1016/S0022-5347(06)00307-7. [DOI] [PubMed] [Google Scholar]
- 12.Martins FE, Boyd SD. Artificial urinary sphincter in patients following major pelvic surgery and/or radiotherapy: are they less favorable candidates? J Urol. 1995;153:1188. [PubMed] [Google Scholar]
- 13.Manunta A, Guille F, Patard JJ, et al. Artificial sphincter insertion after radiotherapy: is it worthwhile? BJU Int. 2000;85:490. doi: 10.1046/j.1464-410x.2000.00484.x. [DOI] [PubMed] [Google Scholar]
- 14.Gomha MA, Boone TB. Artificial urinary sphincter for post-prostatectomy incontinence in men who had prior radiotherapy: a risk and outcome analysis. J Urol. 2002;167:591. doi: 10.1016/S0022-5347(01)69091-8. [DOI] [PubMed] [Google Scholar]
- 15.Lai HH, Hsu EI, Teh BS, et al. 13 Years of experience with artificial urinary sphincter implantation at Baylor College of Medicine. J Urol. 2007;177:1021. doi: 10.1016/j.juro.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 16.Sathianathen N, McGuigan SM, Moon DA. Outcomes of artificial urinary sphincter implantation in the irradiated patient. BJU Int. 2014;113:636. doi: 10.1111/bju.12518. [DOI] [PubMed] [Google Scholar]
- 17.Bordenave M, Roupret M, Taksin L, et al. Long-term results of the treatment of urinary incontinence with bulbar implantation of artificial urinary sphincter in men: a single-center experience. Prog Urol. 2011;21:277. doi: 10.1016/j.purol.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Walsh IK, Williams SG, Mahendra V, et al. Artificial urinary sphincter implantation in the irradiated patient: safety, efficacy and satisfaction. BJU Int. 2002;89:364. doi: 10.1046/j.1464-4096.2001.01759.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang R, McGuire EJ, He C, et al. Long-term outcomes after primary failures of artificial urinary sphincter implantation. Urology. 2012;79:922. doi: 10.1016/j.urology.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 20.Lai HH, Boone TB. Complex artificial urinary sphincter revision and reimplantation cases—how do they fare compared to virgin cases? J Urol. 2012;187:951. doi: 10.1016/j.juro.2011.10.153. [DOI] [PubMed] [Google Scholar]
- 21.Raj GV, Peterson AC, Toh KL, et al. Outcomes following revisions and secondary implantation of the artificial urinary sphincter. J Urol. 2005;173:1242. doi: 10.1097/01.ju.0000152315.91444.d0. [DOI] [PubMed] [Google Scholar]
- 22.Kowalczyk JJ, Nelson R, Mulcahy JJ. Successful reinsertion of the artificial urinary sphincter after removal for erosion or infection. Urology. 1996;48:906. doi: 10.1016/s0090-4295(96)00245-2. [DOI] [PubMed] [Google Scholar]
- 23.Frank I, Elliott DS, Barrett DM. Success of de novo reimplantation of the artificial genitourinary sphincter. J Urol. 2000;163:1702. [PubMed] [Google Scholar]
- 24.Magera JS, Jr, Elliott DS. Tandem transcorporal artificial urinary sphincter cuff salvage technique: surgical description and results. J Urol. 2007;177:1015. doi: 10.1016/j.juro.2006.10.052. [DOI] [PubMed] [Google Scholar]
- 25.Rahman NU, Minor TX, Deng D, et al. Combined external urethral bulking and artificial urinary sphincter for urethral atrophy and stress urinary incontinence. BJU Int. 2005;95:824. doi: 10.1111/j.1464-410X.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 26.Wiedemann L, Cornu JN, Haab E, et al. Transcorporal artificial urinary sphincter implantation as a salvage surgical procedure for challenging cases of male stress urinary incontinence: surgical technique and functional outcomes in a contemporary series. BJU Int. 2013;112:1163. doi: 10.1111/bju.12386. [DOI] [PubMed] [Google Scholar]
- 27.Lee D, Zafirakis H, Shapiro A, et al. Intermediate outcomes after transcorporal placement of an artificial urinary sphincter. Int J Urol. 2012;19:861. doi: 10.1111/j.1442-2042.2012.03034.x. [DOI] [PubMed] [Google Scholar]
- 28.Aaronson DS, Elliott SP, McAninch JW. Transcorporal artificial urinary sphincter placement for incontinence in high-risk patients after treatment of prostate cancer. Urology. 2008;72:825. doi: 10.1016/j.urology.2008.06.065. [DOI] [PubMed] [Google Scholar]
- 29.Smith PJ, Hudak SJ, Scott JF, et al. Transcorporal artificial urinary sphincter cuff placement is associated with a higher risk of postoperative urinary retention. Can J Urol. 2013;20:6773. [PubMed] [Google Scholar]
- 30.Guralnick ML, Miller E, Toh KL, et al. Transcorporal artificial urinary sphincter cuff placement in cases requiring revision for erosion and urethral atrophy. J Urol. 2002;167:2075. [PubMed] [Google Scholar]