Abstract
Benign prostatic hyperplasia (BPH) and associated lower urinary tract symptoms (LUTS) are highly prevalent in older men and represent a substantial challenge to public health. Increasing epidemiologic evidence suggests that diabetes and associated hyperglycemia and insulin resistance significantly increase the risks of BPH and LUTS. Plausible pathophysiologic mechanisms to explain these associations include increased sympathetic tone, stimulation of prostate growth by insulin and related trophic factors, alterations in sex steroid hormone expression, and induction of systemic inflammation and oxidative stress. This article presents a comprehensive update of the current understanding of clinical and epidemiologic research on diabetes and BPH/LUTS, describes hypothesized pathophysiologic mechanisms linking these conditions, and recommends future directions for research.
Keywords: Benign prostatic hyperplasia, BPH, Lower urinary tract symptoms, LUTS, Diabetes, Hyperglycemia, Insulin resistance
Introduction
Benign prostatic hyperplasia (BPH) and associated lower urinary tract symptoms such as weak stream, nocturia, and urinary frequency are a highly prevalent medical condition associated with considerable patient morbidity [1]. BPH/ lower urinary tract symptoms (LUTS) negatively impact health-related quality of life including work productivity, social and family relationships, mental health, and sleep quality [2, 3]. The prevalence of BPH/LUTS is expected to grow sharply in the coming decades [4]. It has been estimated that by the year 2025, over 50 million adults in the USA will have symptoms [4]. Billions are spent annually to treat BPH/LUTS [5, 6].
Despite its significant public health impact, the pathophysiology of BPH/LUTS remains incompletely defined. The causative process likely involves multiple independent and interrelated pathways; metabolic syndrome, advanced age, inflammation, and mental illness have all been implicated [2, 7]. Researchers have postulated the possibility of a link between the metabolic syndrome and BPH/LUTS for two decades [8••]. Metabolic syndrome is defined to include three or more of the following: central obesity (waist circumference greater than 102 cm), HDL less than 40 mg/dl, triglycerides more than 150 mg/dl, blood pressure more than 135/ 85 mmHg, and fasting plasma glucose more than 110 mg/dl.
Mounting evidence suggests that diabetes mellitus is associated with BPH/LUTS [7, 9]. Diabetes mellitus is a chronic disorder associated with insulin resistance and hyperglycemia secondary to abnormal carbohydrate, fat, and protein metabolism. Given that diabetes mellitus can be improved with diet and exercise and is a modifiable risk factor of disease, it suggests that BPH can be prevented or improved through modifications of metabolic pathways. As obesity and diabetes reach epidemic proportions in the USA and globally, understanding the potential causal relationship of diabetes, hyperglycemia, and insulin resistance with BPH/LUTS could produce significant improvements for the health of men. Furthermore, the scope of the diabetes epidemic and the prevalence of BPH/LUTS underscores the need to further understand their relationship. Our objective is to review recent clinical and epidemiologic studies (Table 1) of hyperglycemia and insulin resistance and BPH to suggest a conceptual framework for planning future research and clinical care.
Table 1.
Studies on the association between diabetes, associated hyperglycemia and insulin resistance, and BPH/LUTS
| Year | Authors | Country | Number of samples | BPH definition | Diabetes definition | DM and BPH findings |
|---|---|---|---|---|---|---|
| 2014 | Ferreira et al. | Brazil: cross-sectional | 62 | LUTS (IPSS) | Self-reported | DM associated with LUTS, especially nocturia |
| 2014 | Qu et al. | China: cross-sectional | 117 | LUTS (IPSS); PSA; TRUS PV | FPG (≥7 mmol/l); 2-h oral glucose tolerance test (≥110 mg/dl) | Men with diabetes had increased prostate volume (41.18 versus 51.52 cm3, p=0.005) and increased PSA (1.94 versus 3.23, p=0.013) |
| 2014 | Russo et al. | Italy: cross-sectional | 544 | LUTS (IPSS); PSA; TRUS PV | HOM-IR, FPG>100 mg/dl | Insulin resistance was an independent predictor of severe LUTS (IPSS≥20) (OR=2.0, 95 % CI 1.20–3.34) |
| 2014 | Zhang et al. | China: cross-sectional | 401 | LUTS (IPSS); PSA; TRUS PV | FPG (≥110 mg/dl) | PV was correlated with FINS (r=0.421, p=0.001), but not fasting glucose (r=0.091, p=0.364) or HbA1c levels (r=0.153, p=0.127) |
| 2013 | Gacci et al. | Italy: cross-sectional | 271 | Prostatectomy for moderate/ severe LUTS due to BPH | AHA/NHLBI criteria or previous diagnosis of type 2 diabetes | Inflammatory score (p=0.049), lower uroflowmetric parameters (p=0.008) IPSS (p=0.064) |
| 2013 | Van Den Eeden et al. | USA: retrospective cohort | 63245 | LUTS (IPSS) | DM defined as: fasting glucose (>100 mg/dl) or DM diagnosis | LUTS OR=1.32(95 % CI, 1.26–1.38) No association between DM and new-onset LUTS |
| 2013 | Wallner et al. | USA: cohort | 369 | AUA-SI, maximal urinary flow rate, prostate volume, serum PSA | Self-report, HOMA-IR, FPG, FINS | No significant trends of metabolic disturbances as measured by serum glucose, insulin, or insulin resistance |
| 2012 | Sarma et al. | USA: cross-sectional | 2226 | AUA-SI, maximal urinary flow rate, prostate volume, serum PSA | Self-reported | LUTS OR=1.37 (95 % CI 1.00–1.87), LUTS in patients with no DM medication 2.05 (95 % CI 1.11–3.80) |
| 2011 | Yim et al. | Korea: cross-sectional | 968 | Transrectal ultrasound of prostate P; prostate-specific antigen | FPG; WC | Increased PV is associated FPG and WC: (18.9 versus 16.9 cm3, p=0.001; 19.5 versus 17.5 cm3, p=0.001) |
| 2009 | Wang et al. | Taiwan: case-control convenience sample from diabetes clinic and health fair Age <45 |
DM (n=226); Non-DM (n=183) | IPSS; FR/PVR | DM defined by American Diabetes Association criteria | Patients with diabetes were more. Men with diabetes compared to the control group had worse overall IPSS (6.1 versus 4.1, p<0.001), worse storage (2.7 versus 2.0, p=0.02), and voiding subdomains (3.5 versus 2.1, p<0.001) |
PSA prostate-specific antigen, PV prostate volume, FPG fasting plasma glucose, WC waist circumference, LUTS lower urinary tract symptoms, IPSS International Prostate Symptom Score, FINS fasting insulin, HbA1c glycosylated hemoglobin, FR/PVR flow rate/post-void residual, HOMA-IR homeostasis model of assessment-insulin resistance
Definitions of BPH and LUTS in Clinical Research
Although several pathologies may potentially contribute to BPH and the generation of BPH-associated LUTS, there are two general mechanisms by which BPH may induce bladder outlet obstruction: static and dynamic. The static mechanism involves hyperplastic stromal and epithelial prostate growth, which, over time, compresses the prostatic urethra. The dynamic mechanism entails increased tone of prostate smooth muscle, which is mediated by the alpha-1 adrenergic receptor: stimulation of the alpha-1 receptors induces a contraction and corresponding reduction in urethral lumen diameter. Obstruction of the bladder outlet induces two pathological changes in the structure of the bladder that may produce LUTS. First, decreased bladder compliance causes urinary frequency and urgency. Second, decreased bladder muscle contractility—resulting from chronic tonicity as the bladder labors to overcome increased urethral pressures—may precipitate urinary hesitancy, decreased force of stream, and high residual volumes [2, 3]. In addition to symptoms related to BPH, LUTS can be caused by overactive bladder, bladder stones, urethral stricture, foreign body, and interstitial cystitis, among others. Furthermore, when considering the relationship between diabetes and LUTS, one must consider diabetic cystopathy, an end-organ neuropathy characterized by decreased bladder sensation, increase bladder capacity, and reduced contractility. It is estimated that as many as 45 % of patients with diabetes have diabetic cystography [10].
Still, these relatively straightforward explanations belie the complexity of diagnosing and researching a disease that most often presents with highly subjective symptoms, has few robust objective markers, and overlaps considerably with other conditions that produce urinary symptoms. In fact, a persistent conundrum in both diagnosing and studying BPH is case definition. Case definitions of BPH in epidemiologic studies vary considerably, and measurement and detection biases are acknowledged limitations of BPH research. As a result, clinical and epidemiologic studies have used a number of objective and subjective measures to define BPH. Objective measures include histological analysis of prostate tissue, radiographically determined prostate enlargement, decreased urinary flow rate, prostate-specific antigen (PSA) concentrations, and pressure flow studies consistent with bladder outlet obstruction. Subjective measures include history of noncancer surgery on the prostate, physician-diagnosed BPH, and LUTS assessment with the American Urological Association Symptom Index (AUA-SI) or International Prostate Symptom Score (I-PSS). Importantly, no one definition has been shown to be any more appropriate or robust than the others.
Hypothesized Mechanisms
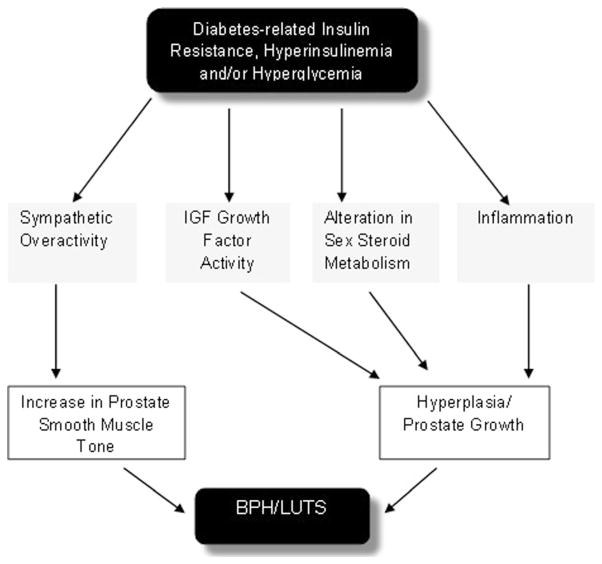
Multiple disparate and interrelated pathways may explain the association of diabetes-induced insulin resistance and hyperglycemia with BPH/LUTS (Fig. 1). First, hyperinsulinemia is associated with increased sympathetic nerve activity [11]. This increased nerve activity may contribute to increased prostate smooth muscle tone and subsequent bladder outlet obstruction. Increased outlet resistance can lead to obstructive symptoms as well as potential future irritative symptoms [12]. In addition, increased insulin concentrations secondary to diabetes may have a trophic affect that leads to enlarged prostate size [13]. McVary et al. demonstrated that autonomic nervous system hyperactivity was associated with increased LUTS and prostate size in a cohort of 38 men [13].
Fig. 1.
Hypothesized mechanisms of diabetes, hyperglycemia, and insulin resistance in the pathogenesis of BPH/LUTS
Second, dysregulation of the insulin-like growth factor (IGF) axis has been implicated in the development of BPH [14] and prostate cancer [15]. The IGF axis regulates the physiologic and pathophysiologic growth of many organs including the prostate [15]. Because of its structural similarity to IGF, insulin combines to IGF receptor to enter prostate cells, possibly causing receptor activation to induce growth and proliferation. Another possibility is as insulin levels increase, IBFBP-1 declines, increasing the bioavailability of IGF [9].
Third, insulin may increase the transcription of genes/ translation of proteins involved in sex hormone metabolism influencing the prostatic hormonal milieu [16]. Alternatively, diabetes-related insulin resistance/hyperinsulinemia/hyperglycemia-induced obesity may cause hormonal changes. Hyperinsulinemia is associated with lower levels of sex hormone-binding globulin, increasing the amount of sex hormone entering prostatic cells thereby influencing growth.
Finally, researchers have suggested the chronic pro-inflammatory state associated with metabolic syndrome, hyperglycemia, and hyperinsulinemia may contribute to BPH/ LUTS [17, 18]. In a recent mouse model of obesity-induced diabetes, pronounced prostatic and urethral tissue fibrosis and voiding dysfunction were associated with diabetic state [18]. Vignozzi, Gacci, and colleagues looked at 244 prostatectomy specimens and determined that metabolic syndrome factors showed a stepwise association with inflammatory histology scores [17]. In the same study, using cell-cultured human myofibroblast BPH cells, the authors showed that oxidized low-density lipoprotein enhanced the production of proinflammatory cytokine/chemokines and growth factors.
Hyperglycemia, Insulin Resistance, and Benign Prostatic Hyperplasia
Research investigating hyperglycemia, insulin resistance, and benign prostatic hyperplasia has produced conflicting results [9]. Although a substantial proportion of the existing literature supports an association between diabetes and BPH/LUTS, many studies have failed to differentiate LUTS from BPH leading to confusion. In the patient with diabetes, both conditions arise through complex, potentially disparate, and overlapping mechanisms mediated through environmental, hormonal, genetic, neuropathic, and microvascular dysfunction. There is clinical overlap between the presence of BPH and LUTS, with LUTS being a potential manifestation of BPH. An improved understanding whether diabetic metabolic dysfunction results in prostate growth will help determine whether diabetes-associated LUTS is related to prostate obstruction or more closely associated with the dynamic components of lower urinary tract function. Previous research in large part has been hampered by reliance on cross-sectional analysis, small sample sizes, narrow/select study populations, and inadequate control of potential confounding [9].
Findings from a number of previous studies suggest that BPH may be directly related to hyperglycemia and insulin resistance [19–21]. Others showed no association between the conditions [22, 23••], while others showed an inverse relationship between diabetes and clinical BPH [24]. Multiple recent studies have explored the association of diabetes, hyperglycemia, and insulin resistance with BPH [25–29, 30•, 31]. In a prospective population-based cohort of men from Michigan (n=2226), Sarma et al. examined the annual change of prostate volume and total PSA in men with and without diabetes. Men with diabetes were stratified by medication status. The annual change in prostate volume in total PSA were not statistically different across groups. In a multivariable logistic regression model, after adjusting for age and race, compared to men without diabetes, men with diabetes on or off medications did not have increased odds of having a prostate volume greater than 30 cm3 or a PSA greater than 2.5 ng/ml [27]. Similarly, in another analysis of the Flint Men’s Health Study subset of the Michigan population, researchers did not observe significant associations between hyperglycemia, hyperinsulinemia, and insulin resistance with the burden and progression of BPH after adjusting for age and BMI [30•]. These authors examined the homeostasis model of assessment-insulin resistance (HOMA-IR), a validated model that assesses the degree of insulin resistance (HOMA-IR= fasting serum glucose×fasting serum insulin/405) and markers of clinical BPH (LUTS severity, maximum urinary flow rate, prostate volume, and serum PSA concentrations). Given that LUTS and DM severity have been associated in this population before, the authors suggest that the presence of diabetes and subsequent poor glycemic control may be less related to prostate growth and more related to the dynamic components of lower urinary tract function [30•].
In an Italian study of 271 men referred to two tertiary centers for simple prostatectomy, increased fasting glycemia level was not associated with a prostate volume greater than 60 cm3 [29]. A convenience sample (n=117) of men older than 60 years of age from China showed that men with diabetes had increased prostate volume (41.18 versus 51.52 cm3, p=0.005) and increased PSA (1.94 v. 3.23, p= 0.013) [28]. In another population of elderly Chinese men (n= 401), prostate volume was correlated with fasting insulin level (r=0.421, p=0.001), but not fasting glucose (r=0.091, p= 0.364) or HbA1c levels (r=0.153, p=0.127) [26]. Yim et al. examined the relationship between metabolic syndrome and prostate volume in men aged 30–49 who underwent transrectal ultrasound over a 1-year period [25]. Overall, having metabolic syndrome was not associated with a statistically significant increase in prostate size compared to not having metabolic syndrome in men (18.4 versus 17.8 cm3, p=0.225). Abnormal fasting plasma glucose (≥100 mg/dl) and waist circumference (≥90 cm) were both associated with larger prostate volume compared with normal men (18.9 versus 16.9 cm3, p=0.001; 19.5 versus 17.5 cm3, p=0.001)
Hyperglycemia, Insulin Resistance, and Lower Urinary Tract Symptoms
It is widely recognized that LUTS is a syndrome with multiple potential etiologies of which BPH is one [9]. BPH is known to cause LUTS by both static and dynamic mechanisms, as mentioned earlier. A number of large high-quality studies have demonstrated the association of hyperglycemia and insulin resistance with LUTS [19, 32, 33]. In the past few years, multiple population-based reports linking diabetes with LUTS have been reported [7, 27, 31, 34•, 35, 36]. In the previously mentioned cohort from Michigan, Sarma et al. found in a model adjusted for age and race at baseline men with diabetes without medication were more likely to report irritative symptoms (odds ratio (OR) 95 % confidence interval (95 % CI) 2.04 (1.08–3.86)) than men with diabetes on medications (OR 95 % CI 1.46 (1.02–2.08)) or men without diabetes (reference group). While LUTS severity based on American Urological Association Symptom Index (AUA-SI) and maximal urinary flow rate less than 12 ml/s reached statistical significance in the unadjusted model, both became nonsignificant after adjustment for age and race. In an analysis of the separate seven domains of the AUA-SI, only nocturia defined as waking to urinate greater than one time per night was associated with history of diabetes. Again, the risk was higher in men with diabetes who took no medications (OR 95 % CI 2.4 (1.26–4.60) than those who were taking medications (OR 95 % CI 2.2 (1.52–3.23) compared to men without diabetes. Interestingly, in an analysis of annual change of AUA-SI score over a 4-year period, diabetes status did not increase or decrease AUA-SI. In another prospective study using data from the California Men’s Health Study and Research Program and Genes, Environment and Health, researchers found type 2 diabetes to be associated with prevalent LUTS (OR 95 % CI 1.32 (1.26–1.38) defined as a AUA-SI score of 8 or more [34•]. Interestingly, no association was observed between type 2 diabetes and new-onset LUTS. Similar to the Michigan cohort where no change in LUTS were seen, the researchers found no influence of diabetes on incident LUTS. In data from the Boston Area Community Health Survey (n=1899), men with either self-reported type 2 diabetes, increased blood sugar level, or diabetes medication use were more likely to have mild (OR 95 % CI 1.95 (1.22–3.12) or severe (OR 95 % CI 2.87 (1.56–5.31) LUTS [7]. Patients with diabetes were more likely to have irritative then storage symptoms. In a case-control cohort of Taiwanese men <45, those with diabetes compared to the control group had worse overall International Prostate Symptom Score (IPSS) (6.1 versus 4.1, p<0.001), worse storage (2.7 versus 2.0, p=0.02), and voiding subdomains (3.5 versus 2.1, p<0.001) [35]. Interestingly, overall quality of life, uroflow Q max, and post-void residual were no different between groups. Men with diabetes had an average voided volume 50 ml greater than men without diabetes (p= 0.04). Finally, in a recent convenience sample from Italy (n= 544), insulin resistance was an independent predictor of severe LUTS (IPSS≥20) (OR=2.0, 95 % CI 1.20–3.34) [31].
Conclusions
Overall, these data suggest that diabetes increases the risks of BPH and LUTS and support the concept that BPH and LUTS to some extent are preventable disorders associated with modifiable exposures, challenging us to revisit traditional paradigms of diagnosis and treatment. Previously, BPH and LUTS have been viewed as immutable processes of aging resulting from relatively nonmodifiable stimuli. This paradigm resulted in reactive approaches to clinical management and research that focused on the categorization and management of symptoms rather than on proactive identification of at-risk individuals or on the design of population-based prevention programs.
Additionally, these data suggest alternative etiologies for BPH outside of sex-steroid hormone growth pathways including sympathetic nervous system and/or insulin-like growth factor activity and systemic inflammation. The elucidation of alternate etiologies necessitates the development of novel descriptions for BPH and LUTS. Although LUTS are the primary clinical manifestation of BPH, they also represent a syndrome generated by a host of bladder-related etiologies that may or may not coexist with true pathological BPH. Distinguishing BPH-associated LUTS from non-BPH LUTS may reveal patterns by which diabetes influences BPH and LUTS phenotypes, suggest new methods for diagnosis, and allow more precise tailoring of treatments.
Regardless of etiology, the prevention of BPH and LUTS is of substantial importance to public health. Current disease trends in the USA suggest that, as the population ages, diabetes, BPH, and LUTS will markedly increase in prevalence and place substantial strains on finite health care resources. Future research is therefore needed to identify the magnitude of onset and progression of BPH associated with diabetes; elucidate mechanisms by which diabetes exerts its effects on BPH; and identify the most effective treatment and prevention strategies for BPH associated with diabetes to reduce the psychosocial, medical, and economic costs of these highly prevalent and chronic disorders affecting men.
Footnotes
Conflict of Interest: Dr. Benjamin N. Breyer and Dr. Aruna V. Sarma each declare no potential conflicts of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Benjamin N. Breyer, Email: BBreyer@urology.ucsf.edu, Department of Urology, University of California, San Francisco, 1001 Potrero Ave, Suite 3A20, San Francisco, CA 94110-1444, USA
Aruna V. Sarma, Email: asarma@umich.edu, Department of Urology and Epidemiology, University of Michigan, 2800 Plymouth Road, Room 109E, Ann Arbor, MI 48109-2800, USA
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Martin S, Lange K, Haren MT, Taylor AW, Wittert G Members of the Florey Adelaide Male Ageing Study. Risk factors for progression or improvement of lower urinary tract symptoms in a prospective cohort of men. J Urol. 2014;191:130–7. doi: 10.1016/j.juro.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Breyer BN, Shindel AW, Erickson BA, Blaschko SD, Steers WD, Rosen RC. The association of depression, anxiety and nocturia: a systematic review. J Urol. 2013;190:953–7. doi: 10.1016/j.juro.2013.03.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int. 2008;101:1388–95. doi: 10.1111/j.1464-410X.2008.07601.x. [DOI] [PubMed] [Google Scholar]
- 4.Litman HJ, McKinlay JB. The future magnitude of urological symptoms in the USA: projections using the Boston Area Community Health survey. BJU Int. 2007;100:820–5. doi: 10.1111/j.1464-410X.2007.07018.x. [DOI] [PubMed] [Google Scholar]
- 5.Saigal CS, Joyce G. Economic costs of benign prostatic hyperplasia in the private sector. J Urol. 2005;173:1309–13. doi: 10.1097/01.ju.0000152318.79184.6f. [DOI] [PubMed] [Google Scholar]
- 6.Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108:1132–8. doi: 10.1111/j.1464-410X.2010.09993.x. [DOI] [PubMed] [Google Scholar]
- 7.Kupelian V, McVary KT, Kaplan SA, et al. Association of lower urinary tract symptoms and the metabolic syndrome: results from the Boston Area Community Health survey. J Urol. 2013;189:S107–14. doi: 10.1016/j.juro.2012.11.026. discussion S15–6. [DOI] [PubMed] [Google Scholar]
- 8••.Hammarsten J, Peeker R. Urological aspects of the metabolic syndrome. Nat Rev Urol. 2011;8:483–94. doi: 10.1038/nrurol.2011.112. Excellent review of the metabolic syndrome and its components and their impact on urologic complications. [DOI] [PubMed] [Google Scholar]
- 9.Sarma AV, Kellogg PJ. Diabetes and benign prostatic hyperplasia: emerging clinical connections. Curr Urol Rep. 2009;10:267–75. doi: 10.1007/s11934-009-0044-5. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki K, Yoshimura N, Chancellor MB. Implications of diabetes mellitus in urology. Urol Clin N Am. 2003;30:1–12. doi: 10.1016/s0094-0143(02)00116-7. [DOI] [PubMed] [Google Scholar]
- 11.Straznicky NE, Grima MT, Sari CI, et al. Neuroadrenergic dysfunction along the diabetes continuum: a comparative study in obese metabolic syndrome subjects. Diabetes. 2012;61:2506–16. doi: 10.2337/db12-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oelke M, Kirschner-Hermanns R, Thiruchelvam N, Heesakkers J. Can we identify men who will have complications from benign prostatic obstruction (BPO)? ICI-RS 2011. Neurourol Urodyn. 2012;31:322–6. doi: 10.1002/nau.22222. [DOI] [PubMed] [Google Scholar]
- 13.McVary KT, Rademaker A, Lloyd GL, Gann P. Autonomic nervous system overactivity in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2005;174:1327–433. doi: 10.1097/01.ju.0000173072.73702.64. [DOI] [PubMed] [Google Scholar]
- 14.Neuhouser ML, Schenk J, Song YJ, et al. Insulin-like growth factor-I, insulin-like growth factor binding protein-3 and risk of benign prostate hyperplasia in the prostate cancer prevention trial. Prostate. 2008;68:1477–86. doi: 10.1002/pros.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampson N, Zenzmaier C, Heitz M, et al. Stromal insulin-like growth factor binding protein 3 (IGFBP3) is elevated in the diseased human prostate and promotes ex vivo fibroblast-to-myofibroblast differentiation. Endocrinology. 2013;154:2586–99. doi: 10.1210/en.2012-2259. [DOI] [PubMed] [Google Scholar]
- 16.Jangir RN, Jain GC. Diabetes mellitus induced impairment of male reproductive functions: a review. Curr Diabetes Rev. 2014;10:147–57. doi: 10.2174/1573399810666140606111745. [DOI] [PubMed] [Google Scholar]
- 17.Vignozzi L, Gacci M, Cellai I, et al. Fat boosts, while androgen receptor activation counteracts, BPH-associated prostate inflammation. Prostate. 2013;73:789–800. doi: 10.1002/pros.22623. [DOI] [PubMed] [Google Scholar]
- 18.Gharaee-Kermani M, Rodriguez-Nieves JA, Mehra R, Vezina CA, Sarma AV, Macoska JA. Obesity-induced diabetes and lower urinary tract fibrosis promote urinary voiding dysfunction in a mouse model. Prostate. 2013;73:1123–33. doi: 10.1002/pros.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parsons JK, Carter HB, Partin AW, et al. Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab. 2006;91:2562–8. doi: 10.1210/jc.2005-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammarsten J, Hogstedt B, Holthuis N, Mellstrom D. Components of the metabolic syndrome-risk factors for the development of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 1998;1:157–62. doi: 10.1038/sj.pcan.4500221. [DOI] [PubMed] [Google Scholar]
- 21.Nandeesha H, Koner BC, Dorairajan LN, Sen SK. Hyperinsulinemia and dyslipidemia in non-diabetic benign prostatic hyperplasia. Clin Chimica Acta; Int J Clin Chem. 2006;370:89–93. doi: 10.1016/j.cca.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Boon TA, Van Venrooij GE, Eckhardt MD. Effect of diabetes mellitus on lower urinary tract symptoms and dysfunction in patients with benign prostatic hyperplasia. Curr Urol Rep. 2001;2:297–301. doi: 10.1007/s11934-001-0067-z. [DOI] [PubMed] [Google Scholar]
- 23••.Burke JP, Jacobson DJ, McGree ME, et al. Diabetes and benign prostatic hyperplasia progression in Olmsted County, Minnesota. Urology. 2006;67:22–5. doi: 10.1016/j.urology.2005.08.010. Excellent prospective cohort study of the effects of diabetes on progression of clinical markers of BPH. [DOI] [PubMed] [Google Scholar]
- 24.Glynn RJ, Campion EW, Bouchard GR, Silbert JE. The development of benign prostatic hyperplasia among volunteers in the Normative Aging Study. Am J Epidemiol. 1985;121:78–90. [PubMed] [Google Scholar]
- 25.Yim SJ, Cho YS, Joo KJ. Relationship between metabolic syndrome and prostate volume in Korean men under 50 years of age. Korean J Urol. 2011;52:390–5. doi: 10.4111/kju.2011.52.6.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Zeng X, Liu Y, Dong L, Zhao X, Qu X. Impact of metabolic syndrome on benign prostatic hyperplasia in elderly Chinese men. Urologia internationalis. 2014 May 22; doi: 10.1159/000357760. [DOI] [PubMed] [Google Scholar]
- 27.Sarma AV, St Sauver JL, Hollingsworth JM, et al. Diabetes treatment and progression of benign prostatic hyperplasia in community-dwelling black and white men. Urology. 2012;79:102–8. doi: 10.1016/j.urology.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qu X, Huang Z, Meng X, Zhang X, Dong L, Zhao X. Prostate volume correlates with diabetes in elderly benign prostatic hyperplasia patients. Int Urol Nephrol. 2014;46:499–504. doi: 10.1007/s11255-013-0555-3. [DOI] [PubMed] [Google Scholar]
- 29.Gacci M, Vignozzi L, Sebastianelli A, et al. Metabolic syndrome and lower urinary tract symptoms: the role of inflammation. Prostate Cancer Prostatic Dis. 2013;16:101–6. doi: 10.1038/pcan.2012.44. [DOI] [PubMed] [Google Scholar]
- 30•.Wallner LP, Hollingsworth JM, Dunn RL, et al. Hyperglycemia, hyperinsulinemia, insulin resistance, and the risk of BPH/LUTS severity and progression over time in community dwelling black men: the Flint Men’s Health Study. Urology. 2013;82:881–6. doi: 10.1016/j.urology.2013.05.034. Nice paper examining comprehensive set of diabetes markers and their influence on BPH/LUTS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo GI, Cimino S, Fragala E, et al. Insulin resistance is an independent predictor of severe lower urinary tract symptoms and of erectile dysfunction: results from a cross-sectional study. J Sex Med. 2014;11:2074–82. doi: 10.1111/jsm.12587. [DOI] [PubMed] [Google Scholar]
- 32.Sarma AV, Burke JP, Jacobson DJ, et al. Associations between diabetes and clinical markers of benign prostatic hyperplasia among community-dwelling Black and White men. Diabetes Care. 2008;31:476–82. doi: 10.2337/dc07-1148. [DOI] [PubMed] [Google Scholar]
- 33.Michel MC, Mehlburger L, Schumacher H, Bressel HU, Goepel M. Effect of diabetes on lower urinary tract symptoms in patients with benign prostatic hyperplasia. J Urol. 2000;163:1725–9. [PubMed] [Google Scholar]
- 34•.Van Den Eeden SK, Ferrara A, Shan J, et al. Impact of type 2 diabetes on lower urinary tract symptoms in men: a cohort study. BMC Urol. 2013;13:12. doi: 10.1186/1471-2490-13-12. Nice paper examining the overall effect of type 2 diabetes on LUTS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang CC, Chancellor MB, Lin JM, Hsieh JH, Yu HJ. Type 2 diabetes but not metabolic syndrome is associated with an increased risk of lower urinary tract symptoms and erectile dysfunction in men aged <45 years. BJU Int. 2010;105:1136–40. doi: 10.1111/j.1464-410X.2009.08913.x. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira FT, Daltoe L, Succi G, et al. Relation between glycemic levels and low tract urinary symptoms in elderly. Aging Male : Off J Int Soc Study Aging Male. 2014;19:1–4. doi: 10.3109/13685538.2014.908461. [DOI] [PubMed] [Google Scholar]