Abstract
Background
Liver-X-receptors, LXRα (NR1H3) and LXRβ (NR1H2), encode two different but highly homologous isoforms of transcription factors belonging to the nuclear receptor superfamily. Whether LXRα and LXRβ subtypes have discrete roles in the regulation of cardiac physiology/pathology is unknown. We determine the role of each LXR subtype in myocardial ischemia/reperfusion (MI/R) injury.
Methods and Results
Mice (wild type; those genetically depleted of LXRα, LXRβ, or both; and those overexpressing LXRα or LXRβ by in-vivo intramyocardial adenoviral vector) were subjected to MI/R injury. Both LXRα and LXRβ were detected in wild type mouse heart. LXRα, but not LXRβ, was significantly upregulated after MI/R. Dual activation of LXRα and LXRβ by natural and synthetic agonists reduced myocardial infarction and improved contractile function after MI/R. Mechanistically, LXR activation inhibited MI/R-induced oxidative stress and nitrative stress, attenuated endoplasmic-reticulum stress and mitochondrial dysfunction, and reduced cardiomyocyte apoptosis in ischemic/reperfused myocardium. The aforementioned cardioprotective effects of LXR agonists were impaired in the setting of cardiac-specific gene silencing of LXRα, but not LXRβ subtype. Moreover, LXRα/β double-knockout and LXRα- knockout mice, but not LXRβ-knockout mice, increased MI/R injury, exacerbated MI/R-induced oxidative/nitrative stress, and aggravated endoplasmic-reticulum stress and mitochondrial dysfunction. Furthermore, cardiac LXRα, not LXRβ, overexpression via adenoviral transfection suppressed MI/R injury.
Conclusions
Our study provides the first direct evidence that the LXRα, but not LXRβ, subtype is a novel endogenous cardiac protective receptor against MI/R injury. Drug development strategies specifically targeting LXRα may be beneficial in treating ischemic heart disease.
Keywords: nuclear receptors, myocardium, reperfusion injury, apoptosis
Acute myocardial infarction is a leading cause of morbidity and mortality worldwide.1 Myocardial ischemia/reperfusion (MI/R) injury, sustained by ischemic myocardium following current reperfusion therapies (including thrombolysis, coronary angioplasty, and coronary bypass surgery), represents an important clinical problem with significant morbidity and mortality.2 Novel pharmacological or molecular interventions mitigating reperfusion injury, adjunctive to current reperfusion therapies, are in great need.
Nuclear hormone receptors are a family of transcription regulators involved in diverse physiological functions, such as cell proliferation, apoptosis, tumorigenesis, and angiogenesis. Several members of this superfamily are expressed in the cardiovascular system, pivotally regulating cardiovascular function.3–6 Livers-X-receptors (LXRs) are ligand-activated transcriptional factors belonging to the nuclear receptor superfamily. The two known receptor subtypes, LXRα (NR1H3) and LXRβ (NR1H2), exhibit different expression patterns, and may perform different functional roles.7 In contrast to LXRβ, a subtype ubiquitously expressed in all cell types, LXRα is selectively expressed in metabolically active tissues (such as liver, kidney, adipose, and intestine).7 As such, LXRα is traditionally viewed as a metabolic regulatory factor. Recent evidence suggests that LXRα/LXRβ are both expressed in the cardiovascular system. The importance of LXRs in vascular cells is well documented, and recent evidence implicates different regulatory functions of LXRα and LXRβ subtypes in the pathogenesis of vascular diseases both in-vitro and in-vivo.8–10 LXRs are also detected in cardiomyocytes, and play important roles in several cardiac diseases.11–13 However, the specific roles of individual LXR subtypes in the regulation of cardiac physiology/pathology are at present unknown. The question of whether LXRα and LXRβ have discrete roles in cardiac physiology/pathology is particularly important, since current strategies for LXR-targeted drugs are focused upon development of tissue- and subtype-selective ligands.
In this study, we determined the regulatory role of two different LXR isoforms in the pathogenesis of MI/R-induced myocardial injury, employing mice genetically depleted of LXRs (LXRα, LXRβ, or both) and in-vivo intramyocardial adenovirus mediated LXRα or LXRβ overexpression.
Methods
This investigation conformed to the National Institutes of Health Guidelines on the Use of Laboratory Animals, and was approved by the Institute’s Animal Ethics Committee. Experiments were performed on wild type (WT) C57BL/6 and LXR-deficient [LXRα-, LXRβ-, or LXRα/β double-knockout (KO)] male mice. Pharmacologic experiments employed LXR endogenous agonist 22(R)-hydroxycholesterol [22(R)-HC] and synthetic agonist GW3965.14, 15 In the acute MI/R protocol, reperfusion commenced for 24 hours following 30 minutes of ischemia. Mice were randomly assigned to the following groups: sham, vehicle, 22(R)-HC (20 mg/kg), or GW3965 (20 mg/kg) by intraperitoneal injection (IP) 15 minutes before reperfusion. To observe the long-term cardioprotective effect of LXRα/β dual agonists, mice were randomly assigned to one of the following groups: sham, vehicle, 22(R)-HC (20 mg/kg), or GW3965 (20 mg/kg) by IP injection 15 minutes before reperfusion, and daily following reperfusion for 4 weeks. Other experiments were designed for MI/R outcome determination in LXRα-, β-, and α/β double-deficiency mice and their WT littermates. Myocardial apoptosis was determined by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) technique and cardiac caspase-3 activity. Myocardial infarct size was determined by Evans blue-TTC double staining methods.16 In-vivo cardiac function was determined by echocardiography.17 Viable myocardium was assessed via micro-positron emission tomography/computed tomography (micro-PET/CT) scanning. Myocardial reactive oxygen species generation was measured by confocal microscopy via in-situ dehydroetidium (DHE) stain or lucigenin-enhanced chemiluminescence. Myocardial nitrative stress was assessed by nitrotyrosine content. In-vivo knockdown of cardiac-specific LXRα or LXRβ expression was achieved by intramyocardial delivery of siRNA. In-vivo cardiac gene overexpression was achieved by adenoviral-encoded LXRα or LXRβ transfection. An expanded Methods appears in the Data Supplement.
Statistical Analysis
All values in the text and figures are presented as the mean±SEM of independent experiments from given n-sizes. Statistical analysis was performed with Mann-Whitney test for two-group comparisons. Statistical significance of multiple treatments was determined by Kruskal-Wallis tests followed by Dunn’s post hoc test. Probabilities of 0.05 or less were considered to be statistically significant (2-tailed).
Results
Both LXRα and LXRβ are expressed in adult heart tissue, but LXRα is selectively upregulated by ischemia/reperfusion
Both LXRα and LXRβ subtypes were detected in cardiac tissue, as demonstrated by both Western blot (Figure 1A) and real-time PCR (Figure 1B), albeit to lesser degree in comparison to those in the liver. More importantly, endogenous LXRα protein level significantly increased after reperfusion in the ischemic risk area, whereas LXRβ expression remained mostly unaffected (Figure 1C and 1D).
Figure 1. Expression of LXRs in heart tissue from control and mice subject to myocardial ischemia/reperfusion (MI/R).
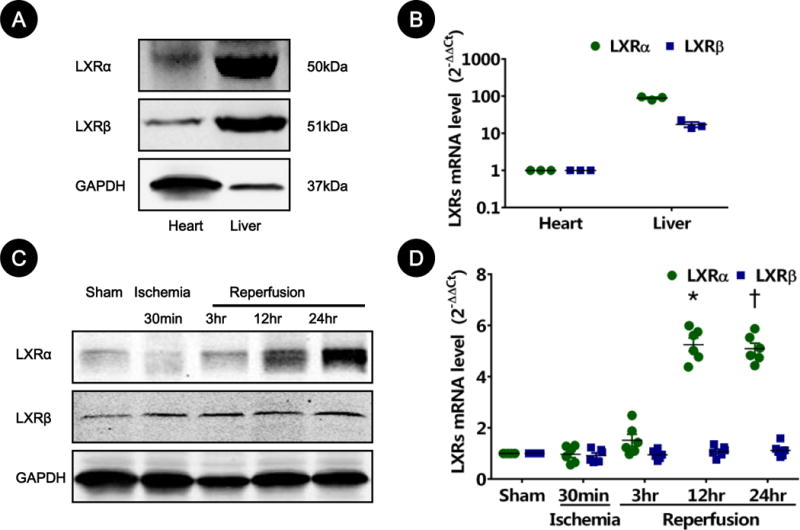
A. LXR protein expression via Western blot from mouse left ventricular tissues. Mice liver lysates served as positive controls. LXRα bands (~50kDa) and LXRβ bands (~51kDa) are representative of three separate experiments.
B. LXR gene expression via real-time PCR from mouse left ventricular tissues. Mice liver lysates served as positive controls. Results are representative of 3 separate tissues.
C–D. Cardiac LXR alteration after MI/R. Time course of LXR alteration from ischemic risk areas detected by Western blot (C) and real-time quantitative PCR (D) from hearts subjected to MI/R for indicated times (n=5–6). Sham-operated animals served as control. Results were normalized against GAPDH and converted to fold induction relative to sham-operated controls. *P=0.002, †P=0.005 vs. sham-operated controls.
LXRα/β dual agonists inhibit MI/R-induced myocardial apoptosis, infarct size, and cardiac dysfunction
To determine whether post MI/R upregulation of LXRα mediates myocardial reperfusion injury or acts as a defensive pro-survival signal, mice were treated with vehicle, 22(R)-HC, or GW3965, and MI/R injury determinants were assessed. Treatment with LXR agonists had no significant effect upon heart rate or mean arterial blood pressure (Supplementary Material, Figure S1). Compared to vehicle, administration of either 22(R)-HC or GW3965 significantly reduced the number of apoptotic nuclei and caspase-3 activity (Figure 2A), decreased infarct size [from 40.2±1.0% in vehicle group to 15.7±1.2% (P=0.01) and 13.0±0.6% (P=0.001) in treated group, Figure 2B], and improved echocardiographic measurements of left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) [65.3±4.7% and 34.3±2.8% in the 22(R)-HC group, 60.7±2.6% and 29.2±2.4% in the GW3965 group, vs. 39.5±2.1% and 20.7±1.6%, in the vehicle group (LVEF: P=0.001, P=0.002 vs. vehicle; LVFS: P=0.002, P=0.04 vs. vehicle, respectively), Figure 2C]. These data suggest LXR activation plays an important cardioprotective role in acute MI/R injury.
Figure 2. LXRα/β dual agonists inhibited acute MI/R-induced myocardial apoptosis, infarct size, and cardiac dysfunction.
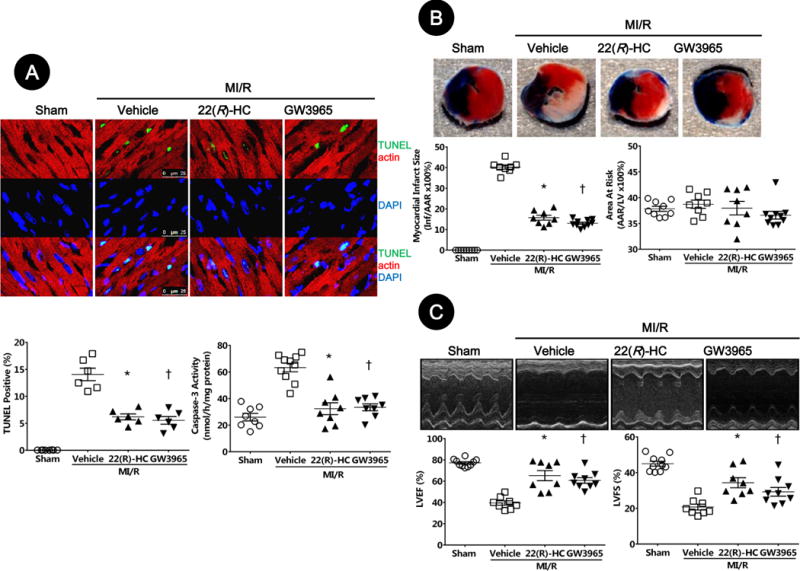
A. Myocardial apoptosis was determined by TUNEL labeling [TUNEL (green), apoptotic nuclei; α-actin (red), myocytes; and DAPI (blue), total nuclei. (n=6–8, bar=25 μm, *P=0.02, †P=0.003 vs. vehicle)] and caspase-3 activation (n=8–10, *P=0.0006, †P=0.002 vs. vehicle).
B. Myocardial infarction was determined by Evans blue/TTC double-staining (n=8–10, *P=0.01, †P=0.001 vs. vehicle).
C. Cardiac function was determined by echocardiography (n=8–10; EF: *P=0.001, †P=0.002 vs. vehicle; FS: *P=0.002, †P=0.04 vs. vehicle). Abbreviations: 22(R)-HC, 22(R)-hydroxycholesterol; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; AAR, area at risk; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening.
To further determine the long-term cardioprotective effect of LXR agonists, mice received agonists 15 minutes before reperfusion, and then daily following reperfusion for 4 weeks. Viable myocardial metabolism was assessed by fluorodeoxyglucose(18F) (18F-FDG) Micro-PET/CT. Cardiac function was determined by echocardiography 4 weeks after reperfusion. MI/R markedly reduced mean myocardial standardized uptake values (SUV) of 18F-FDG. LXR agonists-treated mice manifested significantly increased 18F-FDG uptake compared to vehicle [2.6±0.2 in the 22(R)-HC group, 2.4±0.2 in the GW3965 group, vs. 1.2±0.2 in the vehicle group, P=0.01 and P=0.04 respectively, Figures 3A and 3B]. Furthermore, 22(R)-HC or GW3965 treatment significantly increased echocardiographic-measured LVEF compared to vehicle [53.7±4.7% in the 22(R)-HC-treated group or 52.7±5.6% in the GW3965-treated group, compared to 32.8±3.5% in the vehicle group, P=0.01 and P=0.02 respectively, Figure 3C].
Figure 3. LXRα/β dual agonists improved long-term left ventricular performance and viable myocardium metabolism after MI/R.
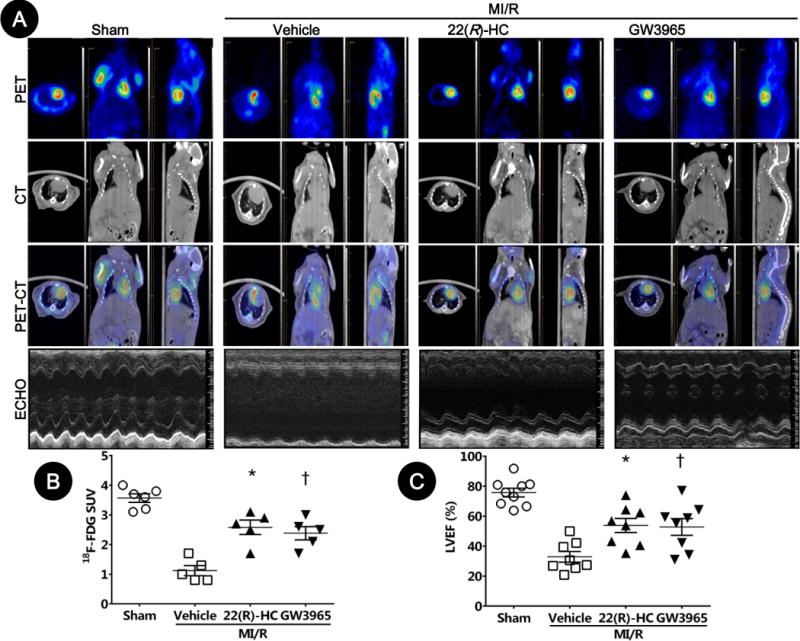
Left ventricular viable myocardium was assessed via 18F-FDG uptake by Micro-PET/CT, and left ventricular performance was determined by echocardiography after 4 weeks of reperfusion.
A. Representative Micro-PET images, Micro-CT images, Micro-PET/CT overlap images, and echocardiographic images.
B. Mean myocardial SUV of 18F-FDG by in-vivo small animal Micro-PET/CT scanning (n=5–6; *P=0.01, †P=0.04 vs. vehicle).
C. Cardiac function was determined by echocardiography (n=8–9; *P=0.01, †P=0.02 vs. vehicle). Abbreviations: 22(R)-HC, 22(R)-hydroxycholesterol; PET, positron emission tomography; CT, computed tomography; ECHO, echocardiography; SUV, standardized uptake values.
LXRα/β dual agonists inhibit the endoplasmic-reticulum (ER) stress- and mitochondrial-mediated apoptosis pathway
To determine the cellular mechanism by which LXR agonists reduces apoptosis and MI/R injury, the effects of LXR activation upon caspase-12 (index of ER stress pathway), caspase-9 (mitochondrial pathway), and caspase-8 (death receptor pathway) activities were analyzed. As expected, all 3 caspases were activated after MI/R. However, administration of LXR agonists significantly inhibited caspase-12 and caspase-9 activity without altering caspase-8 activity (Figure 4A). Moreover, the cleaved caspase-12, CCAAT/-enhancer-binding protein homologous protein (CHOP), cleaved caspase-9, and cytosolic cytochrome c levels were markedly increased in the ischemic/reperfused heart, and significantly inhibited by 22(R)-HC or GW3965 treatment (Figure 4B–E). Taken together, these results suggest that LXR activation reduces post-ischemic myocardial apoptosis primarily by inhibiting the apoptotic pathways mediated by ER stress and mitochondria.
Figure 4. LXRα/β dual agonists inhibited ER stress- and the mitochondrial-mediated apoptosis pathway.
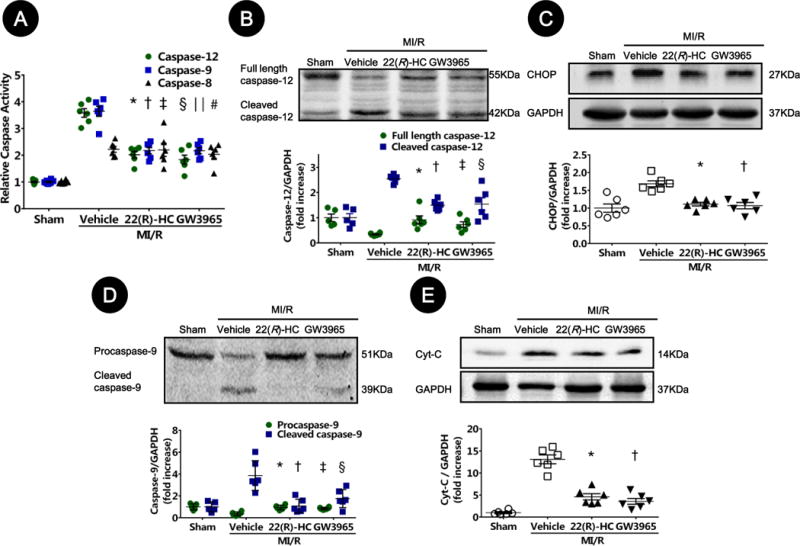
A. Caspase-12, caspase-9, and caspase-8 activation (A) were measured by cleavage of specific substrates from sham-operated and mice subjected to MI/R. Relative AFC fluorescence is fold-increase activity compared to sham (n=6–8; caspase-12: *P=0.02, §P=0.002 vs. vehicle; caspase-9: †P=0.007, ‖P=0.007 vs. vehicle; caspase-8: ‡P>0.99, #P=0.96 vs. vehicle).
B–E. Expressions of caspase-12 (B), CHOP (C), caspase-9 (D), and cytosolic cytochrome c (E) proteins in ischemic/reperfused myocardial tissue were determined by Western blot. GAPDH level served as loading control for total protein expression (n=5–6; full length caspase-12: *P=0.003, ‡P=0.03 vs. vehicle; cleaved caspase-12: †P=0.01, §P=0.01 vs. vehicle; CHOP: *P=0.008, †P=0.006 vs. vehicle; procaspase-9: *P=0.003, ‡P=0.02 vs. vehicle; cleaved caspase-9: †P=0.003, §P=0.04 vs. vehicle; Cyt-C: *P=0.02, †P=0.002 vs. vehicle). Abbreviations: 22(R)-HC, 22(R)-hydroxycholesterol; CHOP, CCAAT/-enhancer-binding protein homologous protein; Cyt-C, cytochrome c.
LXRα/β dual agonists attenuate oxidative/nitrative stress in ischemic/reperfused myocardium
To further determine the molecular mechanisms underlying the ER- and mitochondrial-mediated protective actions of LXR agonists, we investigated the effects of LXR activation upon oxidative/nitrative stress in the ischemic/reperfused myocardium. Both 22(R)-HC and GW3965 attenuated MI/R-induced reactive oxygen species production (Figure 5A and 5B). Furthermore, both LXR agonists inhibited the expression of gp91phox subunit of the NADPH oxidase (Figure 5C). Moreover, LXR agonists significantly reduced tissue nitrotyrosine content (a well-accepted footprint of in-vivo nitrative stress, Figure 5D and 5E) and inhibited inducible nitric oxide synthase (iNOS) expression (Figure 5F). Collectively, these results demonstrate that LXR activation attenuated oxidative/nitrative stress following MI/R, and subsequently inhibited the apoptotic pathways mediated by ER stress and mitochondria.
Figure 5. LXRα/β dual agonists attenuated oxidative/nitrative stresses in ischemic/reperfused myocardium.
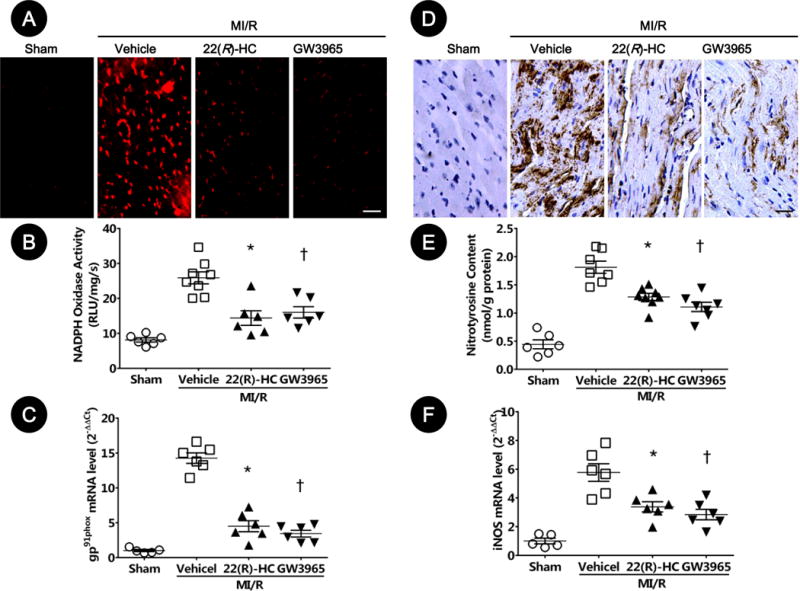
A–C. LXR activation attenuated oxidative stresses in ischemic/reperfused myocardium. A. Myocardial oxidative stress was measured utilizing confocal microscope with in-situ dihydroethidium stain (n=5–6, bar=25 μm). B. NADPH oxidase activity was determined by lucigenin-enhanced chemiluminescence (n=6–8; *P=0.005, †P=0.02 vs. vehicle). C. NADPH oxidase gp91phox gene expression was determined by real-time PCR (n=5–6; *P=0.02, †P=0.002 vs. vehicle). Results were normalized against GAPDH and converted to fold induction relative to sham-operated controls.
D–F. LXR activation attenuated nitrative stress in ischemic/reperfused myocardium. D. Myocardial nitrative stress was assessed via nitrotyrosine levels determined by immunohistochemistry (n=5–6, bar=25 μm). E. Myocardial nitrotyrosine content was determined by ELISA analysis (n=6–8; *P=0.02, †P=0.004 vs. vehicle). F. The gene expression of iNOS was determined by real-time PCR (n=5–6; *P=0.04, †P=0.005 vs. vehicle). Results were normalized against GAPDH and converted to fold induction relative to sham-operated controls. Abbreviations: 22(R)-HC, 22(R)-hydroxycholesterol; RLU, relative light units; iNOS, inducible nitric oxide synthase.
Cardioprotective effects of LXRα/β dual agonist are impaired in cardiac-specific LXRα-knockdown (KD) but not LXRβ-KD mice
The aforementioned experimental results (MI/R upregulates LXRα but not LXRβ, and non-selective LXR agonists protect against MI/R injury) suggest LXRα might be the cardioprotective LXR isotype. To obtain direct evidence supporting this notion, cardiac-specific gene silencing of either LXRα or LXRβ was performed prior to MI/R by in-vivo intramyocardial siRNA transfection (Supplementary Material, Figure S2A). The cardioprotective effects of GW3965 were blunted when endogenous cardiac LXRα was knocked-down (Supplementary Material, Figure S2B–C). However, GW3965 administration in cardiac LXRβ-KD mice remained effective in reducing MI/R induced myocardial apoptosis, decreasing infarct size, and enhancing LVEF (Supplementary Material, Figure S2B–C). Finally, knock-down of LXRα, but not LXRβ, mitigated inhibition of caspase-9 and caspase-12 activation (Supplementary Material, Figure S3A) and the anti-oxidative/anti-nitrative effects of GW3965 (Supplementary Material, Figure S3B–C). These results demonstrate that the cardioprotective effects of GW3965 may be primarily mediated by the LXRα-receptor subtype. Collectively, these results demonstrate that LXRα subtype activation attenuated oxidative/nitrative stress following MI/R, and subsequently inhibited ER- and mitochondrial-mediated apoptosis.
LXRα/β double-KO and LXRα-KO, but not LXRβ-KO, increases MI/R injury
To further confirm the role of the LXRα subtype in the MI/R injury, the effects of LXRα-, LXRβ-, and LXRα/β double-KO upon MI/R injury was investigated. The expression of LXRα and LXRβ subtypes in WT, LXRα-, LXRβ-, and LXRα/β double-KO mice by Western blot and real-time PCR are shown in Figure 6A. Compared to WT controls, LXRα/β double-KO and LXRα-KO mice exhibited increased apoptosis, infarct size, and cardiac dysfunction after acute MI/R (Figure 6B–C). Moreover, 4 weeks after reperfusion, LXRα/β double-KO and LXRα-KO mice exhibited decreased echocardiographic LVEF and 18F-FDG uptake of viable myocardium compared to WT controls (Figure 6D). In contrast, no significant difference was observed between LXRβ-KO and WT mice (Figure 6B–D). Mechanistically, knockout of LXRα/β and LXRα, but not LXRβ, exacerbated caspase-9 and caspase-12 activation, and increased oxidative/nitrative stress after MI/R (Figure 7A–C). Together, these results confirm that LXRα, not LXRβ, is the endogenous cardiac protective receptor against MI/R injury.
Figure 6. LXRα/β double-KO and LXRα-KO mice were more susceptible to MI/R injury.
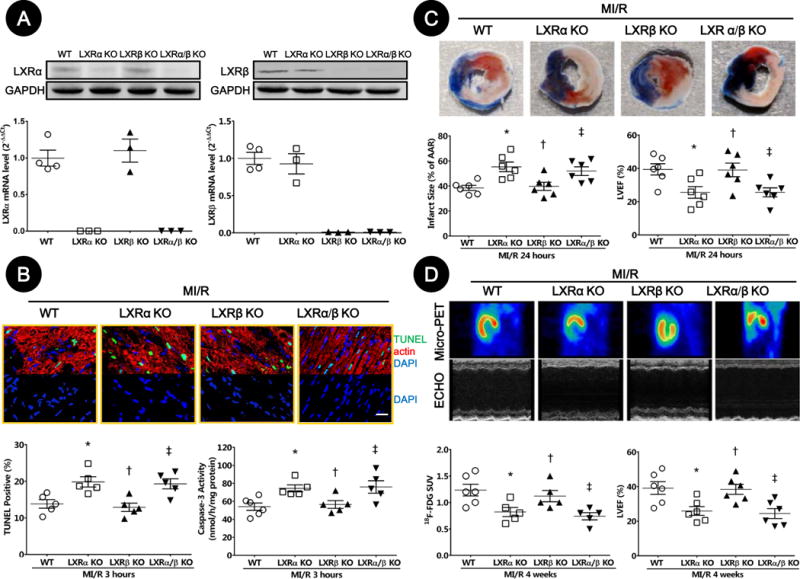
A. LXRα and LXRβ expression in cardiac tissue from WT, LXRα-KO, LXRβ-KO, and LXRα/β double-KO mice were determined by Western blot and real-time PCR.
B. Myocardial apoptosis was determined by TUNEL labeling [TUNEL (green), apoptotic nuclei; α-actin (red), myocytes; and DAPI (blue), total nuclei. (n=5, bar=25 μm; *P=0.03, †P>0.99, ‡P=0.04 vs. WT)] and caspase-3 activation (n=5–6; *P=0.02, †P>0.99, ‡P=0.03 vs. WT).
C. MI/R injury was assessed by myocardial infarction size determined by Evans blue/TTC double-staining (n=6; *P=0.007, †P>0.99, ‡P=0.03 vs. WT), and left ventricular dysfunction determined by echocardiography (n=6; *P=0.03, †P>0.99, ‡P=0.04 vs. WT).
D. Cardiac function was determined by left ventricular viable myocardium assessed via 18F-FDG uptake (Mean SUV) by Micro-PET/CT (n=5–6; *P=0.04, †P>0.99, ‡P=0.01 vs. WT), and left ventricular performance determined by echocardiography (n=6; *P=0.04, †P>0.99, ‡P=0.02 vs. WT).
Abbreviations: TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; AAR, area at risk; LVEF, left ventricular ejection fraction; PET, positron emission tomography; ECHO, echocardiography; SUV, standardized uptake values; WT, wild type; KO, knockout.
Figure 7. LXRα/β double-knockout (KO) and LXRα-KO mice exacerbated oxidative/nitrative stresses in ischemic/reperfused myocardium.
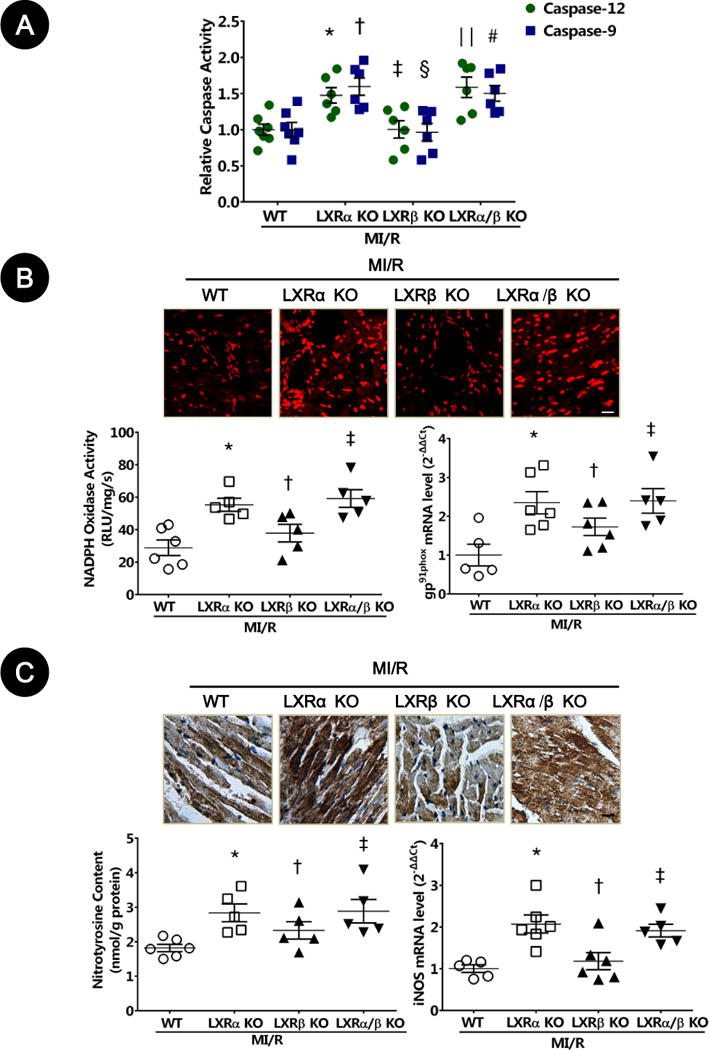
A. Caspase-9 and caspase-12 activity was measured by fluorometric assay (n=5–6; caspase-12: *P=0.03, ‡P>0.99, ‖P=0.007 vs. WT; caspase-9: †P=0.008, §P>0.99, #P=0.03 vs. WT).
B. Myocardial oxidative stress was measured utilizing confocal microscope with in-situ dihydroethidium stain (n=5–6, bar=25 μm). NADPH oxidase activity was determined by lucigenin-enhanced chemiluminescence (n=5–6; *P=0.02, †P>0.99, ‡P=0.005 vs. WT). NADPH oxidase gp91phox gene expression was determined by real-time PCR (n=5–6; *P=0.04, †P=0.61, ‡P=0.02 vs. WT). Results were normalized against GAPDH and converted to fold induction relative to WT mice.
C. Nitrative stress was assessed by nitrotyrosine levels determined by immunohistochemistry (n=5–6, bar=25 μm). Myocardial nitrotyrosine content was determined by ELISA analysis (n=5–6; *P=0.01, †P=0.43, ‡P=0.01 vs. WT). iNOS gene expression was determined by real-time PCR (n=5–6; *P=0.008, †P>0.99, ‡P=0.02 vs. WT). Results were normalized against GAPDH and converted to fold induction relative to WT mice.
Abbreviations: MI/R, myocardial ischemia/reperfusion; WT, wild type; RLU, relative light units; iNOS, inducible nitric oxide synthase.
Cardioprotective effects of LXRα/β dual agonist are lost in LXRα/β double-KO and LXRα-KO but not LXRβ-KO mice
To obtain more direct evidence supporting cardioprotective effects of LXRα subtype, we determined the effects of LXRα-KO, LXRβ-KO, and LXRα/β double-KO upon LXR agonist-mediated cardioprotection. The anti-apoptotic (Figure 8A), infarct sparing (Figure 8B), functional improving (Figure 8C), anti-oxidative (Figure S4A), and anti-nitrative (Figure S4B) effects of LXRα/β dual agonist were abrogated in LXRα/β double-KO and LXRα-KO mice, but not in LXRβ-KO mice.
Figure 8. Cardioprotective effects of LXRα/β dual agonist were lost in LXRα/β double- and LXRα-knockout (KO) mice.
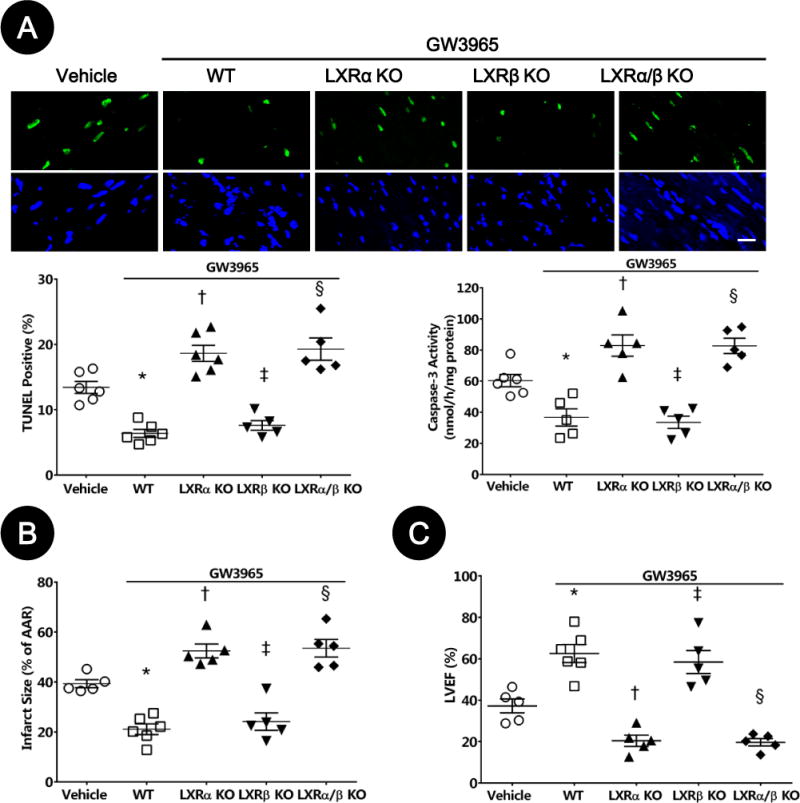
A. Myocardial apoptosis was determined by TUNEL labeling [TUNEL (green), apoptotic nuclei; and DAPI (blue), total nuclei. (n=5–6, bar=25μm; *P=0.002, †P=0.03, ‡P=0.04, §P=0.02 vs. vehicle)] and caspase-3 activity was measured by fluorometric assay (n=5–6; *P=0.02, †P=0.02, ‡P=0.01, §P=0.03, vs. vehicle).
B–C. MI/R injury was assessed by myocardial infarction size as determined by Evans blue/TTC double-staining (n=5–6; *P=0.008, †P=0.02, ‡P=0.04, §P=0.01 vs. vehicle), and left ventricular dysfunction was determined by echocardiography (n=5–6; *P=0.005, †P=0.02, ‡P=0.04, §P=0.02 vs. vehicle). Abbreviations: WT, wild type; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; MI/R, myocardial ischemia/reperfusion; AAR, area at risk; LVEF, left ventricular ejection fraction.
Cardiac overexpression of LXRα, but not LXRβ, suppresses MI/R injury
In a final attempt to obtain additional direct evidence supporting LXRα subtype activation being cardioprotective against MI/R injury, either cardiac LXRα or LXRβ overexpression was performed prior to MI/R by in-vivo intramyocardial adenoviral-encoded LXRα or LXRβ (Ad.LXRα or Ad.LXRβ) transfection respectively. Adenoviral-encoded LacZ (Ad.LacZ) transfection served as control (Figure S5A). Compared to Ad.LacZ transfection controls, Ad.LXRα overexpression mice exhibited decreased apoptosis, infarct size, and cardiac dysfunction after MI/R (Figure S5B–C). However, no significant cardioprotective effects were observed in the cardiac Ad.LXRβ transfection group (Figure S5B–C). These data further demonstrated that LXRα, not LXRβ, is the protective subtype against MI/R injury.
Discussion
Our study provides new insights into understanding the discrete role of two different LXR subtypes in the heart. The novel contributions include the following: (1) Both LXRα and LXRβ subtypes were detected in adult cardiac tissue, but LXRα is selectively upregulated by ischemia/reperfusion; (2) LXRα/β dual agonists inhibited MI/R-induced infarct size and cardiac dysfunction via inhibition of oxidative/nitrative stress, and subsequently reducing the apoptotic pathways mediated by ER stress and mitochondria; (3) The cardioprotective effects of LXRα/β dual agonist were impaired in the setting of cardiac-specific gene silencing via in-vivo intramyocardial transfection of siRNA of LXRα, but not LXRβ, subtype; (4) LXRα/β double-KO and LXRα-KO, but not LXRβ-KO, increased MI/R injury, exacerbated MI/R-induced oxidative/nitrative stress, and ER stress and mitochondrial dysfunction; (5) LXRα/β double-KO and LXRα-KO, but not LXRβ-KO, completely blocked the cardioprotective effects of LXR agonists; and (6) Cardiac LXRα, not LXRβ, overexpression via adenoviral transfection suppressed MI/R injury. In summation, we present the first direct evidence that the LXRα, but not LXRβ, subtype is a novel endogenous cardiac protective receptor against MI/R injury.
LXRs, orphan nuclear hormone receptors originally cloned in liver tissue,18 function as cholesterol sensors with well-recognized function in regulating cholesterol homeostasis.7 Two different but highly homologous LXR isoforms, LXRα (NR1H3) and LXRβ (NR1H2), have been discovered separately. The human LXRα gene is located on chromosome 11p11.2, while the human LXRβ gene is located on chromosome 19q13.3. LXRα expression predominates in metabolically active tissues such as the liver, kidney, adipose, and intestines, whereas LXRβ is more ubiquitously expressed with particularly high levels in the developing brain,7, 19 suggesting differential physiological function regulation for these two receptors. Although LXRα and LXRβ presence in the cardiovascular system had been identified,8–13 little information is available regarding their individual roles in the myocardium. To our knowledge, the current study is the first to investigate the specific roles of two individual LXR subtypes in the cardiac physiology/pathology. The results of our study contributes to the growing body of data emphasizing the distinctively isoform-specific functions of LXR are mediated by two different receptors, by demonstrating that LXRα, a subtype with previously unappreciated cardiovascular significance, but not LXRβ, a subtype known to be ubiquitously expressed (including heart), is a novel endogenous cardiac protective receptor against MI/R injury. This conclusion is supported by the following novel evidence. Firstly, the expression of LXRα (but not LXRβ) was significantly increased in the heart subjected to MI/R. Secondly, MI/R injury was significantly increased in LXRα/β double-KO and LXRα-KO, but not in LXRβ-KO mice. Thirdly, genetic ablation (knockout or cardiac-specific KD) of LXRα, but not LXRβ, blocked the cardioprotective effect of LXRα/β dual agonists. Fourthly, cardiac-specific LXRα, but not LXRβ, overexpression suppressed MI/R injury. Our current observations enrich our understanding of the different biological functions of LXRα and LXRβ in different tissues. The LXRα subtype is the isoform responsible for hepatic lipogenesis,20 with a dominant role in limiting atherosclerosis in-vivo.9 The LXRβ subtype regulates lipogenesis and cholesterol efflux in skeletal muscle,21 with anti-thrombotic effects in human platelets.22 Such data concerning tissue- and isoform-specific effects of LXRs are of practical interest in the development of tissue- or isoform-specific LXR pharmacologic modulators for treating various pathologies.23
To provide mechanistic insight into how LXR activation protected heart against MI/R injury, we investigated the effect of LXR activation upon the apoptosis pathway, a significant contributor to myocardial cell death following ischemia/reperfusion injury. Our study demonstrated that during MI/R, all three apoptosis pathways (the extrinsic, intrinsic, and ER stress-specific apoptotic pathways) were activated, as shown by the activation of different caspase markers (caspase-8, caspase-9, and caspase-12, respectively). LXR activation attenuated the activation of markers of ER stress (cleaved caspase-12 and CHOP) and mitochondrial stress (cleaved caspase-9 and cytochrome c release) without altering cleaved caspase-8 protein levels (the extrinsic apoptotic marker) and its activity. To define the role of the different LXR isoforms in the regulation of cardiomyocyte apoptosis, LXRα-KO, LXRβ-KO, and LXRα/β double-KO mice were subject to MI/R. We observed that, compared to WT mice, ER stress- and mitochondrial-mediated apoptosis was exacerbated after MI/R in LXRα/β double-KO and LXRα-KO, but not LXRβ-KO mice. Furthermore, the ER- and mitochondrial-protective effects of LXR agonist were lost in the setting of genetic ablation of LXRα, but not LXRβ subtype. Thus, LXRα but not LXRβ inhibited the apoptotic pathways mediated by ER stress and mitochondria. Since oxidative/nitrative stress is causatively related to ER stress and mitochondrial dysfunction, we further determined the role of the different LXR isoforms in the regulation of oxidative/nitrative stress. We demonstrated that activation of LXRα, but not LXRβ, significantly decreased myocardial NADPH oxidase expression, attenuated superoxide generation, and reduced both iNOS expression and tissue nitrotyrosine content in ischemic/reperfused myocardium. Taken together, our results demonstrated LXRα subtype protected against MI/R injury via mechanisms inhibiting oxidative/nitrative stresses, subsequently reducing ER- and mitochondrial-mediated apoptosis.
The involvement of LXR in regulating the apoptotic process has been reported in several cell and tissue types. Interestingly, LXR activation was shown to be antiapoptotic in several cell types, and proapoptotic in others. For example, LXR activation inhibited apoptosis in endothelial cells,24 intestine25 and lung tissue,26 but facilitated apoptosis in tumor cells, such as breast, colorectal and prostate carcinomas.27–29 Thus, the role of LXR in inhibiting or promoting apoptosis may be dependent upon cell and tissue type.
Heretofore, several members of the nuclear hormone receptor superfamily have reputed involvement in MI/R injury pathophysiology. Peroxisome proliferator-activated receptors [PPAR-α (NR1C1), -β (NR1C2), and -γ (NR1C3)],3 estrogen receptor (NR3A),30 and androgen receptor (NR3C4)31 have been proposed as the endogenous protective receptors against myocardial apoptosis and MI/R injury, while activation of nuclear receptor farnesoid-X-receptor (NR1H4)6 and Nur77 (NR4A1)5 exacerbates myocardial apoptosis and MI/R injury. Our study adds novel evidence that LXRα (NR1H3) acts as an endogenous protective factor against MI/R injury. Given the distinct regulatory roles of these nuclear receptors in myocardial apoptosis and MI/R injury, it is conceivable that potential regulatory cross-talk among them might maintain the delicate homeostatic balance between cellular death and survival in the heart. Of particular interest is a human macrophage study demonstrating PPARγ activation directly increased LXRα expression via PPAR binding to an LXRα promoter site.32 In a study investigating adipocytes, activated LXRα promoted PPARγ gene expression by directly binding the LXR response element motif in the PPARγ promoter in a positive-feedback loop,33 such that these two nuclear hormone receptors reinforced each other’s expression. Characterization of the molecular cross-talk between these nuclear receptor pathways is requisite for better design of novel therapeutics ameliorating MI/R injury.
Conclusion
In summary, our study provides the first evidence that LXRα, a LXR subtype traditionally viewed as a metabolic regulatory factor, has much stronger cardiac phenotype than ubiquitously expressed LXR subtype, LXRβ. LXRα (but not LXRβ) subtype is a powerful cardiac protection receptor against MI/R injury, via mechanisms (at least in part) inhibiting oxidative/nitrative stresses, and subsequently reducing ER- and mitochondrial-mediated apoptosis. LXRα subtype, therefore, represents a potentially attractive molecular target for the treatment of ischemic heart disease.
Supplementary Material
Acknowledgments
This work was presented in part at the 2012 Annual Scientific Session of the American Heart Association (Los Angeles, CA, USA) and published in abstract form (Circulation 2012;126: A9571).
Sources of Funding: This work was supported by grants from the National Natural Science Foundation of China (81330006, 81170192, 81470389, 81270282, 81070176, and 81200163 to B.H., J.P., and Q.H.), Key Basic Research Program of Shanghai Committee of Science and Technology (14JC1404500 to J.P.), Program for New Century Excellent Talents from Ministry of Education of China (NCET-12-0352 to J.P.), Shanghai Shuguang Program (12SG22 to J.P.), the National Institutes of Health (HL-123404, HL-096686 to X.L.M.), and the American Diabetes Association (7-11-BS-93 to X.L.M.).
Footnotes
Disclosures
None.
References
- 1.Pu J, Mintz GS, Brilakis ES, Banerjee S, Abdel-Karim AR, Maini B, Biro S, Lee JB, Stone GW, Weisz G, Maehara A. In vivo characterization of coronary plaques: novel findings from comparing greyscale and virtual histology intravascular ultrasound and near-infrared spectroscopy. Eur Heart J. 2012;33:372–383. doi: 10.1093/eurheartj/ehr387. [DOI] [PubMed] [Google Scholar]
- 2.Pu J, Mintz GS, Biro S, Lee JB, Sum ST, Madden SP, Burke AP, Zhang P, He B, Goldstein J, Stone GW, Muller JE, Virmani R, Maehara A. Insights into echo-attenuated plaques, echolucent plaques, and plaques with spotty calcification: novel findings from comparisons among intravascular ultrasound, near-infrared spectroscopy, and pathological histology in 2294 human coronary artery segments. J Am Coll Cardiol. 2014;63:2220–2233. doi: 10.1016/j.jacc.2014.02.576. [DOI] [PubMed] [Google Scholar]
- 3.Lotz C, Lazariotto M, Redel A, Smul TM, Stumpner J, Blomeyer C, Tischer-Zeitz T, Schmidt J, Pociej J, Roewer N, Kehl F, Lange M. Activation of peroxisome-proliferator-activated receptors alpha and gamma mediates remote ischemic preconditioning against myocardial infarction in vivo. Exp Biol Med (Maywood) 2011;236:113–122. doi: 10.1258/ebm.2010.010210. [DOI] [PubMed] [Google Scholar]
- 4.Shan P, Pu J, Yuan A, Shen L, Shen L, Chai D, He B. RXR agonists inhibit oxidative stress-induced apoptosis in H9c2 rat ventricular cells. Biochem Biophys Res Commun. 2008;375:628–633. doi: 10.1016/j.bbrc.2008.08.074. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Z, Volkers M, Din S, Avitabile D, Khan M, Gude N, Mohsin S, Bo T, Truffa S, Alvarez R, Mason M, Fischer KM, Konstandin MH, Zhang XK, Heller Brown J, Sussman MA. Mitochondrial translocation of Nur77 mediates cardiomyocyte apoptosis. Eur Heart J. 2011;32:2179–2188. doi: 10.1093/eurheartj/ehq496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pu J, Yuan A, Shan P, Gao E, Wang X, Wang Y, Lau WB, Koch W, Ma XL, He B. Cardiomyocyte-expressed farnesoid-X-receptor is a novel apoptosis mediator and contributes to myocardial ischaemia/reperfusion injury. Eur Heart J. 2013;34:1834–1845. doi: 10.1093/eurheartj/ehs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213–224. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishibashi M, Filomenko R, Rebe C, Chevriaux A, Varin A, Derangere V, Bessede G, Gambert P, Lagrost L, Masson D. Knock-down of the oxysterol receptor LXRalpha impairs cholesterol efflux in human primary macrophages: lack of compensation by LXRbeta activation. Biochem Pharmacol. 2013;86:122–129. doi: 10.1016/j.bcp.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Bischoff ED, Daige CL, Petrowski M, Dedman H, Pattison J, Juliano J, Li AC, Schulman IG. Non-redundant roles for LXRalpha and LXRbeta in atherosclerosis susceptibility in low density lipoprotein receptor knockout mice. J Lipid Res. 2010;51:900–906. doi: 10.1194/jlr.M900096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley MN, Hong C, Chen M, Joseph SB, Wilpitz DC, Wang X, Lusis AJ, Collins A, Hseuh WA, Collins JL, Tangirala RK, Tontonoz P. Ligand activation of LXR beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR alpha and apoE. J Clin Invest. 2007;117:2337–2346. doi: 10.1172/JCI31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu S, Yin R, Ernest R, Li Y, Zhelyabovska O, Luo J, Yang Y, Yang Q. Liver X receptors are negative regulators of cardiac hypertrophy via suppressing NF-kappaB signalling. Cardiovasc Res. 2009;84:119–126. doi: 10.1093/cvr/cvp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuipers I, Li J, Vreeswijk-Baudoin I, Koster J, van der Harst P, Sillje HH, Kuipers F, van Veldhuisen DJ, van Gilst WH, de Boer RA. Activation of liver X receptors with T0901317 attenuates cardiac hypertrophy in vivo. Eur J Heart Fail. 2010;12:1042–1050. doi: 10.1093/eurjhf/hfq109. [DOI] [PubMed] [Google Scholar]
- 13.Lei P, Baysa A, Nebb HI, Valen G, Skomedal T, Osnes JB, Yang Z, Haugen F. Activation of Liver X receptors in the heart leads to accumulation of intracellular lipids and attenuation of ischemia-reperfusion injury. Basic Res Cardiol. 2013;108:323. doi: 10.1007/s00395-012-0323-z. [DOI] [PubMed] [Google Scholar]
- 14.Morales JR, Ballesteros I, Deniz JM, Hurtado O, Vivancos J, Nombela F, Lizasoain I, Castrillo A, Moro MA. Activation of liver X receptors promotes neuroprotection and reduces brain inflammation in experimental stroke. Circulation. 2008;118:1450–1459. doi: 10.1161/CIRCULATIONAHA.108.782300. [DOI] [PubMed] [Google Scholar]
- 15.Kuipers I, van der Harst P, Kuipers F, van Genne L, Goris M, Lehtonen JY, van Veldhuisen DJ, van Gilst WH, de Boer RA. Activation of liver X receptor-alpha reduces activation of the renal and cardiac renin-angiotensin-aldosterone system. Lab Invest. 2010;90:630–636. doi: 10.1038/labinvest.2010.7. [DOI] [PubMed] [Google Scholar]
- 16.Ma H, Gong H, Chen Z, Liang Y, Yuan J, Zhang G, Wu J, Ye Y, Yang C, Nakai A, Komuro I, Ge J, Zou Y. Association of Stat3 with HSF1 plays a critical role in G-CSF-induced cardio-protection against ischemia/reperfusion injury. J Mol Cell Cardiol. 2012;52:1282–1290. doi: 10.1016/j.yjmcc.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Kang YM, Zhang AQ, Zhao XF, Cardinale J, Elks C, Cao XM, Zhang ZW, Francis J. Paraventricular nucleus corticotrophin releasing hormone contributes to sympathoexcitation via interaction with neurotransmitters in heart failure. Basic Res Cardiol. 2011;106:473–483. doi: 10.1007/s00395-011-0155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apfel R, Benbrook D, Lernhardt E, Ortiz MA, Salbert G, Pfahl M. A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol Cell Biol. 1994;14:7025–7035. doi: 10.1128/mcb.14.10.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan X, Kim HJ, Bouton D, Warner M, Gustafsson JA. Expression of liver X receptor beta is essential for formation of superficial cortical layers and migration of later-born neurons. Proc Natl Acad Sci U S A. 2008;105:13445–13450. doi: 10.1073/pnas.0806974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korach-Andre M, Parini P, Larsson L, Arner A, Steffensen KR, Gustafsson JA. Separate and overlapping metabolic functions of LXRalpha and LXRbeta in C57Bl/6 female mice. Am J Physiol Endocrinol Metab. 2010;298:E167–E178. doi: 10.1152/ajpendo.00184.2009. [DOI] [PubMed] [Google Scholar]
- 21.Hessvik NP, Boekschoten MV, Baltzersen MA, Kersten S, Xu X, Andersen H, Rustan AC, Thoresen GH. LXR{beta} is the dominant LXR subtype in skeletal muscle regulating lipogenesis and cholesterol efflux. Am J Physiol Endocrinol Metab. 2010;298:E602–E613. doi: 10.1152/ajpendo.00553.2009. [DOI] [PubMed] [Google Scholar]
- 22.Spyridon M, Moraes LA, Jones CI, Sage T, Sasikumar P, Bucci G, Gibbins JM. LXR as a novel antithrombotic target. Blood. 2011;117:5751–5761. doi: 10.1182/blood-2010-09-306142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faulds MH, Zhao C, Dahlman-Wright K. Molecular biology and functional genomics of liver X receptors (LXR) in relationship to metabolic diseases. Curr Opin Pharmacol. 2010;10:692–697. doi: 10.1016/j.coph.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 24.ElAli A, Hermann DM. Liver X receptor activation enhances blood-brain barrier integrity in the ischemic brain and increases the abundance of ATP-binding cassette transporters ABCB1 and ABCC1 on brain capillary cells. Brain Pathol. 2012;22:175–187. doi: 10.1111/j.1750-3639.2011.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crisafulli C, Di Paola R, Mazzon E, Paterniti I, Galuppo M, Genovese T, Bramanti P, Cappellani A, Cuzzocrea S. Liver X receptor agonist treatment reduced splanchnic ischemia and reperfusion injury. J Leukoc Biol. 2010;87:309–321. doi: 10.1189/jlb.0609438. [DOI] [PubMed] [Google Scholar]
- 26.Crisafulli C, Mazzon E, Paterniti I, Galuppo M, Bramanti P, Cuzzocrea S. Effects of Liver x receptor agonist treatment on signal transduction pathways in acute lung inflammation. Respir Res. 2010;11:19. doi: 10.1186/1465-9921-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Roz A, Bard JM, Huvelin JM, Nazih H. LXR agonists and ABCG1-dependent cholesterol efflux in MCF-7 breast cancer cells: relation to proliferation and apoptosis. Anticancer Res. 2012;32:3007–3013. [PubMed] [Google Scholar]
- 28.Lo Sasso G, Bovenga F, Murzilli S, Salvatore L, Di Tullio G, Martelli N, D’Orazio A, Rainaldi S, Vacca M, Mangia A, Palasciano G, Moschetta A. Liver X receptors inhibit proliferation of human colorectal cancer cells and growth of intestinal tumors in mice. Gastroenterology. 2013;144:1497–1507. doi: 10.1053/j.gastro.2013.02.005. 1507 e1–e13. [DOI] [PubMed] [Google Scholar]
- 29.Pommier AJ, Alves G, Viennois E, Bernard S, Communal Y, Sion B, Marceau G, Damon C, Mouzat K, Caira F, Baron S, Lobaccaro JM. Liver X Receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene. 2010;29:2712–2723. doi: 10.1038/onc.2010.30. [DOI] [PubMed] [Google Scholar]
- 30.Lin J, Steenbergen C, Murphy E, Sun J. Estrogen receptor-beta activation results in S-nitrosylation of proteins involved in cardioprotection. Circulation. 2009;120:245–254. doi: 10.1161/CIRCULATIONAHA.109.868729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsang S, Wu S, Liu J, Wong TM. Testosterone protects rat hearts against ischaemic insults by enhancing the effects of alpha(1)-adrenoceptor stimulation. Br J Pharmacol. 2008;153:693–709. doi: 10.1038/sj.bjp.0707624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laffitte BA, Joseph SB, Walczak R, Pei L, Wilpitz DC, Collins JL, Tontonoz P. Autoregulation of the human liver X receptor alpha promoter. Mol Cell Biol. 2001;21:7558–7568. doi: 10.1128/MCB.21.22.7558-7568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo JB, Moon HM, Kim WS, Lee YS, Jeong HW, Yoo EJ, Ham J, Kang H, Park MG, Steffensen KR, Stulnig TM, Gustafsson JA, Park SD, Kim JB. Activated liver X receptors stimulate adipocyte differentiation through induction of peroxisome proliferator-activated receptor gamma expression. Mol Cell Biol. 2004;24:3430–3444. doi: 10.1128/MCB.24.8.3430-3444.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


